Abstract
Redox homeostasis involves factors that ensure proper function of cells. The excess reactive oxygen species (ROS) leads to oxidative stress and increased risk of oxidative damage to cellular components. In contrast, upon reductive stress, insufficient ROS abundance may result in faulty cell signalling. It may be expected that dietary antioxidants, depending on their standard reduction potentials (E°), will affect both scenarios. In our study, for the first time, we systematically tested the relationship among E°, chemical properties, and biological effects in HT29 cells for a series of structurally different catechins and a major endogenous antioxidant – glutathione (GSH), at both physiological and dietary concentrations. Among chemical antioxidant activity tests, the strongest correlation with E° was seen using a DPPH assay. The values of E° were also highly correlated with cellular antioxidant activity (CAA) values determined in HT29 cells. Our results indicated that physiological concentrations (1–10 µM) of tested catechins stabilized the redox status of cells, which was not exhibited at higher concentrations. This stabilization of redox homeostasis was mirrored by constant, dose and E° independent CAA values, uninhibited growth of HT29 cells, modulation of hydrogen peroxide-induced DNA damage, as well as effects at the genomic level, where either up-regulation of three redox-related genes (ALB, CCL5, and HSPA1A) out of 84 in the array (1 µM) or no effect (10 µM) was observed for catechins. Higher catechin concentrations (over 10 µM) increased CAA values in a dose- and E°-dependent manner, caused cell growth inhibition, but surprisingly did not protect HT29 cells against reactive oxygen species (ROS)-induced DNA fragmentation. In conclusion, dose-dependent effects of dietary antioxidants and biological functions potentially modulated by them may become deregulated upon exposure to excessive doses.
Abbreviations: ACTB, actin beta; ALB, albumin; AOX1, aldehyde oxidase 1; B2M, beta-2-microglobulin; BNIP3, BCL2/adenovirus E1B; C, (+)-catechin; CAA, cellular antioxidant activity; CCL5, chemokine (C-C motif) ligand 5; CYGB, cytoglobin; DCF, dichlorofluorescein; DCFH, dichlorofluorescin; E°, standard reduction potential; EC, (-)-epicatechin; ECG, (-)-epicatechin gallate; EGC, (-)-epigallocatechin; EGCG, (-)-epigallocatechingallate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GSH, reducted glutathione; GSSG, oxidized glutathione; GSTZ1, glutathione transferase zeta 1; HPRT1, hypoxanthine phosphoribosyltransferase 1; HSPA1A, heat shock 70 kDa protein 1A; HT29, human colon adenocarcinoma cell line; NCF1, neutrophil cytosolic factor 1; NOS2, nitric oxide synthase 2; NOX5, NADPH oxidase 5; PRDX1, peroxiredoxin 1; PRDX2, peroxiredoxin 2; ROS, reactive oxygen species; RPLP0, ribosomal protein lateral stalk subunit P0; SEPP1, selenoprotein P, plasma, 1; SHE, standard hydrogen electrode; SRXN1, sulfiredoxin 1; TXNRD2, thioredoxin reductase 2
Keywords: Catechins, Standard reduction potential, Redox homeostasis, Oxidative stress
Graphical abstract
Highlights
-
•
Standard reduction potential (E°) is a good predictor of antioxidant activity of catechins.
-
•
Physiological doses of catechins maintain cellular redox status of cells.
-
•
Redox control by catechins includes regulation of expression of redox-related genes.
-
•
Excessive doses of catechins deregulate redox homeostasis in a dose- and E°-dependent manner.
1. Introduction
The consumption of products rich in catechins, such as tea or cocoa to prevent or ameliorate oxidative stress and to decrease the risk of associated diseases, has been the subject of an impressive number of scientific investigations (reviewed in [1], [2], [3]). Although many mechanisms have been proposed to account for the beneficial effects of catechins, the antioxidant properties of these polyphenols are frequently cited as the most important factor [4]. The evidence supporting the antioxidant function of catechins is mainly derived from assays of their antioxidant activity performed using chemical tests in cell-free systems [5], [6], [7]. Somewhat in contrast, studies using animal models or involving human subjects have been less consistent regarding the direct antioxidant effects of catechins [8], [9], [10]. Therefore, over the past few years, it has been proposed that the chemopreventive properties of catechins may result from more complex modes of action, whereby a variety of mechanisms affecting different targets contribute to the overall cellular redox response. The latter way of reasoning suggests that catechins, or/and their derivatives arising during metabolism, can affect endogenous antioxidant defence systems of cells; for example, by activation of secondary messengers and signal transduction pathways resulting in the modulation of expression of redox related genes [11], [12], [13], [14].
Despite the fact that the ability of catechins to act as oxidative stress ameliorators has received a great deal of attention, the interrelationships between chemical and biological mechanisms of the observed effects remain far from an in-depth understanding. Under conditions of oxidative stress, antioxidants will react with and scavenge ROS, as allowed by their standard reduction potentials (E°), thus preventing oxidative damage of cellular macromolecules accordingly. While values of E° for redox couples of ROS have been well established since the 1990s [15], these values have not been satisfactorily determined for such a widely investigated group as polyphenols, including catechins [16], [17], [18]. The relationship between the values of E° and the impact on cellular redox homeostasis, especially in situations of ROS challenge, has not been adequately recognised. These aspects require detailed clarification, because intracellular redox status has been identified to be involved in regulation of cell proliferation, differentiation, migration and apoptosis [19], [20], [21], [22], i.e. those essential processes that govern cell fate.
The objective of this study was to elucidate the relationship between electrochemical and chemical properties as well as biological effects for structurally different catechins, especially under conditions of oxidative stress. Taking into consideration relatively high concentrations of these antioxidants in ingested foods, but also their low bioavailability, we have selected a cellular model to reflect both situations. The studies were carried out using the colon adenocarcinoma HT29 cell line as a model of the alimentary tract where, on one hand, epithelium is exposed to catechin concentrations matching those in food (e.g. black tea contains on average about 15 mg/g dry weight of catechins [23]) and, on the other hand, where the internal cellular exposure is limited by their uptake, which in the case of polyphenols is relatively low, i.e. only 0.2–2% of consumed catechins reaches plasma of healthy humans [24]. All determinations were compared with the results obtained for corresponding doses of glutathione (GSH). The latter compound is a major endogenous, but at the same time most frequently consumed, antioxidant whose bioavailability is also rather limited [25]. It was shown that, after ingestion of 50 mg (ca. 160 μmoles) per kg body weight of food-derived GSH, the level of this compound in the hepatic portal vein blood of rats amounted to approximately 0.4 μM [26].
The starting point in this study was the determination of electrochemical properties, i.e. standard reduction potentials. In the next stage, antioxidant activity was evaluated using popular chemical tests (ABTS, DPPH, FC), but also taking into account kinetic aspects of reactions between an antioxidant and indicator substances. Subsequently, biological activities, such as cytotoxicity, cellular antioxidant activity, genotoxicity and DNA protection against oxidative damage as well as regulation of expression of 84 redox-related genes, were assessed in HT29 cells.
2. Materials and methods
2.1. Chemicals and reagents
The following redox active compounds were used for the study: (+)-catechin (C), (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECG), (-)-epigallocatechin gallate (EGCG) from Extrasynthese (France) and glutathione (GSH) from Sigma-Aldrich (USA). Solution of potassium hexacyanoferrate (III) from Sigma-Aldrich (USA) was applied as a titrant in potentiometric titration. For spectrophotometric tests 1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azinobis-(ethyl-2,3-dihydrobenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), Folin-Ciocalteu's phenol reagent (FC) and sodium thiosulfate from Sigma-Aldrich (USA), HPLC grade methanol from Merck (Germany) and analytical grade ethanol and methanol from POCH (Poland) were used. Thiazolyl blue tetrazolium bromide (MTT) from Sigma-Aldrich (USA) was applied in MTT test. Solution of 8 M hydrogen peroxide (H2O2), hydrochloric acid (HCl), low melting point agarose (LMP agarose), sodium chloride (NaCl), sodium hydroxide (NaOH), ethylenediaminetetraacetic acid (EDTA), 2-amino-2-(hydroxymethyl)-1,3-propanediol (Trizma-Base), Sybr Green I nucleic acid gel stain, Triton X100 from Sigma-Aldrich (USA) and normal melting point agarose (NMP agarose) from Bioline (UK) were used in comet assays. The OxiSelect™ Cellular Antioxidant Assay Kit was purchased from Cell Biolabs, Inc. (USA). QIAshredder, RNeasy Mini Kit, RNase-Free DNase set, RT2 First Strand Kit, RT2 SybrGreen qPCR Mastermix, and RT2 Profiler PCR Arrays for Oxidative Stress (PAHS 0065) were purchased from Qiagen (Germany). Tablets of phosphate buffered saline (PBS) and dimethyl sulfoxide (DMSO) used during experiments in cell culture were purchased from Sigma-Aldrich (USA). PBS solution was prepared by dissolving one tablet in 200 mL ultrapure water from a Millipore Milli-Q system. Water was purified with a QPLUS185 system from Millipore (USA).
