Abstract
Mobile genetic elements classed as transposons comprise an estimated 45% of the human genome, and 8% of these elements are human endogenous retroviruses (HERVs). Endogenous retroviruses are retrotransposons, containing 5′ and 3′ long terminal repeat sequences and encoding envelope, group-specific antigen and DNA polymerase proteins. The aim of the present study was to analyse genome integration polymorphisms of HERV type K member 6 (HERV-K6) and HERV-K11 by using the retrotransposon based molecular marker technique, inter-retrotransposon amplified polymorphism (IRAP). For this purpose, blood samples of 18 healthy individuals within the age range of 10–79 years (10 females and 8 males) were collected, genomic DNAs were isolated and IRAP-polymerase chain reaction (PCR) was performed. IRAP-PCR analyses demonstrated that there were 0–70% polymorphism rates for HERV-K6, and 0–38% polymorphism rates for HERV-K11 among all the samples. Furthermore, the polymorphism rates were 0–70% among females and 11–60% among males for HERV-K6, and 0–38% among females and 0–25% among males for HERV-K11. Age-associated polymorphism was also investigated, but no age-associated polymorphism was observed among the samples. Therefore, HERV-K6 and HERV-K11 polymorphisms may arise on an individual-specific basis. Various previous studies have investigated the associations between the expression of HERVs and cancer or other major diseases. However, few reports have analysed HERV-K movements among individuals. This is the first report to investigate HERV-K6 and HERV-K11 retrotransposon polymorphisms between the genders and different age groups.
Keywords: mobile genetic elements, human endogenous retrovirus type K, polymorphism, human genome
Introduction
Human endogenous retroviruses (HERVs) belonging to the superfamily of transposable and retrotransposable genetic elements represent ~8% of the human genome (1). HERV type K (HERV-K) is the sole group of endogenous retroviruses that is established to contain human-specific members. Group HERV-K (also known as human mouse mammary tumour virus like-2, HML-2) occupies ~5% of the DNA created by insertions of human-specific transposable elements and is among the most studied groups of human retroelements (2). The HERV-K group may be divided into 10 families. The designation ‘K’ originates from their use of a lysine transfer RNA to prime reverse transcription, while ‘HML-2’ indicates the relationship to the murine betaretrovirus mouse mammary tumour virus (2).
The disease association of HERVs has been a focus of research, and there have been a number of studies on the different expression profiles of HERVs in various clinical situations: According to certain studies, HERVs may serve a significant role in embryonic development, thereby contributing to formation of the placenta, and may have correlation with cancer and autoimmune diseases (3–6). However, there are few studies on the transpositions of HERVs in the human genome. Furthermore, to the best of our knowledge, there have been no studies on age-related polymorphisms of HERVs in the human genome. HERV-K elements are the youngest and most active family among HERVs. There has been specific focus on the ability of these elements to cause or prevent diseases through their expression (7). Taking these into consideration, the present study detected retrotransposon polymorphisms in HERV-K member 6 (HERV-K6) and HERV-K11 in healthy individuals of different ages (between 10 and 79 years old) by using the inter-retrotransposon amplified polymorphism (IRAP) molecular marker technique. The IRAP technique was developed as a molecular marker method due to the abundance and ubiquity of long terminal repeat (LTR) transposons in the plant genome (8). This technique amplifies the sequences between two adjacent retrotransposons with primers facing outward from the LTR sequences (9). Previous studies by our group demonstrated that the IRAP technique could also be applied to the human genome (10,11). Thus, the IRAP technique was the method of choice in the present study for polymorphism analysis of HERV-K transpositions in human subjects.
Materials and methods
Sample collection and DNA extraction
HERV-K6 and HERV-K11 retrotransposon transpositions were analysed in DNA samples of 18 healthy individuals between the ages of 10 and 79 years old. The DNA samples were from Dr Kaniye Sahin's DNA collections at Istanbul University Medical Faculty (Istanbul, Turkey). The subjects from which samples were collected were Turkish, and from the Istanbul University Department of Molecular Biology and Genetics. Subjects completed an information form prior to sample collection. This form requested health information from subjects regarding concomitant diseases, medication and genetic history. Excluded subjects were those presenting with any disease or genetic disorder and/or were on a current course of medication. Table I lists the gender and age information of the subjects. The present study established 5 age groups: 10–19 (n=4), 20–29 (n=4), 40–49 (n=3), 60–69 (n=3) and 70–79 (n=4) years. Genomic DNAs were extracted from 5 ml venous blood samples (intravenous; collected between January and February 2013) using a High Pure PCR Template Preparation kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's protocol. The extracted genomic DNA samples were stored at −20°C until use. The procedures followed were in accordance with the current ethical standards of Istanbul University Medical Faculty, and the information form included a signed statement of written informed consent agreeing to the use of patient materials for research purposes on the condition of anonymity being retained.
