ABSTRACT
Gut bacteria provide a rich source of glycosidases that can recognize and/or hydrolyze glycans for nutrition. Interestingly, some glycosidases have also been found to catalyze transglycosylation reactions in vitro and thus can be used for oligosaccharide synthesis. In this work, six putative and one known exo-α-sialidase genes—three from Bacteroides fragilis NCTC9343, three from Clostridium perfringens ATCC 13124, and one known from Bifidobacterium bifidum JCM1254—were subjected to gene cloning and heterogeneous expression in Escherichia coli. The recombinant enzymes were purified, characterized for substrate specificity, and screened for transglycosylation activity. A sialidase, named BfGH33C, from B. fragilis NCTC9343 was found to possess excellent transglycosylation activity for the synthesis of sialylated human milk oligosaccharide. The native BfGH33C was a homodimer with a molecular weight of 113.6 kDa. The Km and kcat values for 4-methylumbelliferyl N-acetyl-α-d-neuraminic acid and sialic acid dimer were determined to be 0.06 mM and 283.2 s−1, and 0.75 mM and 329.6 s−1, respectively. The enzyme was able to transfer sialyl from sialic acid dimer or oligomer to lactose with high efficiency and strict α2-6 regioselectivity. The influences of the initial substrate concentration, pH, temperature, and reaction time on transglycosylation were investigated in detail. Using 40 mM sialic acid dimer (or 40 mg/ml oligomer) and 1 M lactose (pH 6.5) at 50°C for 10 min, BfGH33C could specifically produce 6′-sialyllactose, a dominant sialylated human milk oligosaccharide, at a maximal conversion ratio above 20%. It provides a promising alternative to the current chemical and enzymatic methods for obtaining sialylated oligosaccharides.
IMPORTANCE Sialylated human milk oligosaccharides are significantly beneficial to the neonate, as they play important roles in supporting resistance to pathogens, gut maturation, immune function, and brain and cognitive development. Therefore, access to the sialylated oligosaccharides has attracted increasing attention both for the study of saccharide functions and for the development of infant formulas that could mimic the nutritional value of human milk. Nevertheless, nine-carbon sialic acids are rather complicated for the traditional chemical modifications, which require multiple protection and deprotection steps to achieve a specific glycosidic bond. Here, the exo-α-sialidase BfGH33C synthesized 6′-sialyllactose in a simple step with high transglycosylation activity and strict regioselectivity. Additionally, it could utilize oligosialic acid, which was newly prepared in an easy, economical way to reduce the substrate cost, as a glycosyl donor. All the studies laid a foundation for the practical use of BfGH33C in large-scale synthesis of sialylated oligosaccharides in the future.
KEYWORDS: exo-α-sialidase, Bacteroides fragilis NCTC9343, transglycosylation, regioselectivity, 6′-sialyllactose
INTRODUCTION
Human milk oligosaccharides (HMOs) are a group of complex unconjugated glycans that are highly abundant in human milk. They are mainly composed of a lactose core elongated with N-acetylglucosamine and galactose moieties and decorated with sialic acid (N-acetyl-α-d-neuraminic acid [Neu5Ac]) and/or fucose residues (1). Numerous reports have detailed the beneficial effects of HMOs on the growth of desirable bacteria, especially members of the genus Bifidobacterium, in the infant intestine (2–4). Moreover, feeding of breast milk to infants directly reduces microbial infections, as HMOs can act as acceptor mimics for pathogen binding and thus prevent pathogen adsorption to the host (5, 6).
The sialylated oligosaccharides, such as 6′-sialyllactose (Neu5Acα2-6Galβ1-4Glc), 3′-sialyllactose (Neu5Acα2-3Galβ1-4Glc), disialyllactose-N-tetraose, and sialyllacto-N-tetraose, are a particularly interesting subgroup of HMOs. They are present in large quantities in colostrum (1 to 3.3 g/liter), especially the dominant 6′-sialyllactose, which accounts for 0.25 to 1.3 g/liter (7). These oligosaccharides are thought to have significant health benefits for the neonate because of their roles in supporting resistance to pathogens, gut maturation, immune function, and brain and cognitive development (7, 8).
Although HMOs are abundant in human milk, only trace amounts are present in infant formula based on bovine milk (9). Recently, some prebiotic oligosaccharides, such as galacto-oligosaccharides and fructo-oligosaccharides, have been successfully supplemented in infant formula to resemble the prebiotic functions of natural oligosaccharides present in human milk. However, they seem unlikely to mimic the sialic acid-involved effects due to a lack of the negatively charged carboxyl group of sialic acid that is critical for some special HMO effects (10). Therefore, the production of natural HMOs has received increasing attention, both for theoretical studies on their functions and for practical applications in infant formulas.
Currently, obtaining homogeneous sialosides by chemical synthesis remains a tremendous task, since sialic acid is a nine-carbon acidic monosaccharide and the three additional carbons make it more complicated for structural modification than the common six-carbon monosaccharides (11, 12). Fortunately, enzymatic approaches employing sialyltransferases and sialidases as synthetic tools have offered an alternative access to the family of sialylated glycostructures. The enzymes possess excellent stereospecificity and varying regioselectivity, depending on the enzyme source. The reactions can be accomplished through a simple step and under mild, environmentally friendly conditions (13). The sialyltransferases are natural enzymes for highly efficient sialylation, but they require rather expensive CMP-sialic acid substrate as the glycosyl donor, and their substrate specificity is strict (14). For large-scale synthesis, exo-α-sialidases (EC 3.2.1.18) are attractive enzyme sources because of their flexible substrate preferences, as well as their capability to use relatively low-cost donor substrates.