2.2. Cell culture
HT29 (human colon adenocarcinoma) cells from the ATCC were maintained in McCoy's medium supplemented with L-glutamine (2 mol/L), sodium pyruvate (200 g/L), foetal bovine serum (100 mL/L), and antibiotics (100 U/mL penicillin and 100 g/L streptomycin). All reagents for cell culture were purchased from Sigma-Aldrich (USA). Stock solutions of antioxidants were sterilized for biological testing by passage through Millex sterile R33 mm (0.22 µm) syringe-driven filters (Millipore). Cells were maintained at 37 °C under a humidified atmosphere with 5% CO2 in a Smart cell incubator (Heal Force). Cultured cells were regularly checked for mycoplasma contamination using a Universal Mycoplasma Detection Kit from ATCC (USA).
2.3. Standard reduction potential by potentiometric titration
Potassium hexacyanoferrate (III) was chosen as a titrant. Its high stability (Kf = 1 × 1042) [33] ensures that chelation of iron ions is impossible, because its stability constant for complexes of C with Fe3+ is equal to 1.6 × 106 [34]. Tested antioxidants (1 mg/mL) and the titrant used as an oxidizing agent (concentration calculated based on the reaction stoichiometry) were dissolved in PBS (pH = 7.4). Concentration of the stock solution of titrant was determined by potassium iodide titration using a strong acid solution [27].
Potentiometric titration was performed vs. 3 M KCl Ag|AgCl reference electrode and a platinum measuring electrode at 37 °C using JENCO 6230N, ORP-146C Micro Oxidation-Reduction equipment (USA). Temperature during measurement was maintained by Ultra Thermostat (PolyScience, USA), while the temperature of the reaction was controlled in the range ± 0.1 °C using a JENCO 6230-AST thermometer. Mixing of the reactants was ensured by bubbling inert, high purity N2 gas. The electrode was stored in 3 M KCl and was washed with distilled water before each measurement. The titrant was added to the analyte in increments of 0.5 mL and potential was read after stabilization. Each potentiometric titration was performed three times in separate experiments. Received titration curves (E [mV] vs. Vtitrant [mL]) were analysed by non-linear regression (Marquardt-Levenberg algorithm) using SigmaPlot (Systat Software Inc., UK) software. The sigmoidal, 5-parameters model was chosen for fitting the curves. Based on this model (with determination coefficient r2 almost equal to 0.999), the curves were plotted based on many more points than the original titration curves. Next, the first derivative maximum, hence the equivalence (inflection) point (EPR) was obtained by numerical differentiation. Finally, the received reduction potentials of tested compounds vs. SHE (standard hydrogen electrode) were calculated. The accurate potential of the used reference electrode was identified in separate measurements by titration of redox couples of known reduction potential: FeCl3 (as an oxidant) and Na2S2O3 (as a reducing agent) in aqueous solution.
2.4. Antioxidant activity by spectrophotometric methods
The colorimetric determination of antioxidant activity was carried out by standard assays employing ABTS and DPPH radicals as described previously [28] with minor modifications, as well as with FC reagent according to the standard ISO 14502-1:2005 method. Briefly, stock solutions of radicals were diluted in methanol before measurements until absorbance amounted to 0.8 ± 0.05 at λ = 734 nm in the case of ABTS radical and 0.9 ± 0.05 at 515 nm for DPPH radical. The commercial FC reagent was diluted with water in the ratio of 1:9 (v/v). All reactions were carried out in 48-well plates at 37 °C. Stock solutions of catechins were prepared in analytical grade ethanol to a concentration of 10 mM. The stock solution of GSH was prepared in distilled water at a concentration of 200 mM just before use. Stock solutions of antioxidants were diluted appropriately with the same solvents to concentrations falling within a linear range of the assay. The ABTS solution (1 mL) was mixed with solutions of catechins or glutathione (10 μL). The absorbance of the mixtures was measured at 734 nm after 10 min. The DPPH solution (1 mL) was mixed with solutions of catechins or glutathione (30 μL) and the absorbance was measured at 515 nm after 10 min. In the case of the FC assay, the solutions of antioxidants (100 μL) were mixed with a solution of FC reagent (500 μL), and after 5 min, 400 μL of water solution of sodium carbonate (7.5% w/v) was added. The reactants were mixed and absorbance was measured at 765 nm after 1 h. All absorbance measurements were performed with the use of a TECAN Infinite M200 spectrophotometer (Tecan Group Ltd., Switzerland).
The results of antioxidant activity determinations for spectrophotomertic tests were expressed as stoichiometry values (n), as described by Villaño et al. [29] with modification. In the case of ABTS and DPPH assays, this parameter was determined as a regression coefficient, which was defined as the slope of the line that represented the relationship between concentrations of a radical scavenger and concentrations of the tested antioxidant present in the mixture after 10 min of reaction (n10). The concentration of radicals scavenged by the tested antioxidants in reaction media was calculated with the use of the Beer–Lambert–Bouguer Law (Beer's Law) according to the equation:
| (1) |
where SR is the concentration of scavenged radicals [M]; A0 is the initial absorbance of the radical solution; Af is the absorbance of the radical solution after reaction time; ε is the molar extinction coefficient of the particular radical (11,240 M−1 cm−1 for DPPH radical at 515 nm [30] and 16,000 M−1 cm−1 for ABTS radical at 734 nm [31]), l is the cuvette optical path [1 cm]. In contrast to the work of Villaño et al. [29], we considered the concentration of radical not after reaching equilibrium with antioxidant, but after 10 min of reaction. In the case of the FC assay, the antioxidant activity of tested compounds was expressed as a slope of the line that represents the relationship between concentration of gallic acid equivalents and concentrations of antioxidants present in reaction mixtures after 60 min (n60).
2.5. MTT cytotoxicity test
The MTT test was performed to assess the inhibition of growth of HT29 cells exposed to different concentrations of the investigated antioxidants. Exponentially growing cells were seeded in 96-well tissue culture plates (104 cells per well in 0.15 mL of medium). The cells were allowed to settle for 24 h at 37 °C, then were treated for 6, 24 or 72 h with 0.05 mL of different concentrations of tested antioxidants. Catechins were dissolved in 60% ethanol, and glutathione in sterile water. Final concentrations of compounds ranged from 10 nM to 10 mM. In the cases of both shorter exposures, the medium was aspirated from the wells, replaced with 0.2 mL of fresh medium and the cells were incubated at 37 °C until 72 h of the total incubation time. Occasionally, the growth of the cells was monitored under an inverted microscope. Treatments were performed as four technical replicates. After 72 h of incubation, MTT solution (4 g/L) was added (0.05 mL per well) and the cells were cultured for another 4 h at 37 °C. Finally, medium was carefully removed from wells and formazan crystals formed by metabolically active cells were dissolved in 0.05 mL of DMSO. The absorption of the resultant solutions was determined at 540 nm with a TECAN Infinite M200 plate reader (Tecan Group Ltd., Switzerland). Three independent replicates of each treatment were performed. Cytotoxicity was expressed as percent growth inhibition of cells exposed to tested antioxidants compared to control cells treated with the appropriate volume of solvent only, whose growth was regarded as 100%.
2.6. CAA assay
The cellular antioxidant activity (CAA) of investigated compounds in HT29 cells was studied using a CAA assay (The OxiSelect™ Cellular Antioxidant Assay Kit, Cell Biolabs, Inc., USA). Cells were seeded in black 96-well tissue culture plates with transparent bottoms dedicated to fluorescence measurements (3 × 104 cells per well in 0.2 mL of medium). The cells were allowed to settle for 24 h at 37 °C and then were treated with 0.05 mL of different concentrations of antioxidants for 1 h. Final concentrations of investigated compounds ranged from 1 to 100 μM. Subsequent steps were performed strictly according to the manufacturer's procedure available from the website: https://www.cellbiolabs.com/sites/default/files/STA-349-cellular-antioxidant-activity-assay-kit_0.pdf. Treatments were performed as four technical replicates. For the experiments, all catechins were dissolved in 10% ethanol, and GSH in sterile water. Control cells were treated with the corresponding solvent. Emission of fluorescence at 538 nm in cell cultures was measured every 5 min for 1 h after excitation at 485 nm using a TECAN Infinite M200 plate reader. Three independent replicates of each treatment were performed. The fluorescence measured over time corresponds to the ability of a given substance to quench free radicals. Quantitation of CAA was achieved by calculation of CAA units according to Eq. (2):
| (2) |
where SA is the area under the fluorescence curve plotted against time corresponding to each concentration of investigated compound, while CA is the area under the control fluorescence curve vs. time for cells treated with the appropriate solvent only.