Table I.
Demographic information of enrolled subjects.
| Subject no. | Sex | Age range (years) |
|---|---|---|
| 1 | Female | 10–19 |
| 2 | Male | 10–19 |
| 3 | Female | 10–19 |
| 4 | Female | 10–19 |
| 5 | Male | 20–29 |
| 6 | Male | 20–29 |
| 7 | Male | 20–29 |
| 8 | Female | 20–29 |
| 9 | Male | 40–49 |
| 10 | Female | 40–49 |
| 11 | Male | 40–49 |
| 12 | Male | 60–69 |
| 13 | Female | 60–69 |
| 14 | Female | 60–69 |
| 15 | Female | 70–79 |
| 16 | Female | 70–79 |
| 17 | Female | 70–79 |
| 18 | Male | 70–79 |
IRAP-polymerase chain reaction (PCR) analysis
Primer sequences of HERV-K6 and HERV-K11 were obtained from the National Centre for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/; accession nos. AF074086.2 and DQ112099.1, respectively; Table II). IRAP-PCR was performed with 2X SapphireAmp Fast PCR Master Mix (Takara Biotechnology Co., Ltd., Dalina, China; RR350A), 10 µM of each primer and 20 ng template genomic DNA. PCR amplification was performed under the following cycling conditions: Denaturation at 95°C for 10 min, followed by 40 cycles of 94°C for 30 sec, 53–56°C for 30 sec and 72°C for 3 min, and a final extension step at 72°C for 10 min (T100TM Thermal Cycler; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PCR products were resolved by 2% agarose gel electrophoresis with ethidium bromide staining. Following electrophoresis, the gel was scanned and photographed on a UV transilluminator.
Table II.
Primer sequences for HERV-K6 and HERV-K11.
| HERV-K member | Primer | Sequence (5′- 3′) | Ta (°C) | Accession no. |
|---|---|---|---|---|
| HERV-K6 | Forward | CCTACAGGTTTCACCATCTTG | 53 | AF074086.2 |
| Reverse | CTTCTTTCTACACAGACACAG | |||
| HERV-K11 | Forward | CCACAGGTGTGGAGGGACAACC | 56 | DQ112099.1 |
| Reverse | CACCGAGACATTCCATTGCCC |
HERV-K, human endogenous retrovirus type K; Ta, annealing temperature.
Determination of polymorphism rates
The polymorphism ratios of samples were calculated using the Jaccard similarity coefficient (12). In brief, bands were scored as a binary value: ‘0’ for absence and ‘1’ for presence; the binary matrix (1/0) was then used to calculate the similarity between the different individuals using Jaccard's coefficient. Additionally, the GelJ v.2.0 programme (Department of Mathematics and Computer Science, University of La Rioja, Logroño, Spain) was used to construct a phylogenetic tree: The unweighted pair group method with arithmetic mean (UPGMA) clustering method of GelJ was used for the gel images to construct dendrograms (13).
Results
Polymorphism analysis
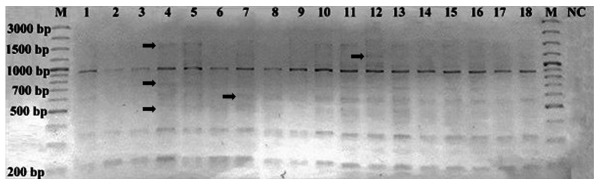
According to the band profiles of HERV-K6, a total of 198 bands were detected, of which 137 were monomorphic bands and 61 were polymorphic bands, ranging from 200 to 3,000 bp (Fig. 1). The polymorphic (−) and monomorphic (+) band numbers in each sample are listed in Table III. As a result of IRAP-PCR, polymorphism ratios were determined as 0–70% for all samples (Table IV). The polymorphism rates were 0–70% among females and 11–60% among males. Age-associated polymorphism was not observed in the study group (data not shown).
Figure 1.
Inter-retrotransposon amplified polymorphism-polymerase chain reaction amplification using human endogenous retrovirus type K member 6-specific primers. Lane numbers correspond to the subjects listed in Table I. M, marker (GeneRulerTM100 bp plus; Fermentas; Thermo Fisher Scientific, Inc., Waltham, MA, USA); NC, negative control (no template DNA). Arrows indicate polymorphic bands.
Table III.