Typically, glycosidases cleave glycosidic linkages in vivo. The sialidases, which are found in higher animals and various microorganisms, including viruses, bacteria, fungi, and protozoa, catalyze the cleavage of terminal sialic acid residues from a variety of glycoconjugates (15, 16). Under appropriate reaction conditions in vitro, the enzymes can catalyze the formation of glycosidic linkages by a transglycosylation reaction. The sialidase from the human pathogen Trypanosoma cruzi (TcTS) exhibits high transglycosylation activity for glycan sialylation (17, 18). However, the enzyme constitutes an important virulence factor in T. cruzi (19). Although mutants of the nonpathogenic Trypanosoma rangeli sialidase (TrSA) have been developed, they show relatively low trans-sialidase activity (20, 21). Additionally, the native TcTS and mutant TrSA form only α2-3 linkages. In contrast, the sialidases of bacteria provide access to diverse glycosyl linkages, but they have the problems of low efficiency and relaxed regioselectivity, the latter of which usually results in a mixture of α2-3- and α2-6-isomer products that are difficult to isolate.
Gut bacteria provide a rich source of glycosidases, among which the sialidases play an important role in catabolism of sialylated glycoconjugates for bacterial nutrition or function as potential virulence factors that can recognize sialic acids exposed on the host cell surface (22). Sialidase genes have been predicted to exist in the genomes of some strains of gut bacteria, such as Bacteroides fragilis, Bifidobacterium bifidum, and Clostridium perfringens (23–27). B. bifidum and B. fragilis are found in the intestinal microbiota of infants, while C. perfringens was isolated from infants born by caesarian section and is an opportunistic pathogen (28–30).
In this work, six novel α-sialidases and one known α-sialidase derived from gut bacteria were subjected to gene cloning, heterogeneous expression, and purification. The hydrolysis preference patterns for various sialylated substrates, as well as the transglycosylation activity for the synthesis of 6′-sialyllactose, were investigated. One enzyme, named BfGH33C, from B. fragilis NCTC9343 was found to display strict α2-6 regioselectivity toward lactose for transglycosylation. The enzyme synthesized 6′-sialyllactose as a single transglycosylation product with high efficiency in the presence of the commercial sialic acid dimer (Neu5Acα2-8Neu5Ac) or the newly prepared oligosialic acid as the glycosyl donor. The use of the BfGH33C enzyme in the synthesis of 6′-sialyllactose would be of practical significance, as it has advantages of low cost and high efficiency compared with the current synthetic methods.
RESULTS
Sequence analysis, gene cloning, and heterogeneous expression of exo-α-sialidases.
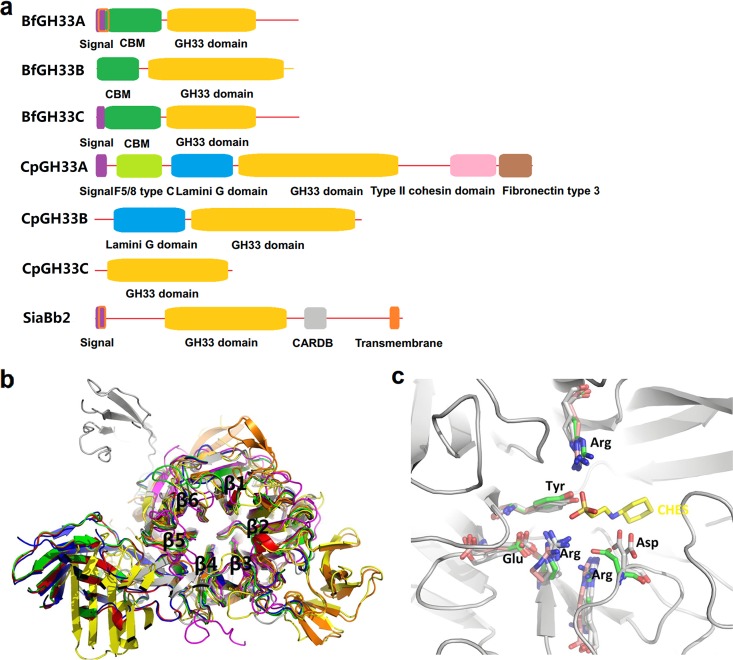
Six putative exo-α-sialidases, BfGH33A, BfGH33B, and BfGH33C from B. fragilis NCTC9343 and CpGH33A, CpGH33B, and CpGH33C from C. perfringens ATCC 13124, were subjected to bioinformatics analysis, in comparison with a known sialidase, SiaBb2 from B. bifidum JCM1254. The results showed that the enzymes differed in the presence of signal peptides and/or membrane-anchored domains, suggesting their natural occurrences were in different patterns. BfGH33C and CpGH33A, with signal peptides at the N terminus, were shown to be extracellular; BfGH33A and SiaBb2, containing signal peptides and transmembrane regions, were predicted to be membrane anchored; BfGH33B, CpGH33B, and CpGH33C, with neither a signal peptide nor a transmembrane region, are likely to be intracellular (Fig. 1a; see Table S1 in the supplemental material).
FIG 1.
Domain analysis (a), putative 3D model (b), and enlarged active center (c) of seven GH33 exo-α-sialidases. The enzymes are BfGH33A, BfGH33B, and BfGH33C from B. fragilis NCTC9343; CpGH33A, CpGH33B, and CpGH33C from C. perfringens ATCC 13124; and SiaBb2 from B. bifidum JCM1254 (GenBank accession no. CAH07505.1, CAH09389.1, CAH09725.1, ABG84247.1, ABG83208.1, ABG84018.1 and BAK26854.1, respectively). (a) The domains were predicted using online tools (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi/). CBM, carbohydrate binding module. (b) Red, BfGH33A (template PDB accession no. 4bbw; 77.8% identity); green, BfGH33B (template PDB accession no. 4bbw; 40.6% identity); blue, BfGH33C (template PDB accession no. 4bbw; 74.6% identity); yellow, CpGH33A (template PDB accession no. 2sli, 38.5% identity); orange, CpGH33B (PDB accession no. 5tsp); purple, CpGH33C (template PDB accession no. 1dim; 36.2% identity); gray, SiaBb2 (template PDB accession no. 1wcq; 47.8% identity). (c) The known structure of CpGH33B (PDB accession no. 5tsp) is shown in gray; the ligand 2-(cyclohexylamino)ethanesulfonic acid (CHES) binds to the catalytic sites of sialidases similarly to the substrate (Neu5Ac) binding site (51). Structure alignment of all the enzymes showing that the arginine triad, the acid/base aspartic acid, the nucleophilic tyrosine, and the conserved glutamic acid overlap in the active site. Homology modeling was performed using online tools (https://swissmodel.expasy.org/), and the resulting structures were visualized with PyMol 1.3.