2.7. Genotoxic effects measured by comet assay
HT29 cells were seeded in 24-well tissue culture plates (105 cells per well in 1.8 mL of McCoy's medium) and left to settle for 24 h at 37 °C and under 5% CO2. After this time, the cells were treated with different concentrations of tested antioxidants (0.2 mL) for 24 h at 37 °C. Final concentrations of investigated compounds ranged from 100 nM to 100 μM. In the case of catechin solutions, the final ethanol concentration in the culture medium did not exceed 3% (v/v). Glutathione was dissolved in sterile water. The cells used as negative controls were treated with the appropriate solvent only. After treatment, the medium was carefully removed from the wells and the cells were detached using 0.2 mL of trypsin (0.5 g/L) solution. The enzymatic action of trypsin was halted by adding 1.8 mL of complete growth medium to the cells. The cells were resuspended and 1 mL of the cell suspension was transferred into 1.5 mL tubes and centrifuged (100 × g, 5 min, 4 °C). The cell pellets were washed with 1 mL of PBS and centrifuged (100 × g, 5 min, 4 °C) again. After centrifugation, approximately 20 μL of the supernatant was left in the tube to resuspend cells. The cell suspension (20 μL) was mixed with 150 μL of 0.5% LMP agarose in water prewarmed to 42 °C and 20 μL of this mixture was placed as two spots on a microscope slide pre-coated with 1% normal melting point agarose (NMP agarose) and left to set on an ice-cold tray. Three slides with two repetitions on each were prepared for every concentration of the tested substances. After overnight lysis in a high salt alkaline buffer (2.5 M NaCl, 0.1 M EDTA, 0.01 M Tris, 1% Triton X100, pH 10), the slides were placed on a Bio-Rad subcell GT electrophoresis platform (UK), covered with cold electrophoresis buffer (0.3 M NaOH, 1 mM EDTA, pH 13) and DNA was allowed to unwind for 20 min before electrophoresis. Electrophoresis was conducted at 26 V and 300 mA for 30 min in darkness at 4–8 °C. After electrophoresis, the slides were transferred to neutralizing buffer (0.4 M Tris, pH 7.5) for 5 min. This step was repeated twice, then slides were washed using distilled water and fixed in 70% ethanol. Subsequently, the DNA was stained with SybrGreen in TE buffer (0.1 M Trizma-Base, 1 mM EDTA, pH 10) for 30 min. After staining, the slides were washed with distilled water for 5 min. Finally, DNA “comets” were examined under a fluorescence microscope (Zeiss ImagerZ2, USA) connected to a computerized slide scanning system (Metafer4, Germany). Comet analysis involved counting 200 consecutive nuclei per sample. The mean %DNA in the comet tail was a measure of genotoxic potency of compounds tested. Three independent replicates of each treatment were performed.
2.8. Protection against genotoxic effects by comet assay
HT29 cells were seeded in 24-well tissue culture plates (105 cells per well in 1.8 mL of McCoy's medium) and incubated for 24 h at 37 °C and 5% CO2 to settle. Then, the cells were treated with 0.2 mL of different concentrations of the tested antioxidants for 24 h at 37 °C. Final concentrations of investigated compounds were in the range 100 nM to 100 μM. After incubation of cells with tested compounds, the medium was carefully removed from wells and replaced with 1 mL of 150 μM solution of H2O2 in complete medium. The cells were incubated with H2O2 for 1 h and then submitted to comet assays. The cells serving as positive controls were treated with 150 μM H2O2 for 1 h. Further steps of the comet assay procedure, as well as DNA fragmentation evaluation, were performed as described in Section 2.7.
2.9. Microarray analysis
HT29 cells were seeded in 24-well tissue culture plates (6 × 104 cells per well in 1.8 mL of McCoy's medium) and incubated for 24 h at 37 °C and 5% CO2. After this time, the cells were treated for 24 h at 37 °C with 0.2 mL of different concentrations of tested chemicals. Final concentrations of investigated compounds ranged from 1 μM to 10 μM. The cells treated with catechins dissolved in ethanol were exposed to 3% (v/v) of this solvent. GSH was dissolved in sterile water. The cells used as negative controls were treated with the appropriate solvent only.
Isolation of total RNA was performed according to the RNeasy Mini Kit protocol with QIAshredder to homogenize cells and an additional step of on-column gDNA elimination using RNase-Free DNase Set. RNA concentration and purity were checked using the ratio of absorbance at 260/280 nm, as well as 260/230 nm by spectrophotometric measurement with Nanodrop 2000c (Thermo Scientific, USA). From each cell culture, an equal amount of mRNA (0.5 μg) was reverse transcribed to cDNA using a RT2 First Strand kit according to the manufacturer's instructions available from the website: https://www.qiagen.com/pl/resources/resourcedetail?id=6161ebc1-f60f-4487-8c9e-9ce0c5bc3070&lang=en. Samples were diluted in qPCR master mix using RT2 SYBR Green Kit according to the supplier's instructions mentioned above and pipetted (25 μL/well) into 96-well PCR array plates. Real-time PCR for the human oxidative stress PCR array consisting of 84 genes involved in oxidative stress response and antioxidant defence with 3 reverse transcription controls, 3 positive PCR controls, a gDNA contamination control, and 5 constitutively expressed housekeeping genes (ACTB, B2M, GAPDH, HPRT1, RPLP0) was performed according to the manufacturer's instructions using Roche LightCycler 96 (Switzerland). Quality controls confirmed no gDNA contamination. Tests for RNA quality and PCR performance were successfully passed. Three independent replicates of each treatment of cells with investigated concentrations of antioxidants were carried out.
Data obtained from gene expression experiments were analysed using software available from the Qiagen website: http://www.qiagen.com/pl/shop/genes-and-pathways/data-analysis-center-overview-page/. Relative changes in gene expression were calculated using the comparative threshold cycle method (ΔΔCt). This method requires that from the Ct value of a gene of interest (GOI), the gene-average Ct of the 5 housekeeping genes (HKG) is first subtracted to normalize the amount of RNA for both the sample and controls. Subsequently, the ΔΔCt was calculated as the difference between the normalized average Ct of GOI of sample and the normalized average Ct of the controls. This ΔΔCt value was raised to the power of 2 to calculate the degree of change.
2.10. Statistical analysis
All values are expressed as means ± SD of three independent experiments unless stated otherwise. Correlations between values of standard reduction potentials as well as antioxidant activity determined by chemical (ABTS, DPPH, FC) and biological (CAA) tests were examined using Pearson's coefficients. The statistical significance of determinations of antioxidant activity in a cell model obtained by CAA testing was examined by one-way ANOVA with Tukey-Kramer test, while the results of genotoxicity and protection against DNA fragmentation measured by comet assays were examined by one-way ANOVA with Dunnett's test. All statistical analyses were performed using Prism 4.0 software package (GraphPad Software, Inc., USA). The statistical significance of changes in gene expression between samples and controls was evaluated by unpaired Student's t-test for each GOI. The level of statistical significance was set at p ≤ 0.05.
3. Results
3.1. Standard reduction potentials
Standard reduction potential describes the ability of a compound to accept electrons. The lower the value of the standard reduction potential of a compound, the better an electron donor it is, which means that the compound exhibits stronger antioxidant properties. Table 1 contains the values of standard reduction potentials E° [V] vs. standard hydrogen electrode (SHE) for catechin derivatives and GSH determined using redox potentiometric titration.
Table 1.
Standard reduction potentials for catechins and GSH (vs. SHE at 37 ± 0.1 °C). When there are more inflection points on the titration curve, they are marked as 1st infl. and 2nd infl.
| Antioxidant | Standard reduction potential E° [V] |
|---|---|
| GSH | 0.310 ± 0.003 |
| C | 0.281 ± 0.008 |
| EC | 0.277 ± 0.005 |
| EGC | 0.287 ± 0.003 |
| ECG (1st infl.) | 0.098 ± 0.002 |
| ECG (2nd infl.) | 0.146 ± 0.003 |
| EGCG (1st infl.) | 0.104 ± 0.002 |
| EGCG (2nd infl.) | 0.153 ± 0.001 |
The redox couple GSH/GSSG displayed the highest value of reduction potential as compared to other tested compounds. It follows that GSH exhibited the weakest antioxidant activity. When comparing different catechin derivatives, it can be seen that their standard reduction potentials depended not only on the number of hydroxyl groups, but also on the site of substitution (Fig. 1). Among catechins, the parent catechin structure (C) displayed the lowest antioxidant activity (Table 1). The introduction of a hydroxyl group at position 3′ (EGC) did not improve antioxidant properties. C exhibited better antioxidant properties than its stereoisomer – EC. These differences were not large, however. The esterification of a hydroxyl group at position 3 with gallic acid markedly decreased the values of standard reduction potentials of both EGCG and ECG, the latter becoming the strongest antioxidant among the catechins tested.
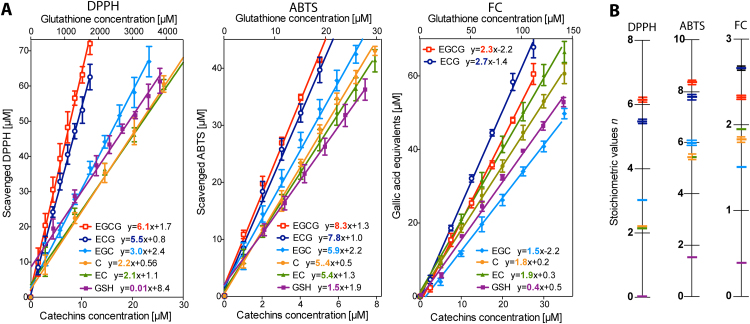
Fig. 1.
Chemical structures of tested compounds. Characteristic substituents linked to parent flavan-3-ol structure present in catechin derivatives are highlighted in red. The acronyms refer to: C – (+)-catechin, EC – (-)-epicatechin, EGC – (-)-epigallocatechin, ECG – (-)-epicatechin gallate, EGCG – (-)-epigallocatechin gallate.