Polymorphic (−) and monomorphic (+) band numbers of HERV-K6.
| HERV-K6 | ||
|---|---|---|
| Subject no. | + | − |
| 1 | 5 | 6 |
| 2 | 4 | 7 |
| 3 | 4 | 7 |
| 4 | 8 | 3 |
| 5 | 9 | 2 |
| 6 | 7 | 4 |
| 7 | 9 | 2 |
| 8 | 6 | 5 |
| 9 | 6 | 5 |
| 10 | 9 | 2 |
| 11 | 10 | 1 |
| 12 | 10 | 1 |
| 13 | 10 | 1 |
| 14 | 8 | 3 |
| 15 | 8 | 3 |
| 16 | 8 | 3 |
| 17 | 8 | 3 |
| 18 | 8 | 3 |
HERV-K6, human endogenous retrovirus type K member 6; +, monomorphic; -, polymorphic.
Table IV.
Polymorphism rates (%) of human endogenous retrovirus type K member 6 determined by Jaccard coefficient.
| Subject no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | |||||||||||||||||
| 2 | 20 | – | ||||||||||||||||
| 3 | 20 | 0 | – | |||||||||||||||
| 4 | 56 | 50 | 50 | – | ||||||||||||||
| 5 | 60 | 56 | 56 | 11 | – | |||||||||||||
| 6 | 50 | 43 | 43 | 13 | 22 | – | ||||||||||||
| 7 | 44 | 56 | 56 | 30 | 36 | 22 | – | |||||||||||
| 8 | 63 | 57 | 57 | 25 | 33 | 14 | 33 | – | ||||||||||
| 9 | 57 | 50 | 50 | 56 | 44 | 50 | 60 | 43 | – | |||||||||
| 10 | 60 | 70 | 70 | 30 | 20 | 40 | 36 | 33 | 44 | – | ||||||||
| 11 | 50 | 60 | 60 | 20 | 10 | 30 | 27 | 40 | 50 | 10 | – | |||||||
| 12 | 50 | 60 | 60 | 36 | 27 | 30 | 10 | 40 | 50 | 27 | 18 | – | ||||||
| 13 | 50 | 60 | 60 | 20 | 10 | 30 | 27 | 40 | 50 | 10 | 0 | 18 | – | |||||
| 14 | 38 | 50 | 50 | 22 | 30 | 13 | 11 | 25 | 56 | 30 | 20 | 20 | 20 | – | ||||
| 15 | 38 | 50 | 50 | 22 | 30 | 13 | 11 | 25 | 56 | 30 | 20 | 20 | 20 | 0 | – | |||
| 16 | 38 | 50 | 50 | 22 | 30 | 13 | 11 | 25 | 56 | 30 | 20 | 20 | 20 | 0 | 0 | – | ||
| 17 | 38 | 50 | 50 | 22 | 30 | 13 | 11 | 25 | 56 | 30 | 20 | 20 | 20 | 0 | 0 | 0 | – | |
| 18 | 38 | 50 | 50 | 22 | 30 | 13 | 11 | 25 | 56 | 30 | 20 | 20 | 20 | 0 | 0 | 0 | 0 | – |
Emboldened, maximum polymorphism rate.
Analysis of HERV-K11 band profiles identified 162 scorable bands in all samples: 130 monomorphic bands and 32 polymorphic bands ranging from 200 to 3,000 bp (Fig. 2). The polymorphic (−) and monomorphic (+) band numbers in each sample are listed in Table V. As a result of IRAP-PCR, polymorphism ratios were determined as 0–38% for all samples (Table VI). The polymorphism rates were 0–38% among females and 0–25% among males. Similar to HERV-K6, age-associated polymorphism was not observed in the study group (data not shown).
Figure 2.
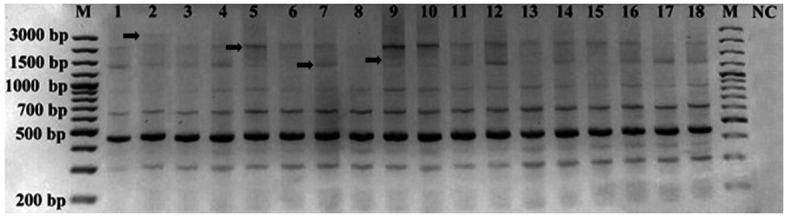
Inter-retrotransposon amplified polymorphism-polymerase chain reaction amplification using human endogenous retrovirus type K member 11-specific primers. Lane numbers correspond to the subjects listed in Table I. M, marker (GeneRulerTM100 bp plus; Fermentas; Thermo Fisher Scientific, Inc., Waltham, MA, USA); NC, negative control (no template DNA). Arrows indicate polymorphic bands.
Table V.