All the enzymes shared a common GH33 characteristic domain responsible for catalysis and differed in other functional domains that might assist with substrate binding, such as carbohydrate binding modules (CBM), the F5/8 type C domain, the type II cohesin domain, the fibronectin type 3 domain, and the cell adhesion-related domain (CARDB) (Fig. 1a) (31). The putative 3-dimensional (3D) model of these enzymes exhibited six-bladed β-propeller folds in the conserved catalytic domains (Fig. 1b). Multiple-sequence alignment of the enzymes further disclosed the characteristic consensus motif of nonviral exo-α-sialidases, the RIP (Arg-Ile/Leu-Pro), and the five repeats of an Asp box motif (Ser/Thr-X-Asp-X-Gly-X-Thr-Trp/Phe [where X represents any amino acid]) (Fig. 2). The predicted catalytic amino acids (the nucleophilic Tyr and the acid/base Asp), as well as the triarginine cluster important for enzyme activity, were shown to be located in the catalytic cavity and proved to be highly conserved in the putative 3D model (Fig. 1c).
FIG 2.
Multiple alignments of seven GH33 exo-α-sialidases. The alignment was performed using the ClustalX program. The enzymes Bfra_BfGH33A, Bfra_BfGH33B, Bfra_BfGH33C, Cper_CpGH33A, Cper_CpGH33B, Cper_CpGH33C, and Bbif_SiaBb2 represent BfGH33A, BfGH33B, BfGH33C, CpGH33A, CpGH33B, CpGH33C, and SiaBb2, respectively. Gray, similar residues; black, identical residues. RIP and Asp box motifs are boxed; the acid/base aspartic acid is marked by the star, the nucleophilic tyrosine by the filled arrow, the conserved glutamic acid by the open arrowhead, and the arginine triad by the open arrows.
The genes for these enzymes were obtained by PCR, and the recombinant enzymes were successfully expressed in Escherichia coli after removal of signal peptides and transmembrane regions. The enzyme proteins were fused to C-terminal 6×His tags and purified by nickel affinity chromatography. They migrated as single protein bands in SDS-PAGE (see Fig. S1 in the supplemental material), with molecular weights in agreement with the calculated masses (Table 1).
TABLE 1.
Screening of transglycosylation abilities of various exo-α-sialidases
| Enzyme source | GenBank accession no. | Enzyme | Molecular wt (kDa) | Transglycosylation activitya |
|
|---|---|---|---|---|---|
| Methanol | Lactose | ||||
| B. fragilis NCTC9343 | CAH07505.1 | BfGH33A | 56.5b | + | + |
| CAH09389.1 | BfGH33B | 58.8 | + | − | |
| CAH09725.1 | BfGH33C | 57.9b | + | + | |
| C. perfringens ATCC 13124 | ABG84247.1 | CpGH33A | 126.8b | + | − |
| ABG83208.1 | CpGH33B | 77.4 | + | − | |
| ABG84018.1 | CpGH33C | 43.2 | + | − | |
| B. bifidum JCM1254 | BAK26854.1 | SiaBb2 | 81.1b | + | − |
The transglycosylation reaction was performed by using oligosialic acid as the glycosyl donor and methanol or lactose as the acceptor. +, positive transglycosylation ability; −, negative transglycosylation ability.
Molecular weight was calculated without putative signal peptides and transmembrane regions.
Substrate specificities of exo-α-sialidases for hydrolysis.
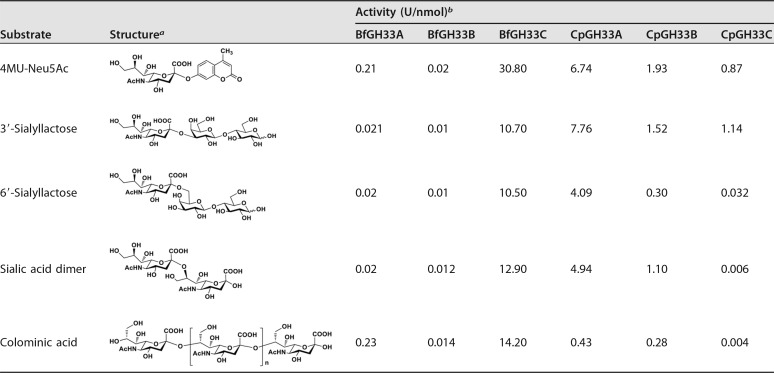
The recombinant enzymes were determined to possess sialidase activity, as they hydrolyzed sialyl linkages. Nonetheless, they showed different substrate specificities for various natural oligosaccharides and artificial glycosides (Table 2). All of them showed higher hydrolytic activity toward the artificial 4-methylumbelliferyl (4MU)-Neu5Ac than toward the natural oligosaccharide, except the CpGH33A and CpGH33C enzymes, which showed the highest cleavage rate for α2-3-linked sialyllactose.
TABLE 2.
Substrate specificities of various exo-α-sialidases
The chemical structures were drawn using ChemDraw Ultra.
One unit of enzyme activity was defined as the amount of enzyme required to liberate 1 μmol of Neu5Ac per minute under the assay conditions.
In the case of B. fragilis NCTC9343, two sialidase isoforms, BfGH33B and BfGH33C, could hydrolyze α2-3- and α2-6-linked sialyllactose, as well as α2-8-linked disaccharides and polysaccharides, but they had a preference for α2-8-linked saccharides over α2-3- and α2-6-linked substrates, whereas the other tested enzymes, like BfGH33A from B. fragilis NCTC9343 and CpGH33A, CpGH33B, and CpGH33C from C. perfringens ATCC 13124, had higher hydrolysis activity toward substrates bearing α2-3 linkages than those harboring α2-6- or α2-8-linkages, consistent with the property of the reported SiaBb2 from B. bifidum JCM1254 (25). Despite the substrate preference, these enzymes were capable of catalyzing the hydrolysis of a broad range of sialoglycoconjugates containing α2-3-, α2-6-, or α2-8-linked sialic acids. This relaxed substrate specificity was beneficial for glycoside synthesis, since the selection of a glycosyl donor was flexible.