Both catechin gallates are compounds with complicated chemical structures with respect to the number and localization of hydroxyl groups. Consequently, the curves obtained by potentiometric titration reflecting their redox behaviour gave somewhat ambiguous results. Therefore, two inflection points were taken into consideration. The first inflection point (1st infl.) may be ascribed to the gallic acid ester moiety. The second inflection point (2nd infl.) may be ascribed to the catechin core of esters (EC, EGC). Considering the 2nd inflection points, ECG displayed higher antioxidant activity than EGCG. Similarly, unbound EC possessed lower standard reduction potential than EGC. Moreover, the difference between standard reduction potential of compounds (EC, EGC), which constitute the catechin core of esters, and the second inflection points (2nd infl.) of ECG and EGCG (E°EC–E°ECG 2nd infl. and E°EGC–E°EGCG 2nd infl.) was in both cases similar and equalled to 131 and 134 mV, respectively. It seems that the presence of the gallic ester group decreased the standard reduction potential of the core structure, thus improving the antioxidant activity of the parent compound. The sequence of the antioxidant activity at 37 °C based on the standard reduction potentials determined by redox titration of the 1st and 2nd inflection points for the studied group was: GSH < C = EGC < EC < EGCG < ECG.
3.2. Antioxidant activity by spectrophotometric methods
The ABTS and DPPH assays have been widely used to determine the free radical scavenging activity of pure compounds. In these tests, antioxidants are usually characterized by their EC50 value, i.e. the concentration necessary to reduce 50% of ABTS•+ or DPPH• or by TEAC index (Trolox Equivalent Antioxidant Capacity), which expresses antioxidant capacity of a given substance as equivalent to a certain Trolox concentration. However, we propose here to use a stoichiometric value n initially described by Villaño et al. [29] for steady state oxidation-reduction reactions. In contrast to the orginal definition, we decided to also incorporate kinetic elements, and in our hands, the stoichiometry value describes the number of oxidant molecules reduced by one molecule of antioxidant after 10 min of reaction in ABTS and DPPH tests (n10). In the case of the presented results, the stoichiometric values n10 were calculated for antioxidant concentrations within a linear range of the assay and were expressed as the slope of a line describing the relationship between concentration of reduced oxidant and concentration of tested antioxidant. The concentration response lines obtained after an appropriate reaction time (10 min), and calculated on their basis stoichiometric values determined at 37 °C, are presented in Fig. 2. The results indicate the same trend for the two tests applied, where antioxidant activity of tested compounds increased in the following order: GSH < EC < C < EGC < ECG < EGCG. When comparing antioxidant activity of GSH to catechins, it is striking that GSH exhibited a much lower n10 value in the DPPH assay than in ABTS assays.
Fig. 2.
The linear relationship between concentration of reduced radicals or gallic acid equivalents and concentration of flavan-3-ols and GSH – Panel A, comparison of stoichiometric values n10 calculated based on ABTS and DPPH methods as well as n60 in the case of the FC method – Panel B. The results are means ± SD of three independent determinations.
The FC method was another considered in this study. It is based on the transfer of electrons in an alkaline medium from phenolic compounds to phosphomolybdenic phosphotungstic acid complexes to form blue coloured complexes. The reducing ability in this case was expressed as the number of gallic acid molecules which formed blue complexes equivalent to one molecule of antioxidant under study. The dependencies calculated on the basis of linear regression are shown in Fig. 2. The outcome in FC thus was strongly affected not only by the number of hydroxyls, but by their localization in the antioxidant structure as well. The obtained antioxidant activity values using the FC method increased in the following order: GSH < EGC < C < EC < EGCG < ECG. In comparison to the former series, there were some shifts observed: C and EC more effectively reduced the FC reagent than EGC, and ECG seemed a better antioxidant than EGCG. The observed shifts may stem from the different capacity to chelate metal ions among catechins. High chelation activity is strongly related to catechol moieties and this may interfere with measurements of antioxidant activity by FC method [32].
3.3. Cytotoxicity by MTT test
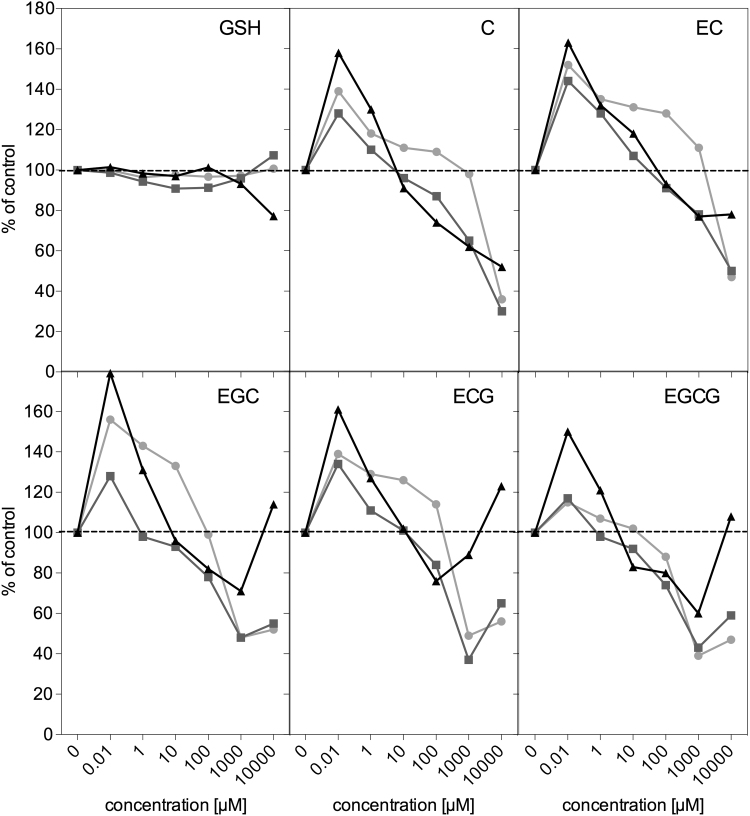
Determination of cell growth inhibition using the MTT test is a standard approach to assess biological potential of substances via detection of metabolic activity in exposed cells. Here, this test was applied to study the impact of exogenous GSH and catechins on HT29 cell growth. The human colon adenocarcinoma HT29 cell line was chosen as a model of the alimentary tract, a tissue being in direct contact with ingested food components, including phytochemicals such as catechins. The dose response curves for 6, 24 and 72 h treatments are presented in Fig. 3.
Fig. 3.
Inhibition of growth of HT29 cells determined by MTT test after 6 (circles), 24 (squares) and 72 h (triangles) exposure to exogenous GSH and catechins. Results represent means of three independent experiments carried out in triplicate (SD are lower than 15%).
GSH, the main cellular redox stabilizer, did not influence the cell growth at any of the investigated concentrations, for neither short nor prolonged treatments. Low concentrations of catechins (0.01–1 μM), which may influence epithelial cells of the alimentary tract, as well as being reachable in the blood stream, caused significant cell growth stimulation for all exposure times tested. The concentration of 10 µM after 24 and 72 h of treatments seemed to be a sort of turning point, i.e. it did not cause cell growth stimulation, but rather maintained (C, EC, EGC, ECG) or inhibited (EGCG) cell growth, by no more than 20%, however. In the case of higher doses, inhibition of cell growth up to 70% was observed for the 6 and 24 h treatments, with the exception of EC, for which the stimulatory effects were seen even at 1 mM after 6 h of exposure. The seemingly increased cell viability after 72 h of incubation observed at the highest concentration of EGC, ECG, and EGCG is an artefact. The observation of these cell cultures under the inverted microscope did not reveal higher number of cells, on the contrary, hardly any colonies were seen (Fig. S1 in Supplementary materials). It seems that catechins or their derivatives exhibited such strong antioxidant activity that, despite rinsing the plates before addition of the MTT dye, direct non-enzymatic reduction to formazan crystals occurred in the cell culture medium.
3.4. Cellular antioxidant activity
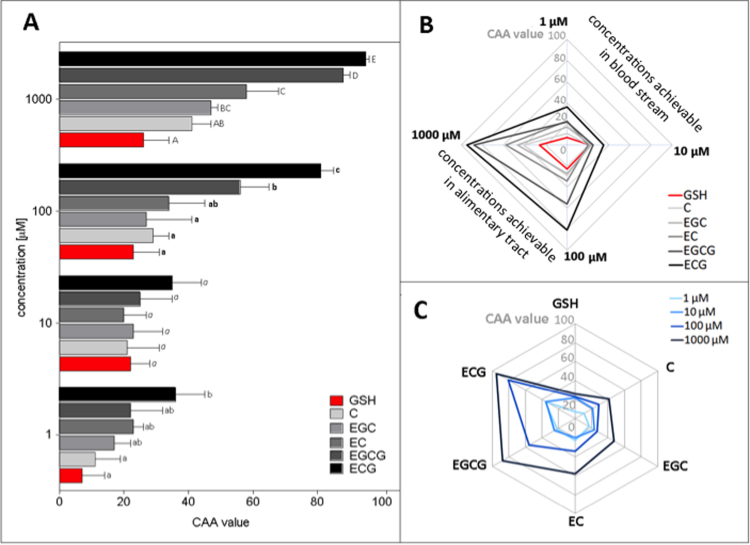
To quantify the antioxidant properties of catechins and exogenous GSH in the HT29 cell line model, the CAA assay was applied, which is said to successfully emulate the redox conditions existing in the human body, including several aspects of uptake, transport and cellular metabolism [33]. In this method, the assessment of antioxidant effect relies on the ability of antioxidants to inhibit oxidation of the specific probe (DCFH) absorbed by cells to its fluorescent form (DCF). Because the degree of probe oxidation is proportional to fluorescence levels, its decrease can be a measure of reducing capacity of an antioxidant in the cellular setting [33]. The results of fluorescence measurements are recalculated into so-called CAA values as described in Section 2.6. Higher CAA values after exposure to antioxidants suggests stronger reducing status of treated cells.