Polymorphic (−) and monomorphic (+) band numbers of HERV-K11.
| HERV-K11 | ||
|---|---|---|
| Subject no. | + | − |
| 1 | 7 | 2 |
| 2 | 8 | 1 |
| 3 | 7 | 2 |
| 4 | 7 | 2 |
| 5 | 8 | 1 |
| 6 | 6 | 3 |
| 7 | 7 | 2 |
| 8 | 5 | 4 |
| 9 | 8 | 1 |
| 10 | 8 | 1 |
| 11 | 7 | 2 |
| 12 | 8 | 1 |
| 13 | 8 | 1 |
| 14 | 7 | 2 |
| 15 | 7 | 2 |
| 16 | 8 | 1 |
| 17 | 7 | 2 |
| 18 | 7 | 2 |
HERV-K11, human endogenous retrovirus type K member 11; +, monomorphic; -, polymorphic.
Table VI.
Polymorphism rates (%) of human endogenous retrovirus type K member 11 determined by Jaccard coefficient.
| Subject no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | |||||||||||||||||
| 2 | 13 | – | ||||||||||||||||
| 3 | 0 | 13 | – | |||||||||||||||
| 4 | 0 | 13 | 0 | – | ||||||||||||||
| 5 | 13 | 22 | 13 | 13 | – | |||||||||||||
| 6 | 14 | 25 | 14 | 14 | 25 | – | ||||||||||||
| 7 | 0 | 13 | 0 | 0 | 13 | 14 | – | |||||||||||
| 8 | 29 | 38 | 29 | 29 | 38 | 17 | 29 | – | ||||||||||
| 9 | 13 | 22 | 13 | 13 | 0 | 25 | 13 | 38 | – | |||||||||
| 10 | 13 | 22 | 13 | 13 | 0 | 25 | 13 | 38 | 0 | – | ||||||||
| 11 | 0 | 13 | 0 | 0 | 13 | 14 | 0 | 29 | 13 | 13 | – | |||||||
| 12 | 13 | 22 | 13 | 13 | 0 | 25 | 13 | 38 | 0 | 0 | 13 | – | ||||||
| 13 | 13 | 22 | 13 | 13 | 0 | 25 | 13 | 38 | 0 | 0 | 13 | 0 | – | |||||
| 14 | 0 | 13 | 0 | 0 | 13 | 14 | 0 | 29 | 13 | 13 | 0 | 13 | 13 | – | ||||
| 15 | 0 | 13 | 0 | 0 | 13 | 14 | 0 | 29 | 13 | 13 | 0 | 13 | 13 | 0 | – | |||
| 16 | 13 | 22 | 13 | 13 | 0 | 25 | 13 | 38 | 0 | 0 | 13 | 0 | 0 | 13 | 13 | – | ||
| 17 | 0 | 13 | 0 | 0 | 13 | 14 | 0 | 29 | 13 | 13 | 0 | 13 | 13 | 0 | 0 | 13 | – | |
| 18 | 0 | 13 | 0 | 0 | 13 | 14 | 0 | 29 | 13 | 13 | 0 | 13 | 13 | 0 | 0 | 13 | 0 | – |
Emboldened, maximum polymorphism rate.
Clustering analysis
The UPGMA clustering method was performed for HERV-K6 and HERV-K11 profiles in the samples. According to the band profiles of HERV-K6, the 18 analysed samples were grouped in two clusters. The first group consisted of the numbered samples 1 and 10–18, while the second group consisted of the numbered samples 2–9 (Fig. 3). As a result of UPGMA analysis of HERV-K11, the 18 analysed samples were grouped in two clusters. Samples 1–11 were grouped in the first cluster, while 12–18 were grouped in the second (Fig. 4).
Figure 3.
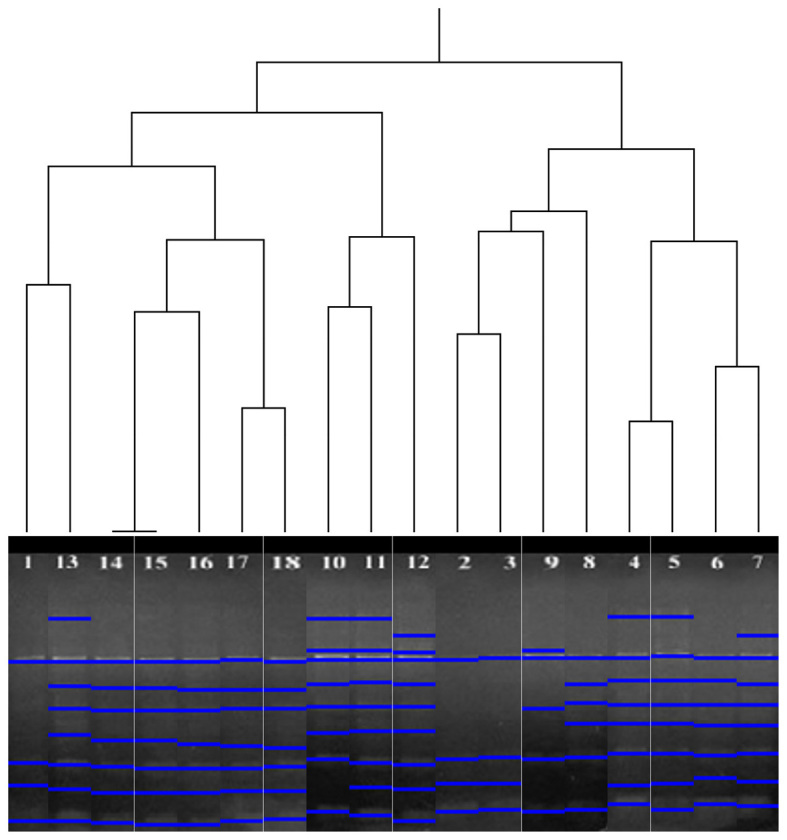
Clustering of subjects based on inter-retrotransposon amplified polymorphism-polymerase chain reaction amplification using human endogenous retrovirus type K member 6 primers (UPGMA analysis). Lane numbers correspond to the subjects listed in Table I.