BfGH33C showed the highest hydrolysis activity toward all the tested substrates. Strikingly, it displayed significant activity toward colominic acid [a polysialic acid of (Neu5Acα2-8Neu5Ac)n with an average molecular weight of 30,000], an inexpensive and easily available polysaccharide. For glycoside synthesis, the price of substrates affects the overall production cost. Considering that most enzymes showed relatively low activity for polysaccharide but moderate activity for α2-8-linked sialic acid dimer, a low-cost preparation of oligosialic acid by the hydrolysis of colominic acid was subsequently performed for use as the glycosyl donor in the subsequent screening of transglycosylation activity. Thin-layer chromatography (TLC) and mass spectrometry (MS) analyses showed the oligosialic acid was a mixture of di-, tri-, and tetrasaccharides, with characteristic signals of [M-H]− ions at 599.19, 890.29, and 1181.17, respectively (see Fig. S2 in the supplemental material).
Screening of exo-α-sialidases for transglycosylation.
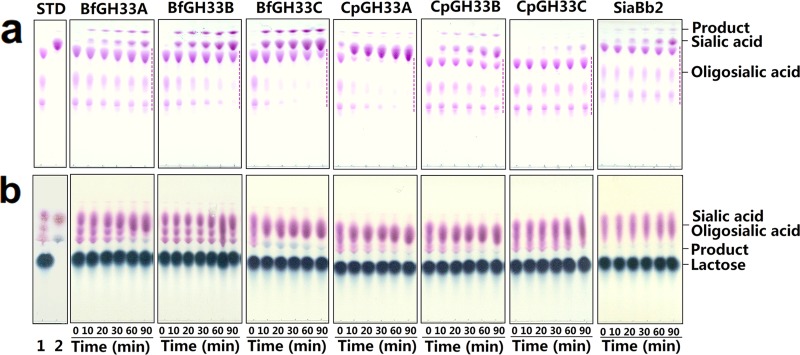
The recombinant enzymes were incubated with oligosialic acid as a glycosyl donor and methanol or lactose as an acceptor to detect transglycosylation activity. The results showed that all the enzymes exhibited transglycosylation activities toward methanol, whereas only two enzymes, BfGH33A and BfGH33C from B. fragilis NCTC9343, showed transglycosylation activities toward lactose (Fig. 3 and Table 1). The BfGH33C enzyme clearly produced the largest amount of glycosides when lactose was used as the acceptor. Interestingly, BfGH33C seemed to have strict regioselectivity toward lactose, since only one product spot appeared on the TLC plate. In addition, BfGH33C exhibited the highest transglycosylation activity regardless of the kind of glycosyl donor when sialic acid dimer and colominic acid were tested (data not shown). Hence, this enzyme was chosen for further studies.
FIG 3.
TLC analysis of transglycosylation activities using methanol (a) and lactose (b) as glycosyl acceptors. STD, standard saccharides. Lanes: 1, oligosialic acid (a) and a mixture of oligosialic acid and lactose (b); 2, standard sialic acid (a) and a mixture of sialic acid and 6′-sialyllactose (b). (a) The dashed lines indicate remaining oligosialic acid. The spots of glycoside products derived from methanol are located at the tops of the plates, followed by the spots of sialic acid and the remaining donor oligosialic acid. (b) The spots of the glycoside products derived from lactose appeared dark blue, whereas the spots of the sialic acid and donors appeared purple. The transglycosylation reactions were performed by incubating the various enzymes with 33 mg/ml oligosialic acid as the glycosyl donor and 20% (by volume) methanol or 300 mM lactose as the glycosyl acceptor at 37°C within 90 min. The solvent systems used were chloroform-methanol-0.02% aqueous CaCl2 (3:4:1) (a) and 1-propanol–1 M ammonia water–0.02% aqueous CaCl2 (6:3:2) (b) (by volume).
Characterization of recombinant BfGH33C.
The native molecular weight of the recombinant BfGH33C was about 113.6 kDa, as determined by gel filtration chromatography (see Fig. S3 in the supplemental material), and its subunit molecular weight was 57.9 kDa, as analyzed by SDS-PAGE (see Fig. S1 in the supplemental material), indicating the enzyme was a homodimer protein. The substrate specificity of the enzyme for various glycosides with different sugar residues was examined. The result showed that it strictly released the sialic acid residue from 4MU-Neu5Ac and had no activities toward other substrates with β-linkages or lacking sialic acid in the glycan moieties, including 4MUα- or 4MUβ-d-galactopyranoside, p-nitrophenyl (pNP)α- or pNPβ-d-galactopyranoside, oNPα- or oNPβ-d-galactopyranoside, mNPα-d-galactopyranoside, pNPα- or pNPβ-d-glucopyranoside, pNPα- or pNPβ-d-mannopyranoside, pNPα-l-mannopyranoside, pNPβ-d-mannopyranoside, pNP-2-acetylamino-2-deoxy-α- or pNP-2-acetylamino-2-deoxy-β-d-galactopyranoside, and pNP-2-acetylamino-2-deoxy-β-d-glucopyranoside.
BfGH33C was highly active at pH 6.5, and it was stable at pHs between 4.5 and 7.5 (see Fig. S4a in the supplemental material). The optimal temperature for enzyme activity was 45°C, and it was stable below 40°C (see Fig. S4b in the supplemental material). Hg2+, Cu2+, and Ag+ completely inactivated the enzyme activity, whereas NH4+, K+, Na+, Co2+, Mn2+, Ca2+, and EDTA slightly affected the enzyme activity (by less than 18.34%). Mg2+ and Ni2+ exhibited slight promotion of enzyme activity, while Fe2+, Fe3+, and Zn2+ caused partial inhibition of enzyme activity, by 76.35, 72.57, and 66.34%, respectively (see Fig. S5 in the supplemental material).