As shown in Fig. 4A, for potential physiological concentrations of the investigated compounds, significant differences in CAA values were observed only between GSH and ECG as well as C and ECG at 1 µM concentration. The latter compound exhibited the strongest cellular antioxidant activity for all doses applied to cells. At a concentration of 10 µM, all compounds increased cellular antioxidant capacity to the same extent. At a concentration of 100 µM, the increase of cellular reducing status by ECG stood out from other catechins and was followed by EGCG, while treatment with other compounds did not significantly influence the CAA value. The most diversified impact was observed at the highest concentration used. In this case, ECG remained the strongest stimulant in terms of cellular antioxidant activity and was followed by EGCG and EC.
Fig. 4.
Cellular antioxidant activity of exogenous GSH and catechins in HT29 cells – Panel A, Panel B – grouping antioxidant potential of tested compounds as regards physiological concentrations potentially occurring in blood (1–10 µM) and concentrations reachable in alimentary tract (100–1000 µM); Panel C – the impact of individual compounds on cellular antioxidant activity in HT29 cells. Results are means ± SD of three independent experiments. Different letters for the same concentration indicate a significant difference in the one way ANOVA with Tukey test (p < 0.05).
Panel B of Fig. 4 shows that the reducing status of intestinal cells exposed to catechins is bimodal; somewhat influenced by lower compound concentrations and strongly enhanced by higher ones. Both esters (ECG, EGCG), especially at higher concentrations and relevant only for intestinal epithelium, seemed to ensure a much better antioxidant barrier for cells than other compounds (Fig. 4, panel C).
3.5. Genotoxic effects and antigenotoxic protection
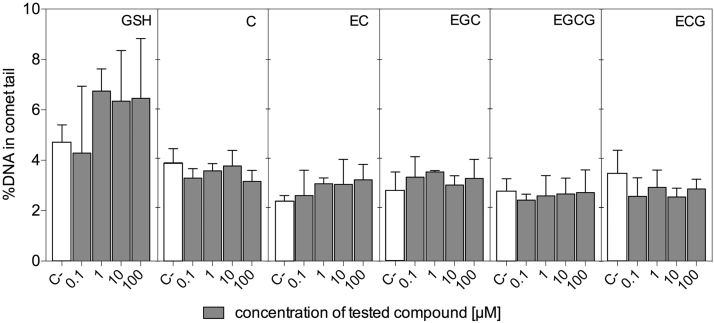
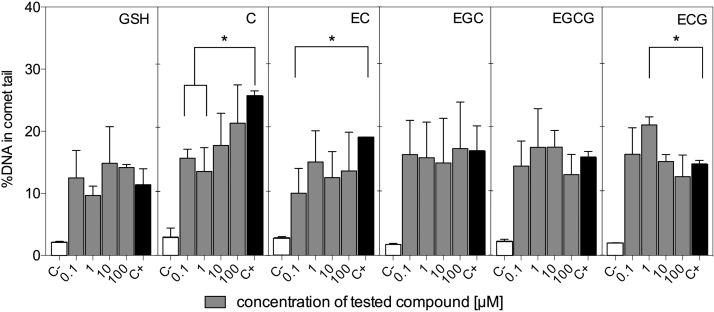
It has been shown that antioxidants may protect DNA from H2O2-mediated damage, however some may actually escalate oxidative damage caused by H2O2, or may even be genotoxic themselves [34], [35]. The comet assay, a useful method for detecting DNA strand breaks at the single-cell level, has been used by us to study the impact of exogenous GSH and catechins on DNA integrity in both mentioned situations.
Fig. 5 shows that under the treatment conditions used here, none of the investigated compounds was genotoxic per se to HT29 cells, regardless of concentration. The compounds differed however, when the comet assay was used to determine their ability to protect DNA from oxidative damage caused by H2O2 (Fig. 6). Compared to non-treated cells (negative control C−), this oxidant, at 150 µM and after 1 h of treatment, increased DNA fragmentation in treated HT29 cells by at least 5 times (positive control C+). The cytotoxicity assessment using the MTT test showed that 0.1–10 µM H2O2 caused cell growth stimulation after both 1 and 24 h of exposure. A concentration of 100 µM maintained cell growth at control level, while 150 µM inhibited cell proliferation (Fig. S2 in Supplementary Materials). The significant decrease in DNA fragmentation following 1 h exposure to 150 µM H2O2 was observed only for cells previously treated for 24 h with C at 0.1 and 1 µM, as well as for EC at 0.1 µM. In contrast, ECG at 1 µM significantly potentiated the genotoxic effect caused by H2O2. These slight genotoxic effects were not reflected by increased cytotoxicity (Fig. S2), which suggests that DNA repair systems successfully removed DNA lesions induced by H2O2 in cells that were pre-exposed to antioxidants. The combined treatment, i.e. 24 h exposure to catechins followed by 1 h exposure to H2O2, prevented, however, the stimulation of cell growth observed for HT29 cells treated with catechins only (Fig. 3), thereby supporting our hypothesis that reduction of oxidative stress by antioxidants may create an intracellular environment conducive to proliferation.
Fig. 5.
Genotoxicity of tested antioxidants expressed as %DNA in comet tail evaluated in HT29 cells. Results represent means ± SD of three independent experiments. Negative control (C−) – non-treated cells. No significant differences between treated and non-treated cells were observed according to one-way ANOVA with Dunnett's test.
Fig. 6.
The ability of tested antioxidants to protect DNA of HT29 cells from H2O2-induced oxidative damage expressed as %DNA in comet tail. Results represent means ± SD of three independent experiments. Negative control (C−) – non-treated cells, positive control (C+) – cells treated with 150 μM H2O2. Significantly different values determined by one-way ANOVA with Dunnett's test are marked as (*) p < 0.05.
3.6. Microarray analysis
Owing to the pleiotropic biological properties of flavan-3-ols, the present work also aimed at the determination of the impact of catechins and exogenous GSH on the expression of a wide spectrum of genes associated with the oxidative stress response. The investigated genes (84 included in the microarray used, as indicated in Table S1 in Supplementary materials) were categorized as those relevant for: antioxidant activity, superoxide release and metabolism, activity of peroxidases and oxidoreductases, as well as being relevant for inflammation, apoptosis, regulation of cell cycle, and other processes implicated in oxidative stress. Table 2 summarizes the -fold changes in gene expression determined for HT29 cells in response to 24 h incubation with the investigated compounds at 1 and 10 µM concentrations compared to control cells treated with the appropriate solvent only. The selection of concentrations was based on previous tests, in particular on cytotoxicity (Section 3.3) where 1 and 10 µM catechins displayed very different biological behaviours.
Table 2.
Changes in expression of oxidative stress response and antioxidant defence genes in HT29 cells after treatment with exogenous GSH and catechins at concentrations of 1 and 10 μM for 24 h at 37 °C.
| Antioxidant | Concentration [μM] | Gene | Description | Fold change | p-value |
|---|---|---|---|---|---|
| Up-regulation | |||||
| GSH | 1 | GSTZ1 | Glutathione transferase zeta 1 | 2.242 | 0.006 |
| NOX5 | NADPH oxidase 5 | 5.114 | 0.016 | ||
| SEPP1 | Selenoprotein P, plasma, 1 | 3.948 | 0.004 | ||
| TXNRD2 | Thioredoxin reductase 2 | 2.126 | 0.009 | ||
| 10 | BNIP3 | BCL2/adenovirus E1B | 3.813 | 0.030 | |
| NCF1 | Neutrophil cytosolic factor 1 | 5.570 | 0.001 | ||
| NOS2 | Nitric oxide synthase 2 | 2.792 | 0.013 | ||
| NOX5 | NADPH oxidase 5 | 3.625 | 0.019 | ||
| SEPP1 | Selenoprotein P, plasma, 1 | 2.831 | 0.012 | ||
| C | 1 | ALB | Albumin | 3.303 | 0.002 |
| CCL5 | Chemokine (C-C motif) ligand 5 | 3.492 | 0.021 | ||
| HSPA1A | Heat shock 70-kDa protein 1A | 2.629 | 0.040 | ||
| 10 | – | – | – | – | |
| EC | 1 | ALB | Albumin | 2.001 | 0.023 |
| 10 | HSPA1A | Heat shock 70-kDa protein 1A | 2.189 | 0.019 | |
| EGC | 1 | ALB | Albumin | 3.049 | 0.035 |
| CCL5 | Chemokine (C-C motif) ligand 5 | 2.767 | 0.020 | ||
| 10 | – | – | – | – | |
| ECG | 1 | AOX1 | Aldehyde oxidase 1 | 2.103 | 0.034 |
| HSPA1A | Heat shock 70-kDa protein 1A | 3.751 | 0.013 | ||
| 10 | – | – | – | – | |
| EGCG | 1 | ALB | Albumin | 2.836 | 0.014 |
| HSPA1A | Heat shock 70-kDa protein 1A | 3.351 | 0.005 | ||
| 10 | – | – | – | – | |
| Down-regulation | |||||
| GSH | 1 | CYGB | Cytoglobin | − 2.506 | 0.008 |
| 10 | – | – | – | – | |
| C, EC, ECG, EGCG | 1, 10 | – | – | – | – |
| EGC | 1 | – | – | – | – |
| 10 | SRXN1 | Sulfiredoxin 1 | − 2.542 | 0.012 | |
The obtained results showed that treatment with exogenous GSH and catechins had very different impacts on the cellular transcriptome. GSH caused up regulation of a broad range of genes in exposed cells. Both 1 and 10 µM GSH influenced the genes SEPP1 and NOX5. SEPP1 encodes selenoprotein P, an extracellular glycoprotein that has an antioxidant role and appears to be associated with endothelial cells [36], while NOX5 codes for a calcium-dependent NADPH oxidase that generates superoxide [37]. The set of genes up-regulated by 1 µM GSH was expanded to genes important for cellular defence against oxidative stress, such as GSTZ1 and TXNRD2. GSTZ1 is a member of the glutathione S-transferase gene super-family, which encodes key enzymes implicated in the detoxification of electrophilic molecules by conjugation with GSH [38]. The other gene codes for thioredoxin reductase 2, a protein that plays a key role in maintaining thioredoxin in a reduced state [39]. In contrast, GSH at 10 µM enhanced the expression of proteins critical for the emergence of oxidative stress in a cell. Except for up-regulation of NOX5, an increase in expression of NCF1 was observed. This gene encodes a cytosolic protein required for the activation of the NADPH oxidase (NOX2) system responsible for superoxide production [40]. Furthermore, GSH at 10 µM was found to up-regulate expression of nitric oxide synthase 2 (NOS2). The final up-regulated gene related to oxidative stress was BNIP3, which encodes a proapoptotic protein that may act via a mitochondrial pathway. This gene is silenced in tumours by DNA methylation [41]. Only one gene, CYGB, was significantly down-regulated by GSH at 1 µM. The protein encoded by this gene may be involved in intracellular oxygen storage or transfer [42].