Figure 4.
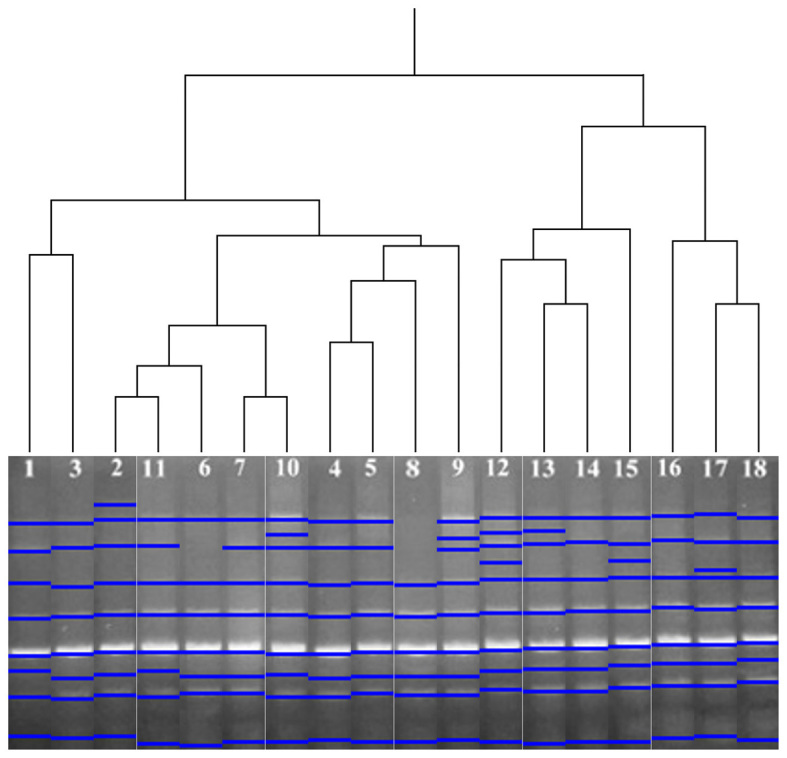
Clustering of subjects based on inter-retrotransposon amplified polymorphism-polymerase chain reaction amplification using human endogenous retrovirus type K member 11 primers (UPGMA analysis). Lane numbers correspond to the subjects listed in Table I.
Discussion
The presence of HERVs in the human genome is considered to be a result of insertion of their exogenous ancestors into primate germ-line cells (14). Further amplification via retrotranspositions likely resulted in the formation of the HERV families identified to date, which have been suggested to serve a significant role in primate evolution (14–16). The HERV-K family is established as the most functionally active group of endogenous retroviruses (17). In the present study, the IRAP molecular marker technique was used to assess HERV-K6 and HERV-K11 transpositions in the human genome, and determined that HERV-K6 and HERV-K11 elements may still be active in the human genome. According to the IRAP analysis results, HERV-K6 and HERV-K11 exhibited notably similar band profiles (137 monomorphic bands for HERV-K6; 130 monomorphic bands for HERV-K11). However, the small numbers of polymorphic bands varied between the HERV-K6 and HERV-K11 profiles of the different DNA samples (61 polymorphic bands for HERV-K6; 32 polymorphic bands for HERV-K11). When band profiles of samples were compared between different age groups and same age groups, no marked differences were determined regarding polymorphism age specificity. Furthermore, as the study group was composed of males and females, the band patterns of males and females were compared; however, no gender specific polymorphisms were determined. Therefore, polymorphisms were considered to be individual specific. Additionally, the study group did not include any family members. Thus, polymorphism rates may also be family specific.