The Km and kcat values of BfGH33C for 4MU-Neu5Ac and sialic acid dimer were determined to be 0.06 mM and 283.2 s−1, and 0.75 mM and 329.6 s−1, respectively. Thus, the kcat/Km values of BfGH33C for 4MU-Neu5Ac and sialic acid dimer were determined to be 4,857.3 mM−1 s−1 and 438.0 mM−1 s−1, respectively (see Table S2 in the supplemental material).
Synthesis of 6′-sialyllactose by recombinant BfGH33C.
The preliminary transglycosylation reaction of BfGH33C was performed using sialic acid dimer as the glycosyl donor for convenient qualitative analysis and quantitation. As shown in Fig. S6a in the supplemental material, a novel spot of oligosaccharide product (designated PLac) appeared below lactose, which had the same migration distance as the standard 6′-sialyllactose on the TLC plate. The result of high-performance anion-exchange chromatography (HPAEC) analysis also showed that the peak of PLac shared identical retention time with the standard 6′-sialyllactose (see Fig. S6b in the supplemental material). These results suggested the PLac formed by BfGH33C might be 6′-sialyllactose. In addition to PLac, sialic acid also appeared as a by-product resulting from the hydrolysis of sialic acid dimer by the enzyme.
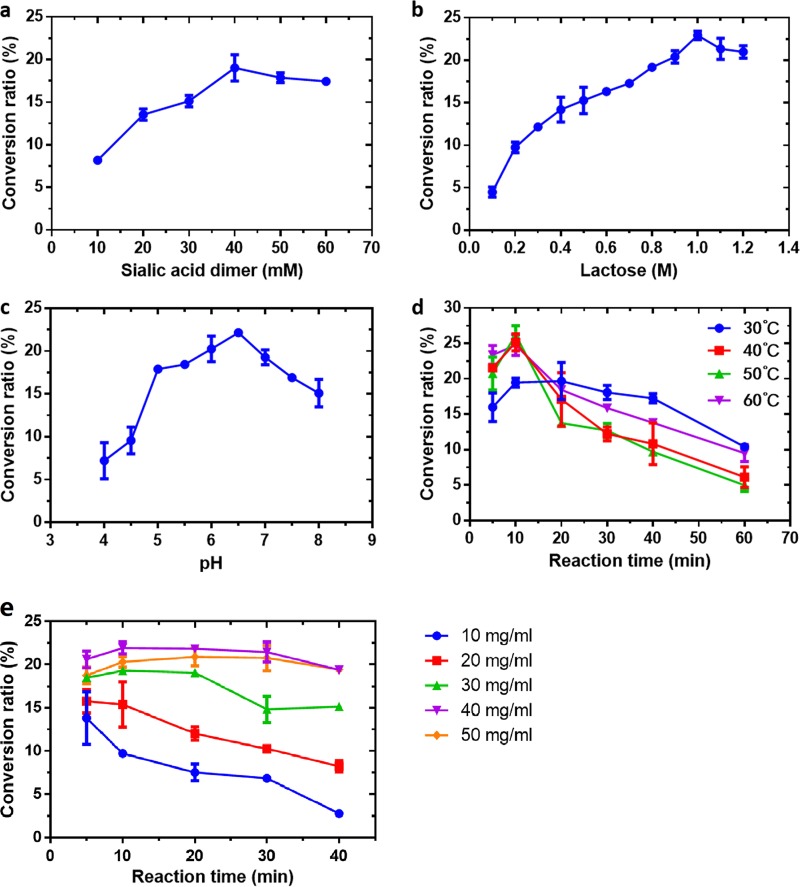
The influences of various factors on the formation of the product PLac by BfGH33C were further evaluated, including substrate concentration, pH, temperature, and reaction time. Figure 4a showed the effects of the donor concentration on oligosaccharide synthesis. The conversion ratio of PLac was increased when the sialic acid dimer concentrations were raised from 10 to 40 mM, and then it decreased when the sialic acid dimer was changed to 60 mM (Fig. 4a). The maximum product conversion ratio of 19.0% occurred at 40 mM donor. Therefore, the subsequent reactions were performed at 40 mM sialic acid dimer. Figure 4b shows the influence of the acceptor concentration on oligosaccharide synthesis. The amount of product increased when lactose concentrations were increased from 0.1 to 1 M. The product conversion ratio reached a maximum of 22.9% at 1 M acceptor. Continuous increase of the acceptor concentration from 1 to 1.2 M led to a slight decrease in the product conversion ratio. Therefore, the subsequent reactions were performed using 40 mM sialic acid dimer and 1 M lactose.
FIG 4.
Effects of substrate concentrations (a, b, and e), pH (c), and temperature (d) on the conversion ratio of PLac. (a) The concentrations of the donor sialic acid dimer were tested from 10 to 60 mM at pH 6.5 and 37°C in the presence of 0.8 M lactose for 20 min. (b) The acceptor concentration was tested from 0.1 to 1.2 M by incubation with 40 mM sialic acid dimer at pH 6.5 and 37°C for 20 min. (c) Reactions were carried out at 37°C by incubating the enzyme with 40 mM sialic acid dimer and 1 M lactose and for 20 min in buffers from pH 4.0 to 8.0. (d) Temperature and reaction time were tested at pH 6.5 by incubation of the enzyme with 1 M lactose and 40 mM sialic acid dimer at 30 to 60°C and detected within 60 min. (e) The concentrations of the donor oligosialic acid were tested from 10 to 50 mg/ml in the presence of 1 M lactose at pH 6.5 and 50°C, following a time course of 40 min. The data represent means ± standard deviations (SD) of three replicates.