In the case of catechins, we found 3 genes (ALB, CCL5, HSPA1A) out of 84 in the array to be significantly up-regulated by most of catechins at a concentration of 1 µM. This increase in expression was not observed at higher concentrations (10 µM). The increase in expression of ALB was caused by C, EC, EGC and EGCG. This gene encodes albumin, that constitutes a part of the non-enzymatic antioxidant system which, along with antioxidant enzymes, forms a line of defence against oxidation [43]. CCL5, belonging to a group of inflammation-relevant genes, was up-regulated by C and EGC. The protein encoded by it is classified as a chemotactic cytokine or chemokine, a secreted protein involved in immunoregulatory and inflammatory processes [44]. Within the group of genes up-regulated by 1 µM of C, ECG and EGCG, HSPA1A was also identified, which codes for a heat shock protein. Up regulation of HSPA1A expression may support host resistance to protein misfolding caused by oxidative stress and inflammation [45]. EC increased the expression of this gene at the higher concentration evaluated (10 µM). Among genes associated with metabolism of ROS, an increase in expression of AOX1 after cell exposure to ECG was observed. AOX1 is an enzyme that catalyses the conversion of an aldehyde in the presence of oxygen and water to a carboxylic acid, which is, however, accompanied by hydrogen peroxide release [46]. It is difficult to assess whether the presence of usually very toxic and stable aldehyde or the ROS generated as a result of aldehyde metabolism are more dangerous for the cell. Only EGC at the higher concentration tested caused a drop in expression of one gene, namely SRXN1. This down regulation may have a negative impact on redox homeostasis because the protein encoded by this gene contributes to oxidative stress resistance by reducing cysteine-sulfinic acid formed under exposure to oxidants such as peroxiredoxins [47].
4. Discussion
This study examined the relationship between electrochemical properties and biological behaviour of catechins and exogenous GSH, as well as molecular implications for oxidative stress responses and antioxidant defence systems in HT29 cells exposed to these compounds. In the initial stage of statistical analysis of the results, we examined the connections between standard reduction potentials and antioxidant capacity of compounds assessed by common chemical tests (DPPH, ABTS, FC all carried out at 37 °C) and the biologically more relevant CAA assay (Table 3). In calculations, the stoichiometry values n10 or n60 and CAA values were used as indicators of antioxidant activity. In the case of catechin esters, values of E° for 1st and 2nd inflection points (Table 1) were taken into consideration for calculations. The strongest (inverse) correlation with E° was seen for n10 values obtained by the DPPH assay and for CAA values determined for high concentrations of the studied antioxidants. In contrast, CAA values obtained at physiologically-relevant concentrations showed substantially weaker correlation (inverse) with E° values. In general, when only catechins were considered, better correlations between chemical or biological antioxidant tests were determined for E° based on the 1st inflection point. This suggests that the major effect on reducing activity of catechin gallate esters is the galloyl moiety, whose oxidation is reflected by the 1st inflection point. Thus, the final antioxidant activity of esters does not seem to depend strongly on oxidation of the catechin core, as reflected by E° calculated based on the 2nd inflection point.
Table 3.
Correlations between values of standard reduction potentials and antioxidant activity of GSH and catechins or only catechin series determined by chemical (ABTS, DPPH, FC) as well as biological (CAA) tests examined using Pearson's coefficients.
| Pearson's coefficients |
||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | DPPH n10 | ABTS n10 | FC n60 | CAA (1 µM) | CAA (10 µM) | CAA (100 µM) | CAA (1000 µM) | |
| E° (1st infl.) | GSH, catechins | − 0.930 | − 0.807 | − 0.778 | − 0.781 | − 0.792 | − 0.940 | − 0.956 |
| catechins | − 0.966 | − 0.966 | − 0.903 | − 0.727 | − 0.794 | − 0.928 | − 0.969 | |
| E° (1st and 2nd infl.) | GSH, catechins | − 0.929 | − 0.837 | − 0.812 | − 0.772 | − 0.750 | − 0.907 | − 0.951 |
| catechins | − 0.940 | − 0.938 | − 0.886 | − 0.697 | − 0.737 | − 0.884 | − 0.951 | |
Among the antioxidants studied, GSH possesses the highest value of standard reduction potential, meaning the lowest antioxidant activity, which was confirmed by all chemical and cellular tests used. Moreover, for this physiological antioxidant, when applied exogenously to HT29 cells in CAA tests, no dose dependence was observed (Fig. 4). In the case of catechins, our results have shown that flavanols bearing a catechol moiety exhibited higher antioxidant activity compared to those with a pyrogallol moiety, although structures of the latter contain more hydroxyl groups. In the cellular model, in particular, it was seen that esters with catechol moieties (ECG, EGCG) were the most efficient in protection against ROS. In general, it may be concluded that under conditions of undisturbed redox homeostasis, the absorbed catechins will exert little influence on the antioxidant barrier of human tissues due to low bioavailability that limits internal exposure. However, they may play a role in the event of, for example, GSH depletion occurring due to xenobiotic detoxification via the mercapturic acid pathway. Certainly, intestinal cells being in direct contact with ingested food may be exposed to much higher concentrations of catechins and are thus potentially more effectively protected against ROS.
In the next stage of statistical analysis of the results, we examined correlations between the chemical (DPPH, ABTS, FC) and cellular (CAA) tests used for the assessment of reducing capacity of the investigated compounds (Table 4). The strongest correlation with the CAA test was seen for the DPPH assay when all antioxidants were considered, and for FC tests in the case of the catechin-only series. However, the strength of these correlations was highly dependent on the concentration of compounds applied to cells in the CAA assay. In almost all cases, the highest values of Pearson's coefficient were calculated for the highest concentration of antioxidants (1 mM) in CAA tests. The weakest correlation was observed for physiologically-relevant concentrations: 1 µM in the case of the catechin series or 10 µM when all compounds were considered. At the latter concentration in CAA tests, antioxidant activity of all catechins and exogenous GSH did not differ significantly (Fig. 4).
Table 4.
Correlations between antioxidant activity of GSH and catechins or catechin-only series determined by chemical (ABTS, DPPH, FC) tests and CAA assay examined using Pearson's coefficients.
| Pearson's coefficients |
||||||
|---|---|---|---|---|---|---|
| DPPH n10 |
ABTS n10 |
FC n60 |
||||
| GSH, catechins | catechins | GSH, catechins | catechins | GSH, catechins | catechins | |
| CAA (1 µM) | 0.772 | 0.628 | 0.759 | 0.613 | 0.808 | 0.731 |
| CAA (10 µM) | 0.664 | 0.725 | 0.531 | 0.710 | 0.580 | 0.807 |
| CAA (100 µM) | 0.834 | 0.831 | 0.732 | 0.824 | 0.786 | 0.940 |
| CAA (1000 µM) | 0.948 | 0.920 | 0.888 | 0.917 | 0.870 | 0.872 |
Altogether, the results of the above described experiments seem to suggest that exposure to antioxidants at physiologically-relevant concentrations does not have any substantial impact on cellular redox homeostasis. However, an alternative interpretation of this observation seems to better fit the results of biological assays presented in this paper. One could hypothesize that the treatment with catechins at around 10 µM brings cellular redox status to the peak of its resistance to changes in the concentration of redox-active species. In other words, to a state of equilibrium buffered by cellular anti- and pro-oxidants, as proposed by Jacob et al. [48], [49], for cysteine-modifying agents and thiol/disulfide balance in their thiolstat concept. Indeed, both cytotoxicity and gene expression (at least in the case of the specific gene array analysed) for catechin concentrations around 10 µM in culture medium appeared to make HT29 cells non-responsive to the treatment. Neither was mitochondrial respiration of HT29 cells measured using a Seahorse Agilent XFp metabolic flux analyser affected at this dose of studied antioxidants (data not shown), though one could expect respiratory processes to be sensitive to the presence of redox active substances. It has been demonstrated that polyphenolic antioxidants are able to affect mitochondrial electron transport chain and ATP synthesis by modulating activity of complexes I–V (reviewed in [50]). One could speculate that catechins should also behave in a similar manner, yet at the concentration studied we did not observe this.