There have been a number of studies on HERV polymorphisms (11,18–20). A study by Mamedov et al (18) identified a novel HERV-K solo LTR insertion polymorphism, suggesting a recent retrovirus insertion followed by a recombination event between two retroviral LTRs. Additionally, the allele frequencies were studied in 88 human DNA samples among different ethnic groups: Mordvinians, Bashkirs and Kalmyks (all from Russia) and five samples of African origin (all from Guinea-Bissau) (18). Another study by Guliyev et al (11) observed integration polymorphism patterns for HERV-H in the tested individuals (n=20) of diverse ethnic origin. Furthermore, a study by Kahyo et al (19) investigated HML-2 (HERV-K) insertional polymorphisms in a small Japanese population. They compared reference genomes obtained from genome projects and genomic PCR sequences. Sequencing of the preintegration sites identified a HML-2 site, located at 7p21.2. Another insertionally polymorphic site for a non-human-specific HML-2 site was also identified at 6p25.2 (19). In a study conducted by Belshaw et al (20), 113 human-specific HERV-K (HML2) elements were identified in the human genome sequence, 8 of which were insertionally polymorphic. Furthermore, it has been determined that the number of polymorphic elements was not significantly different from that predicted by a standard population genetic model that assumes constant genetic activity of the family (19). This suggests that the HERV-K family may be active in humans.
According to the results of the present UPGMA analysis, re-ordered samples exhibited similar band patterns. When investigating the dendrograms of HERV-K6 and HERV-K11, it was observed that all re-ordered samples were not included in the same or different age groups (data not shown). For instance, samples 1, 13 and 14 were in the same group on UPGMA analysis of HERV-K6 profiles, despite the samples belonging to different age groups. Similarly, while samples 6, 7 and 11 were in the same group on UPGMA analysis of HERV-K11, they belonged to different age groups; by contrast, samples 1–3 were in the same age group and also the same group determined by UPGMA clustering. These results indicated that the polymorphisms were not age-associated. Thus, the polymorphisms may be individual specific.
High-throughput sequencing technologies have also provided information on insertionally polymorphic sites of HERV-K in different studies (21–23). A study by Shin et al (24) analysed insertions of HERV-K elements including HERV-K101 and -K132. They concluded that HERV-K activity served an important role in genomic divergence within the human population. Movements of human retrotransposons may differ between normal somatic tissue and somatic tumour genomes (25). The present HERV-K6 and HERV-K11 insertion polymorphism analyses were similar to those reported previously with regard to determining insertion polymorphisms, while they differ from some studies due to the different techniques used (11,18–21). Guliyev et al (11) determined polymorphisms with the IRAP technique whereas Mamedov et al (18) tested distribution of the LTR-containing allele in Africans and Russian populations. Kahyo et al (19) identified insertional polymorphisms with genomic PCR analysis in a Japanese group. Belshaw et al (20) and Lee et al (21) determined insertional polymorphisms with sequencing and bioinformatics.
HERVs are inactivated by mutations, deletions or recombinations (26). Studies have indicated that certain active copies may express proteins or virions, and have pathogenic effects or physiological roles (2). Furthermore, there may be an association between the expression of HERV-K elements and the development of cancer and autoimmune diseases (24). A study performed by Li et al (27) detected HERV-K envelope protein expression in pancreatic cancer cell lines and patient biopsies, but not in normal pancreatic cell lines or uninvolved normal tissues. Maze et al (28) demonstrated that HERV-K envelope, capsid, Rec and Np9 proteins were overexpressed in human primary schwannoma cells and tissues. They also identified that anti-HERV-K antibodies reduced p53 expression and schwannoma proliferation; furthermore, pre-incubation of schwannoma cells with HERV-K antibodies prior to treatment with cancer drugs (AZD6244 with/without Sorafenib and BEZ235) potentiated the drug efficiency. Thus, they suggested that HERV-K has a pathogenic role in schwannoma and may be a promising therapeutic target (28). Additionally, a HERV-K-related insert may serve as an enhancer for the schizophrenia-linked gene proline dehydrogenase, a candidate gene for sensitivity to schizophrenia and other neurological diseases (29). Another clinical study on HERV-K indicated that HERV-K expression was markedly higher in leukaemia patients when compared with its expression in healthy donors of a similar median age (30). To date, HERV studies have analysed the association between expression and neurological diseases, cancer or autoimmune diseases (24). There have been few reports related to HERV retrotransposon movements in the human genome (31). To the best of our knowledge, the present analyses were the first to focus on HERV-K6 and HERV-K11 transpositions in different healthy individuals. In the present study, polymorphisms were also investigated in healthy subjects according to different age groups. Although polymorphism was identified among all subjects, the HERV-K6 and HERV-K11 endogenous retrovirus polymorphisms exhibited no age-associations.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- HERVs
human endogenous retroviruses
- HERV-K
human endogenous retrovirus type K
- HML-2
human mouse mammary tumour virus like-2
- IRAP
inter-retrotransposon amplified polymorphism
- LTR
long terminal repeat
- PCR
polymerase chain reaction
- UPGMA
unweighted pair group method with arithmetic mean
Funding
The present study was supported by the Istanbul University Scientific Research Projects Coordination Unit (grant no. 22142).