As shown in Fig. 4c, the effects of pH on transglycosylation showed a trend similar to that for hydrolysis of 4MU-Neu5Ac by BfGH33C. The conversion ratio reached maximum (22.2%) at pH 6.5 and dropped outside the pH range. The influences of temperature and reaction time were studied by measuring time curves at different temperatures. As shown in Fig. 4d, the reaction temperature affected product formation remarkably. Product synthesis at all tested temperatures rapidly reached peak values for about 10 min. The products formed at 50°C clearly showed the highest peak value, but when the reaction times were prolonged, the conversion ratio sharply decreased as a result of secondary hydrolysis. Thus, the optimal conditions for product synthesis by BfGH33C were a final concentration of sialic acid dimer of 40 mM, a final lactose concentration of 1 M, and 10-min incubation at pH 6.5 and 50°C, at which the highest conversion ratio of PLac reached 26.2%.
The transglycosylation reactions using oligosialic acid as the glycosyl donor were subsequently investigated under the optimal conditions, except for the donor concentration. The effects of the concentration of oligosialic acid on synthesis were tested from 10 to 50 mg/ml. The conversion ratio reached a maximum of 21.9% at 10 min using oligosialic acid at 40 mg/ml, which was comparable to the use of sialic acid dimer. Notably, the product formation was relatively stable within the initial 30 min, and its reduction beyond 30 min was slow and slight (Fig. 4e).
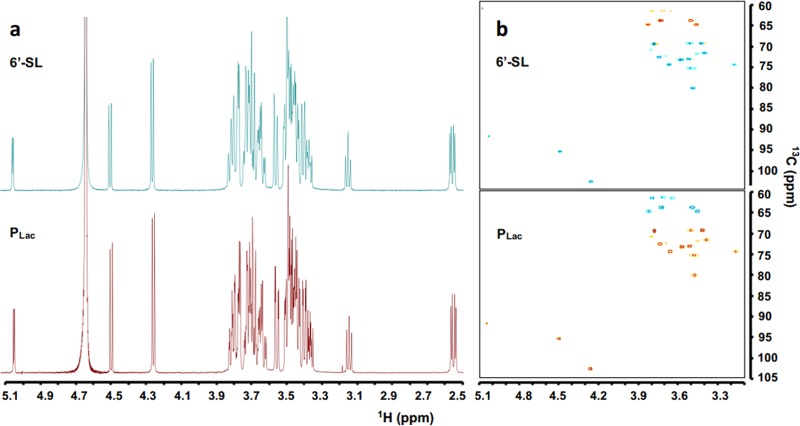
Then, the synthesis reaction by BfGH33C was performed at a scale of 10 ml. The resulting transglycosylation product was purified and analyzed by MS and nuclear magnetic resonance (NMR) spectroscopy. The negative-ion electrospray ionization (ESI) mass spectrum of the product PLac showed a peak of [M-H]− ion at m/z 632.29 (see Fig. S7 in the supplemental material), consistent with the molecular weight of sialyllactose (633.21). The 1H NMR and 1H and 13C heteronuclear single quantum coherence (HSQC) spectra of PLac were fully in agreement with those of the standard 6′-sialyllactose (Fig. 5). Accordingly, the transglycosylation product was completely confirmed to be 6′-sialyllactose. The enzymatic synthesis process and the product structure are shown in Fig. 6.
FIG 5.
1H NMR and 1H and 13C HSQC of standard 6′-sialyllactose and PLac synthesized by BfGH33C. The NMR data were collected on an Agilent DD2 600-MHz instrument at room temperature in D2O at 600 MHz for 1H and 150 MHz for 13C. Chemical shifts are given in parts per minute downfield from the internal TMS of D2O.
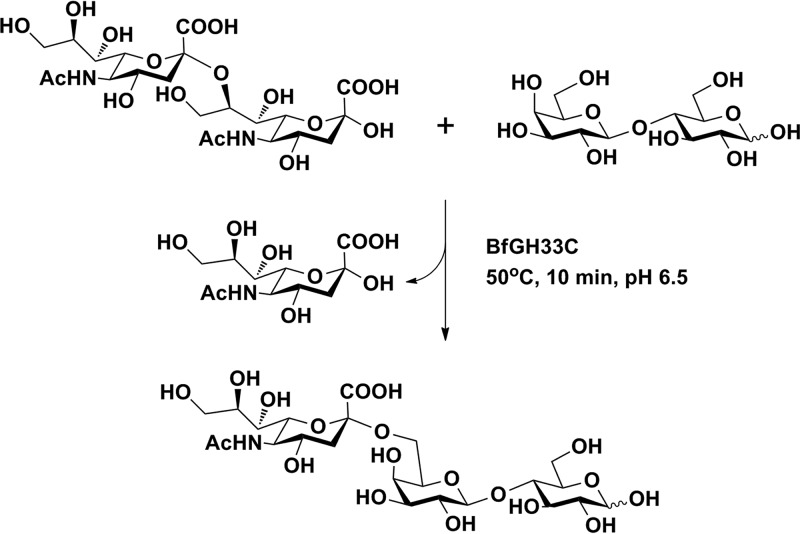
FIG 6.
Synthesis of 6′-sialyllactose by recombinant BfGH33C. The enzyme synthesized 6′-sialyllactose in one step, using sialic acid dimer as a glycosyl donor and lactose as a glycosyl acceptor.
DISCUSSION
Development of an efficient route to the large-scale synthesis of sialylated oligosaccharides is necessary for the biological functional study of HMOs, as well as the prebiotic fortification of infant formula in the near future. Currently, sialidases have shown great potential in the enzymatic synthesis of functional sialylated oligosaccharides and glycomimetics. In this work, a sialidase, namely, BfGH33C from B. fragilis NCTC9343, was found to possess excellent transglycosylation activity for the synthesis of sialylated HMOs.