The dual impact of catechin concentrations on cell growth inhibition was also clearly observed and perhaps may be explained by gradual alterations in the redox status of cells. It is known that cancer cells overproduce ROS, which on one hand are key molecules in activation of signalling pathways important for proliferation but, on the other, may impair cell growth due to oxidative stress [51], [52], [53]. Both of these effects seemed to be influenced by the treatment of HT29 cells with catechins. Low (physiologically achievable) concentrations of catechins stimulated cellular growth. This could be interpreted as a protective effect of these antioxidants, which helps cells to combat internal residual oxidative stress by restoring optimal redox balance. A concentration of 10 µM maintained cell growth at control levels, probably owing to the ensured stability of redox-dependent signalling at the suggested “redox buffered” equilibrium state. Higher concentrations, i.e. above 10 μM, possible only in the case of intestinal cells being in direct contact with ingested food, inhibited HT29 cell growth. This inhibition was not associated with induction of apoptosis, because no genomic DNA fragmentation was observed by comet assay for any dose of antioxidants applied to cells (Fig. 5). Hence, it can be presumed that decline in cell growth was due to deregulation of proliferation which depends on the constitutive activation of redox-sensitive targets, e.g. protein kinase C (PKC), protein kinase B (Akt), mitogen-activated protein kinases (MAPK), and ataxia telangiectasia mutated (ATM) kinase [54], [55], [56]. Such effects might be brought about by the disturbance of redox balance known as reductive stress. Cellular levels of ROS might be decreased to a point which shuts down ROS-dependent signalling pathways involved in proliferation [57].
The results of the CAA test justified the conclusion that treatment of HT29 cells with catechins should enhance the cellular antioxidative barrier, at least for concentrations above 10 µM. Thus, somewhat surprising were observations made with the aid of the comet assay under conditions of oxidative stress induced by H2O2 (150 µM) treatment. The protective effects against ROS-induced DNA fragmentation were determined only in a few cases and only for low concentrations (Fig. 6). C and EC, which possess higher values of E°, were the two catechins for which some decreasing trend of DNA fragmentation in ROS challenged cells was detected, reaching statistical significance at physiological concentrations (0.1 or 1 µM). Both catechin esters (ECG and EGCG), which displayed the strongest antioxidant activity in CAA tests, under the situation of oxidative stress tended to enhance DNA fragmentation, again especially at lower concentrations. These findings, though somewhat unexpected, are consistent with our previous results obtained in a cell-free system for a DNA amplicon exposed to 500 mM H2O2 in the presence of increasing catechin concentrations. At lower concentrations, (25 mM) this flavanol increased the level of DNA amplicon modification, but at higher concentrations (75 mM) this effect was reversed [58]. Although the mechanism behind these observations was not investigated, one could try to explain these results taking electrochemical properties of the studied catechins into consideration. Due to the low value of standard reduction potentials, these compounds may rapidly reduce H2O2 and acquire oxidized, intermediate form(s). Then, one may speculate, the disproportionation effect takes place, where an intermediate oxidation state converts to two different derivatives with opposing genotoxic effects towards H2O2. In the case of catechin esters, it was even possible to determine two values of E° (Table 1). Depending on reduction potentials, for strong reductants such as ECG and EGCG, at lower concentrations applied to cells along with H2O2, pro-oxidant effects will prevail, leading to the enhancement of DNA damage; at higher concentrations, the pro-oxidant derivative will be effectively sequestered by its antioxidant counterpart in a reaction called comproportionation. Following this scenario, for compounds characterized by higher values of E° such as C and EC, the abundance of pro-oxidant derivatives will increase with concentration and, accordingly, the protection of cellular DNA against oxidative damage will weaken with the dose of antioxidant applied to cells. It is often said in discussions regarding polyphenols that their antioxidant properties in biological systems depend on the concentration used; the above reasoning explains why, based on electrochemical grounds, this may indeed be the case (see Fig. 5).
However, the pro-oxidative activity of ECG may also be explained in a different way. We have found that only this ester, bearing a catechol moiety and at a concentration of 1 µM, up-regulated AOX1 expression, encoding a xenobiotic metabolizing enzyme, which transfers electrons to O2 with concomitant production of O2•- and H2O2. Slightly elevated levels of these ROS could additionally enhance DNA lesions caused primarily by exogenous H2O2. No such up-regulation was seen for higher concentrations (10 µM) or for other catechins.
The strong impact of the concentration of the antioxidants studied on mechanisms involved in redox homeostasis was observed in genomic studies as well. In the case of catechins, basically only the lower concentration investigated (1 µM) affected gene expression. We found 3 genes (ALB, CCL5, HSPA1A) to be significantly up-regulated by most catechins at this dose, at which increased proliferation of HT29 cells was also determined (Fig. 3). In particular, the enhanced expression of ALB and HSPA1A may have positive effects on ameliorating oxidative stress and its effects on cancer cells, whose increased metabolic rate may lead to elevations in ROS abundance, because they are mainly produced by the mitochondrial respiratory chain. The higher, 10 µM concentration of catechins, as mentioned before, seemed to ensure proper redox balance in cells and the expression of antioxidant genes was no longer stimulated.
GSH, which, in contrast to catechins, did not affect the rate of proliferation of HT29 cells or the cellular antioxidant barrier, regardless of concentration, also displayed different, this time more variable, influences on expression of several genes associated with oxidative stress responses. Again, at the genomic level, this antioxidant seemed to work as a buffer, which sustains redox balance in cells, but this time in a dose dependent manner. At the lower 1 µM concentration, it caused up-regulation of expression of antioxidant genes (GSTZ1, SEPP1, TXNRD2), thus preventing oxidative stress, whereas at the 10 µM concentration, it stimulated expression of key genes involved in ROS production such as NOX5, NOS2 and NCF1, thereby preventing reductive stress. These observations are in line with Zhang et al. discovery that excessive GSH levels and depletion of ROS blunts the antioxidant defence system in induced pluripotent stem cells of aged tissue donors [59].
In conclusion, our study demonstrated that the standard reduction potential of redox active compounds may be a helpful, chemically defined, unambiguous predictor of their impact on a number of biological activities in a cellular model, and thus probably also in vivo. In addition, we have found that the presence of a catechol moiety exerts significant influence on antioxidant properties and consequently biological potential of the tested catechins. Because a catechol moiety is often present in a variety of substances found in dietary products, this may be another factor missing in our understanding of redox regulation of cellular functions by bioactive phytochemicals. The presented results may also have certain practical implications. The chemopreventive potential of catechins, as documented in numerous studies carried out in several experimental models, is considered as being limited in humans, because of the low bioavailability of these phytochemicals attributed to poor intestinal absorption. However, our results point to the fact that such low physiological concentrations around 10 µM seemed to stabilize the redox status of cells, whereas higher concentrations relevant for intestinal epithelium, do not show this feature. It follows that all dose-dependent effects associated with electrochemical properties of redox active food components, such as catechins and biological functions potentially controlled by them, may become deregulated upon exposure to excessive doses. This puts in question attempts to increase the bioavailability of antioxidant phytochemicals as a way of improving their health-promoting properties.
Acknowledgments
This work was supported by National Science Centre (Poland) in a programme MAESTRO 6 (application no. 2014/14/A/ST4/00640).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.05.005.