Availability of data and materials
The DNA samples were from Dr Kaniye Sahin's DNA collections at Istanbul University Medical Faculty (Istanbul, Turkey). All data generated or analyzed during this study are included in this published article.
Authors' contributions
BCG, EK and SM analysed and interpreted the data, and wrote the draft manuscript. NG revised the manuscript for important intellectual content and gave final approval of the version to be published. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Patient donors of the DNA samples provided written informed consent agreeing to the use of patient materials for research purposes.
Consent for publication
Patient donors of the DNA samples provided written informed consent permitting publication of relevant data on the condition of anonymity being retained.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Perron H, Lang A. The human endogenous retrovirus link between genes and environment in multiple sclerosis and in multifactorial diseases associating neuroinflammation. Clin Rev Allergy Immunol. 2010;39:51–61. doi: 10.1007/s12016-009-8170-x. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian RP, Wildschutte JH, Russo C, Coffin JM. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology. 2011;8:90. doi: 10.1186/1742-4690-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downey RF, Sullivan FJ, Wang-Johanning F, Ambs S, Giles FJ, Glynn SA. Human endogenous retrovirus K and cancer: Innocent bystander or tumorigenic accomplice? Int J Cancer. 2015;137:1249–1257. doi: 10.1002/ijc.29003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Cao M, Iduma P, Karachaliou N, Santarpia M, Blanco J, Rosell R. Human endogenous retroviruses and cancer. Cancer Biol Med. 2016;13:483–488. doi: 10.20892/j.issn.2095-3941.2016.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurst TP, Magiorkinis G. Epigenetic control of human endogenous retrovirus Expression: Focus on regulation of long-terminal repeats (LTRs) Viruses. 2017;9:9. doi: 10.3390/v9060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer D, Glanzman R, Traboulsee A, Nath A, Groc L, Horwitz M, Göttle P, Perron H, Gold J, Hartung HP, et al. Prehistoric enemies within: The contribution of human endogenous retroviruses to neurological diseases. Meeting report: ‘Second International Workshop on Human Endogenous Retroviruses and Disease’, Washington DC, March 13th and 14th 2017. Mult Scler Relat Disord. 2017;15:18–23. doi: 10.1016/j.msard.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol. 2001;2:S1017. doi: 10.1186/gb-2001-2-6-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalendar R, Grob T, Regina M, Suoniemi A, Schulman A. IRAP and REMAP: Two new retrotransposon-based DNA fingerprinting techniques. Theor Appl Genet. 1999;98:704–711. doi: 10.1007/s001220051124. [DOI] [Google Scholar]
- 9.Gozukirmizi N, Yilmaz S, Marakli S, Temel A. Retrotransposon-based molecular markers; Tools for variation analysis in plants. In: Taski-Ajdukovic K, editor. Applications of Molecular Markers in Plant Genome analysis and Breeding. Research Signpost; Kerala: 2015. pp. 19–45. [Google Scholar]
- 10.Cakmak B, Marakli S, Gözükirmizi N. Sukkula retrotransposon movements in the human genome. Biotechnol Biotechnol Equip. 2017;31:900–905. [Google Scholar]
- 11.Guliyev M, Yılmaz S, Şahin K, Maraklı S, Gözükirmizi N. Human endogenous retrovirus-H insertion screening. Mol Med Rep. 2013;7:1305–1309. doi: 10.3892/mmr.2013.1295. [DOI] [PubMed] [Google Scholar]
- 12.Jaccard P. Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat. 1908;44:223–270. [Google Scholar]
- 13.Heras J, Domínguez C, Mata E, Pascual V, Lozano C, Torres C, Zarazaga M. GelJ - a tool for analyzing DNA fingerprint gel images. BMC Bioinformatics. 2015;16:270. doi: 10.1186/s12859-015-0703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sverdlov ED. Retroviruses and primate evolution. BioEssays. 2000;22:161–171. doi: 10.1002/(SICI)1521-1878(200002)22:2<161::AID-BIES7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.van de Lagemaat LN, Landry JR, Mager DL, Medstrand P. Transposable elements in mammals promote regulatory variation and diversification of genes with specialized functions. Trends Genet. 2003;19:530–536. doi: 10.1016/j.tig.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Kazazian HH., Jr Mobile elements: Drivers of genome evolution. Science. 2004;303:1626–1632. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- 17.Seifarth W, Spiess B, Zeilfelder U, Speth C, Hehlmann R, Leib-Mösch C. Assessment of retroviral activity using a universal retrovirus chip. J Virol Methods. 2003;112:79–91. doi: 10.1016/S0166-0934(03)00194-0. [DOI] [PubMed] [Google Scholar]