The complete gene for the BfGH33C enzyme comprised 1,635 nucleotides, which encoded 544 amino acids with a calculated molecular weight of about 60 kDa. The deduced protein sequence of BfGH33C revealed high homology with four known exo-α-sialidases from B. fragilis YCH46 (GenBank accession no. BAA05853.1), Bacteroides thetaiotaomicron VPI-5482 (GenBank accession no. AAO75562.1), Tannerella forsythia 92A2 ATCC 43037 (GenBank accession no. AEW22573.1), and Capnocytophaga canimorsus Cc5 (GenBank accession no. AEK22601.1), with identities of 78%, 75%, 64%, and 39%, respectively. It showed low similarity to other confirmed exo-α-sialidases, with identities of less than 32%. Based on the phylogenetic analysis of GH33 exo-α-sialidases (see Fig. S8 in the supplemental material), BfGH33C and the enzyme from B. thetaiotaomicron VPI-5482 were distributed in the same evolutionary branch, suggesting that they might have evolved from a common ancestor. These enzymes were located far from the exo-α-sialidases from pathogens such as C. perfringens, Salmonella enterica serovar Typhimurium, Pasteurella multocida, T. cruzi, and Streptococcus pneumoniae (see Fig. S8 in the supplemental material).
It has been reported that GH33 exo-α-sialidases proceed through a retaining mechanism in which two catalytic amino acids act in tandem (32, 33). In BfGH33C, Tyr510 and Asp229 were predicted to be the putative nucleophilic and acid/base residues, respectively (Fig. 1c). The multiple alignment of BfGH33C also displayed five Asp box motifs in the putative catalytic domain (Fig. 2). The bacterial sialidases are commonly found to have three to five copies of Asp box motifs that occur on the outside of the β-propeller fold and are remote from the active sites (34). The functional significance of the motifs is still unclear (22). In addition, a RIP (Arg-Ile/Leu-Pro) motif was observed upstream of the first Asp box, with the Arg residue involved in the formation of the arginine triad at the active site. BfGH33C possessed three conserved arginine residues, Arg204, Arg223, and Arg415 (Fig. 1c), the possible triarginine cluster that could interact with the carboxylate group of Neu5Ac, with the help of a conserved glutamic acid, Glu526, that might stabilize the triarginine cluster (35).
Although bacterial sialidases share many structural features, their biochemical properties, especially the substrate specificities for hydrolysis, vary widely. BfGH33C released sialic acid from α2,3-, α2,6-, and α2,8-linked substrates and showed 1.2-fold higher activity for α2,8 linkage than for α2,3 or α2,6 linkages, indicating that the specificity is biased toward the α2,8 linkage. The preference for α2,8-linked substrates was also found in several other sialidases, such as the enzymes from Pseudomonas fluorescens JK-0412 and bacteriophage K1F (36, 37). In contrast, the sialidases CpGH33A, CpGH33B, and CpGH33C from C. perfringens ATCC 13124 and SiaBb2 from B. bifidum JCM1254 showed preference for the α2,3 linkage, which was consistent with the hydrolysis specificity of the sialidases from Pasteurella multocida and Salmonella Typhimurium (38, 39). In the case of the sialidases from Corynebacterium diphtheriae and Micromonospora viridifaciens, a preference for α2,6-linked sialic acid over α2,3-linked sialic acid was observed (22). As a whole, the sialidases from the gut bacteria shown in Table 2 displayed broad substrate specificity and could cleave α2,3-, α2,6-, and α2,8-linked terminal sialic acids. This property might contribute to the survival of these bacteria in the gut. In the gastrointestinal (GI) tract, sialic acid is commonly found in terminal residues of mucins, which can serve as metabolic substrates for providing nutrition to gut bacteria (40, 41). The release of sialic acid from nonreducing ends by sialidases is an initial step in the sequential degradation of mucins, since the location of the terminal sialic acid would prevent the actions of other glycoside hydrolases (35). The exo-α-sialidase SiaBb2 from B. bifidum JCM1254 has been found to remove sialic acid from mucins and HMOs and may promote bifidobacterial adhesion to the mucosal surface (42). We therefore speculated that the sialidases from B. fragilis NCTC9343 and C. perfringens ATCC 13124 might contribute to bacterial survival and colonization in the gut via the removal of sialic acids from mucin O-glycans.
Interestingly, the BfGH33C enzyme could efficiently transfer a sialyl residue to lactose by transglycosylation, using sialic acid dimer or oligomer as the glycosyl donor. A variety of sialic acid-containing donor substrates have been employed for sialoside synthesis. The production yields of the sialosides typically varied, depending on the type of sialic acid donor, in the following order: pNP-Neu5Ac > sialic acid dimer > colominic acid (22, 43). It seemed the smaller the donor that was used, the higher the yield that was achieved. However, neither pNP-Neu5Ac nor sialic acid dimer was an ideal donor for sialylation, since they were expensive and not economical for large-scale synthesis of glycosides. In this work, the preparation of oligosialic acid from hydrolysis of the low-cost colominic acid was developed, and the resulting oligosialic acid was used as the donor substrate. The process was simple, easy, and practical for industrial applications. More importantly, the BfGH33C enzyme exhibited transglycosylation activity with the newly prepared oligosialic acid that was similar to that of the commercial sialic acid dimer.
During glycosidase-catalyzed synthesis, the hydrolysis of products usually competes with the transglycosylation reaction. The concentrations of glycosyl acceptors have been reported to reduce water activity, which tends to favor the transglycosylation reaction over hydrolysis (44, 45). In the BfGH33C-catalyzed reaction, the yield of transglycosylation product was improved, with the lactose increased from 100 to 1,000 mM and then decreased, in the presence of sialic acid dimer (40 mM) as the donor. The optimal ratio of the acceptor to the donor proved to be 25:1. Such high acceptor/donor ratios have been found in other sialidase-catalyzed reactions, such as 16.7:1 for the synthesis of 6′-sialyllactose by the enzyme from Arthrobacter ureafaciens and 12.7:1 for the synthesis of sialylated LewisX by the enzyme from S. Typhimurium LT2 (43, 46). It seemed that higher acceptor/donor ratios might increase the possibility of nucleophilic attack by the acceptor on the enzyme to form a glycosyl-enzyme intermediate, thus facilitating subsequent trans-sialylation reactions (44).