Appendix A. Supplementary material
Supplementary material
References
- 1.Clement Y. Can green tea do that? A literature review of the clinical evidence. Prev. Med. 2009;49:83–87. doi: 10.1016/j.ypmed.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Yang C.S., Jin H., Guan F., Chen Y.K., Wang H. Cancer preventive activities of tea polyphenols. J. Food Drug Anal. 2012;20:318–322. [Google Scholar]
- 3.Crespy V., Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2018;134:3431–3440. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury A., Sarkar J., Chakraborti T., Pramanik P.K., Chakraborti S. Protective role of epigallocatechin-3-gallate in health and disease: a perspective. Biomed. Pharmacother. 2016;78:50–59. doi: 10.1016/j.biopha.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Šeruga M., Novak I., Jakobek L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011;124:1208–1216. [Google Scholar]
- 6.Todorovic V., Milenkovic M., Vidovic B., Todorovic Z., Sobajic S. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J. Food Sci. 2017;82:1020–1027. doi: 10.1111/1750-3841.13672. [DOI] [PubMed] [Google Scholar]
- 7.Grzesik M., Napar K., Bartosz G., Sadowska-Bartosz I. Antioxidant properties of catechins: comparison with other antioxidants. Food Chem. 2018;241:480–492. doi: 10.1016/j.foodchem.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 8.Le Gal K., Ibrahim M.X., Wiel C., Sayin V.I., Akula M.K., Karlsson C., Dalin M.G., Akyürek L.M., Lindahl P., Nilsson J., Bergo M.O. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015;7:1–8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 9.James K.D., Kennett M.J., Lambert J.D. Potential role of the mitochondria as a target for the hepatotoxic effects of (-)-pigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2018;111:302–309. doi: 10.1016/j.fct.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bast A., Haenen G.R.M.M. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013;34:430–436. doi: 10.1016/j.tips.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Kim H., Sakamoto K. (-)-Epigallocatechin gallate suppresses adipocyte differentiation through the MEK/ERK and PI3K/Akt pathways. Cell Biol. Int. 2012;36:147–153. doi: 10.1042/CBI20110047. [DOI] [PubMed] [Google Scholar]
- 12.Lee I., Lin C., Lee C., Hsieh P., Yang C. Protective effects of (−)-epigallocatechin-3-gallate against TNF-α-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J. Nutr. Biochem. 2013;24:124–136. doi: 10.1016/j.jnutbio.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.S., Quon M.J., Kim J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa-diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koppenol W.H. Oxyradical reactions: from bond-dissociation energies to reduction potentials. FEBS Lett. 1990;264:165–167. doi: 10.1016/0014-5793(90)80239-f. [DOI] [PubMed] [Google Scholar]
- 16.Janeiro P., Brett A.M. Oliveira. Catechin electrochemical oxidation mechanisms. Anal. Chim. Acta. 2004;518:109–115. [Google Scholar]
- 17.Vivas N., Nonier M.F., de Gaulejac N.V. Vivas. Titrimetric method based on potentiometric titration to evaluate redox couples in wine and polyphenols. Vitis – J. Grapevine Res. 2004;43:205–208. [Google Scholar]
- 18.Kilmartin P.A., Zou H., Waterhouse A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001;49:1957–1965. doi: 10.1021/jf001044u. [DOI] [PubMed] [Google Scholar]
- 19.Autréaux B.D., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 20.Ray P.D., Huang B., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad S., Gupta S.C., Tyagi A.K. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105. doi: 10.1016/j.canlet.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 22.In I., Chio C., Chio D.A. ROS in cancer: the burning question. Trends Mol. Med. 2017;23:411–429. doi: 10.1016/j.molmed.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khokhar S., Magnusdottir S.G.M. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food Chem. 2002;50:565–570. doi: 10.1021/jf010153l. [DOI] [PubMed] [Google Scholar]
- 24.Yashin A., Nemzer B., Yashin Y. Bioavailability of tea components. J. Food Res. 2012;1:281–290. [Google Scholar]
- 25.Omca D., Craig A., Nuran E. Biologically important thiols in various vegetables and fruits. J. Agric. Food Chem. 2004;52:8151–8154. doi: 10.1021/jf040266f. [DOI] [PubMed] [Google Scholar]
- 26.Yamada H., Ono S., Wada S., Aoi W., Park E.Y., Nakamura Y., Sato K. Statuses of food-derived glutathione in intestine, blood, and liver of rat. Npj Sci. Food. 2018;2:1–6. doi: 10.1038/s41538-018-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds W.L. The reaction between potassium ferrocyanide and iodine in aqueous solutions. J. Am. Chem. Soc. 1958;80:1830–1835. [Google Scholar]
- 28.Kusznierewicz B., Piekarska A., Mrugalska B., Konieczka P., Namieśnik J., Bartoszek A. Phenolic composition and antioxidant properties of polish blue-berried honeysuckle genotypes by HPLC-DAD-MS, HPLC postcolumn derivatization with ABTS or FC, and TLC with DPPH visualization. J. Agric. Food Chem. 2012;60:1755–1763. doi: 10.1021/jf2039839. [DOI] [PubMed] [Google Scholar]
- 29.Villaño D., Fernández-Pachón M.S., Moyá M.L., Troncoso A.M., García-Parrilla M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71:230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 30.Goupy P., Dufour C., Loonis M., Dangles O. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem. 2003;51:615–622. doi: 10.1021/jf025938l. [DOI] [PubMed] [Google Scholar]
- 31.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 32.Mira L., Fernandez M.T., Santos M., Rocha R., Florêncio M.H., Jennings K.R. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic. Res. 2002;36:1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe K.L., Rui H.L. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007;55:8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 34.Hobbs C.A., Swartz C., Maronpot R., Davis J., Recio L., Koyanagi M., mo Hayashi S. Genotoxicity evaluation of the flavonoid, myricitrin, and its aglycone, myricetin. Food Chem. Toxicol. 2015;83:283–292. doi: 10.1016/j.fct.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 35.George V.C., Rupasinghe H.P.V. Apple flavanoids suppress carcinogens-induced DNA damage in normal human bronchial epithelial cells. Oxid. Med. Cell. Longev. 2017;2017:1–34. doi: 10.1155/2017/1767198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciofani G., Genchi G.G., Mazzolai B., Mattoli V. Transcriptional profile of genes involved in oxidative stress and antioxidant defense in PC12 cells following treatment with cerium oxide nanoparticles. Biochim. Biophys. Acta - Gen. Subj. 2014;1840:495–506. doi: 10.1016/j.bbagen.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Fulton D.J.R. Nox5 and the regulation of cellular function. Antioxid. Redox Signal. 2009;11:2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharjee P., Paul S., Banerjee M., Patra D., Banerjee P., Ghoshal N., Bandyopadhyay A., Giri A.K. Functional compensation of glutathione S-transferase M1 (GSTM1) null by another GST superfamily member, GSTM2. Sci. Rep. 2013;3:1–6. doi: 10.1038/srep02704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshioka J. Thioredoxin reductase 2 (Txnrd2) regulates mitochondrial integrity in the progression of age-related heart failure. J. Am. Heart Assoc. 2015;4:2–4. doi: 10.1161/JAHA.115.002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babior B.M., Lambeth J.D., Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Ney P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S., Li X., Jourd’Heuil F.L., Qu S., Devejian N., Bennett E., Jourd’Heuil D., Cai C. Cytoglobin promotes cardiac progenitor cell survival against oxidative stress via the upregulation of the NFκB/iNOS signal pathway and nitric oxide production. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-11342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roche M., Rondeau P., Singh N.R., Tarnus E., Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 44.Shah D., Wanchu A., Bhatnagar A. Interaction between oxidative stress and chemokines: possible pathogenic role in systemic lupus erythematosus and rheumatoid arthritis. Immunobiology. 2011;216:1010–1017. doi: 10.1016/j.imbio.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Xin L., Wang J., Wu Y., Guo S., Tong J. Increased oxidative stress and activated heat shock proteins in human cell lines by silver nanoparticles. Hum. Exp. Toxicol. 2014;34:315–323. doi: 10.1177/0960327114538988. [DOI] [PubMed] [Google Scholar]
- 46.Kundu T.K., Velayutham M., Zweier J.L. Aldehyde oxidase functions as a superoxide generating NADH oxidase: an important redox regulated pathway of cellular oxygen radical formation. Biochemistry. 2012;51:2930–2939. doi: 10.1021/bi3000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzony E.T., Ghanei M., Panahi Y. Oxidative stress and altered expression of peroxiredoxin genes family (PRDXS) and sulfiredoxin-1 (SRXN1) in human lung tissue following exposure to sulfur mustard. Exp. Lung Res. 2016;42:217–226. doi: 10.1080/01902148.2016.1194501. [DOI] [PubMed] [Google Scholar]
- 48.Jacob C., Battaglia E., Burkholz T., Peng D., Bagrel D., Montenarh M. Control of oxidative posttranslational cysteine modifications: from intricate chemistry to widespread biological and medical applications. Chem. Res. Toxicol. 2012;25:588–604. doi: 10.1021/tx200342b. [DOI] [PubMed] [Google Scholar]
- 49.Jacob C. Redox signalling via the cellular thiolstat. Biochem. Soc. Trans. 2011;39:1247–1253. doi: 10.1042/BST0391247. [DOI] [PubMed] [Google Scholar]
- 50.Sandoval-Acuña C., Ferreira J., Speisky H. Polyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014;559:75–90. doi: 10.1016/j.abb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Manda G., Isvoranu G., Victoria M., Manea A., Debelec B., Sami K. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol. 2015;5:347–357. doi: 10.1016/j.redox.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panieri E., Santoro M.M. ROS homeostasis and metabolism: a dangerous liaison in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J., Luo B., Li X., Lu W., Yang J., Hu Y., Huang P., Wen S. Inhibition of cancer growth in vitro and in vivo by a novel ROS-modulating agent with ability to eliminate stem-like cancer cells. Cell Death Dis. 2017;8:e2887. doi: 10.1038/cddis.2017.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benhar M., Engelberg D., Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen-Heininger Y.M.W., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T., Stamler J.S., Goo S., Van Der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marengo B., Nitti M., Furfaro A.L., Colla R., De Ciucis C., Marinari U.M., Pronzato M.A., Traverso N., Domenicotti C. Redox homeostasis and cellular antioxidant systems: crucial players in cancer growth and therapy. Oxid. Med. Cell. Longev. 2016;2016:1–16. doi: 10.1155/2016/6235641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pouysségur J. The central role of amino acids in cancer redox homeostasis: vulnerability points of the cancer redox code. Front. Oncol. 2017;7:319. doi: 10.3389/fonc.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kołodziejski D., Brillowska-Dąbrowska A., Bartoszek A. The extended version of restriction analysis approach for the examination of the ability of low-molecular-weight compounds to modify DNA in a cell-free system. Food Chem. Toxicol. 2015;75:118–127. doi: 10.1016/j.fct.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C., Skamagki M., Liu Z., Ananthanarayanan A., Zhao R., Li H., Kim K. Biological significance of the suppression of oxidative phosphorylation in induced pluripotent stem cells report biological significance of the suppression of oxidative phosphorylation in induced pluripotent stem cells. Cell Rep. 2017;21:2058–2065. doi: 10.1016/j.celrep.2017.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material