- 18.Mamedov I, Lebedev Y, Hunsmann G, Khusnutdinova E, Sverdlov E. A rare event of insertion polymorphism of a HERV-K LTR in the human genome. Genomics. 2004;84:596–599. doi: 10.1016/j.ygeno.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Kahyo T, Yamada H, Tao H, Kurabe N, Sugimura H. Insertionally polymorphic sites of human endogenous retrovirus-K (HML-2) with long target site duplications. BMC Genomics. 2017;18:487. doi: 10.1186/s12864-017-3872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belshaw R, Dawson ALA, Woolven-Allen J, Redding J, Burt A, Tristem M. Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family HERV-K(HML2): Implications for present-day activity. J Virol. 2005;79:12507–12514. doi: 10.1128/JVI.79.19.12507-12514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee E, Iskow R, Yang L, Gokcumen O, Haseley P, Luquette LJ, III, Lohr JG, Harris CC, Ding L, Wilson RK, et al. Cancer Genome Atlas Research Network: Landscape of somatic retrotransposition in human cancers. Science. 2012;337:967–971. doi: 10.1126/science.1222077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchi E, Kanapin A, Magiorkinis G, Belshaw R. Unfixed endogenous retroviral insertions in the human population. J Virol. 2014;88:9529–9537. doi: 10.1128/JVI.00919-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wildschutte JH, Williams ZH, Montesion M, Subramanian RP, Kidd JM, Coffin JM. Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc Natl Acad Sci USA. 2016;113:E2326–E2334. doi: 10.1073/pnas.1602336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin W, Lee J, Son SY, Ahn K, Kim HS, Han K. Human-specific HERV-K insertion causes genomic variations in the human genome. PLoS One. 2013;8:e60605. doi: 10.1371/journal.pone.0060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, Kazazian HH., Jr L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev. 2009;23:1303–1312. doi: 10.1101/gad.1803909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel G. The human genome. Objection #2: Why sequence the junk? Science. 2001;291:1184. doi: 10.1126/science.291.5507.1184. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Radvanyi L, Yin B, Li J, Chivukula R, Lin K, Lu Y, Shen J, Chang DZ, Li D, et al. Down-regulation of human endogenous retrovirus type K (HERV-K) viral env RNA in pancreatic cancer cells decreases cell proliferation and tumour growth. Clin Cancer Res. 2017;23:5892–5911. doi: 10.1158/1078-0432.CCR-17-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maze E, Reeves S, Hilton D, Provenzano L, Belshaw R, Ammoun S. Abstract 4627: The role of human endogenous retroviral proteins in the development of Merlin-deficient tumors and as potential drug targets. In: Proceedings of the AACR 107th Annual Meeting 2016, New Orleans, LA. Cancer Res. 2016;76(Suppl 14) Abstract nr 4627. [Google Scholar]
- 29.Suntsova M, Gogvadze EV, Salozhin S, Gaifullin N, Eroshkin F, Dmitriev SE, Martynova N, Kulikov K, Malakhova G, Tukhbatova G, et al. Human-specific endogenous retroviral insert serves as an enhancer for the schizophrenia-linked gene PRODH. Proc Natl Acad Sci USA. 2013;110:19472–19477. doi: 10.1073/pnas.1318172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergallo M, Montanari P, Mareschi K, Merlino C, Berger M, Bini I, Daprà V, Galliano I, Fagioli F. Expression of the pol gene of human endogenous retroviruses HERV-K and -W in leukemia patients. Arch Virol. 2017;162:3639–3644. doi: 10.1007/s00705-017-3526-7. [DOI] [PubMed] [Google Scholar]
- 31.Iskow RC, McCabe MT, Mills RE, Torene S, Pittard WS, Neuwald AF, Van Meir EG, Vertino PM, Devine SE. Natural mutagenesis of human genomes by endogenous retrotransposons. Cell. 2010;141:1253–1261. doi: 10.1016/j.cell.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The DNA samples were from Dr Kaniye Sahin's DNA collections at Istanbul University Medical Faculty (Istanbul, Turkey). All data generated or analyzed during this study are included in this published article.