It is worth noting that BfGH33C synthesized 6′-sialyllactose with high transglycosylation activity and strict regioselectivity, since the current sialidases capable of forming 6′-sialyllactose were either nonregioselective or inefficient. Tanaka et al. (47) reported that the partially purified sialidase from B. fragilis SBT3182 produced a mixture of 3′-sialyllactose and 6′-sialyllactose from colominic acid and lactose. The relaxed regioselectivity for the synthesis resulted in isomer products that shared similar chemical or physical properties and would be rather hard to individually isolate. In addition, the total products had a rather low yield of 0.14% at 24 h. Maru et al. (48) found that the sialidase from A. ureafaciens was able to synthesize 6′-sialyllactose by reverse hydrolysis. The enzyme still had low regioselectivity and produced an additional branched trisaccharide with the sialyl residue linked to the glucose moiety. Ajisaka et al. (43) investigated several commercial sialidases for synthesis of 6′-sialyllactose using various donors. All the tested enzymes formed glycosides in low conversion ratios of less than 10% at 5 h. In this study, BfGH33C transferred sialyl from sialic acid dimer or oligomer to lactose and synthesized 6′-sialyllactose at a conversion ratio above 20% at 10 min. The method seemed more advantageous, as it was regioselective, utilized low-cost donor substrate, and was accomplished within a short time.
In conclusion, a novel exo-α-sialidase from B. fragilis NCTC9343 was found to be a powerful synthetic tool capable of transferring sialyl from sialic acid dimer or oligomer to lactose and producing an important human milk oligosaccharide, 6′-sialyllactose. The enzyme could achieve the specific synthesis in one step at low cost and high efficiency, suggesting it is a good candidate for use in the production of sialylate oligosaccharide on a large scale. The oligosialic acid was newly prepared in an economical way for use as the glycosyl donor for the transglycosylations catalyzed by sialidases.
MATERIALS AND METHODS
Materials.
4-Methylumbelliferyl N-acetyl-α-d-neuraminic acid (4MU-Neu5Ac), 6′-sialyllactose (Neu5Acα2-6Galβ1-4Glc), 3′-sialyllactose (Neu5Acα2-3Galβ1-4Glc), sialic acid dimer (Neu5Acα2-8Neu5Ac), colominic acid [a polysialic acid of (Neu5Acα2-8Neu5Ac)n with an average molecular weight of 30,000], and other 4-methylumbelliferyl glycosides were purchased from Carbosynth (Compton, United Kingdom). p-Nitrophenyl (pNP) or o-nitrophenyl (oNP) glycosides were obtained from Sigma (St. Louis, MO, USA). Ni2+ Sepharose high performance was purchased from GE Healthcare (Uppsala, Sweden). Bio-Gel P2 was purchased from Bio-Rad Laboratories (Hercules, CA, USA). Restriction endonucleases were purchased from New England BioLabs (USA). T4 DNA ligase was purchased from TaKaRa Corporation (Tokyo, Japan). FastPfu Fly DNA polymerase was obtained from Transgen (China). The other chemicals used were of analytical grade.
Strains and media.
B. bifidum JCM1254 was grown anaerobically at 37°C in medium containing 10 g of glucose, 5 g of peptone, 5 g of yeast extract, 4 g of K2HPO4, 5 g of sodium acetate (NaOAc), 0.2 g of MgSO4, 6.8 g of ascorbic acid, and 0.4 g of cysteine hydrochloride in 1,000 ml of water (pH 7.0). B. fragilis NCTC9343 was grown anaerobically at 37°C in medium containing 5 g of yeast extract, 20 g of peptone, 5 g of NaCl, 60 g of glucose, 5 mg of hemin, and 0.5 mg of vitamin K1 in 1,000 ml of water (pH 7.2). C. perfringens ATCC 13124 was grown anaerobically at 40°C in medium containing 5 g of yeast extract, 5 g of peptone, 5 g of sodium acetate, 0.2 g of MgSO4, 4 g of K2HPO4, 0.4 g of cysteine hydrochloride, and 6.8 g of ascorbic acid in 1,000 ml of water (pH 7.0). Anaerobic culture was performed in a Forma anaerobic system (Thermo) under a mixture of nitrogen-hydrogen-carbon dioxide at a ratio of 84.9:10:5.1 (vol/vol/vol).
E. coli strains DH5α and BL21(DE3) were grown at 37°C in LB medium containing 5 g of yeast extract, 10 g of peptone, and 7 g of NaCl in 1,000 ml of water (pH 7.0). The medium for the E. coli cells containing pET-21b or pET-28b plasmid was supplemented with 50 μg/ml ampicillin or 30 μg/ml kanamycin. The pET-21b and pET-28b plasmids (Novagen) were used to construct an expression vector with a His tag.
Gene cloning and heterogeneous expression.
Six putative and one known exo-α-sialidase genes deposited in the Carbohydrate-Active enZYmes database (http://www.cazy.org/) were obtained by PCR using the primers listed in Table 3. The primers for the genes for the BfGH33A, BfGH33B, and BfGH33C enzymes were designed based on the B. fragilis NCTC9343 genome sequence (GenBank accession no. CR626927.1); the primers for the genes for the CpGH33A, CpGH33B, and CpGH33C enzymes were designed based on the C. perfringens ATCC 13124 genome sequence (GenBank accession no. CP000246.1); the primer for the gene for SiaBb2 was designed based on the B. bifidum JCM1254 genome sequence (GenBank accession no. AB278567.1). Among these, the genes encoding the truncated enzymes BfGH33A, BfGH33C, CpGH33A, and SiaBb2 were obtained by removal of putative signal peptides and transmembrane regions (see Table S1 in the supplemental material).
TABLE 3.
Primers and vectors used in this study
| Enzyme and source | Vector | Primer | DNA sequence (5′–3′)a | Restriction enzyme |
|---|---|---|---|---|
| B. fragilis NCTC9343 | ||||
| BfGH33A | pET-21b | BfGH33AF | CAGCCATATGCGTGAAACACGTATTCCTAT | NdeI |
| BfGH33AR | CGCGCTCGAGTTTAATAATGTCTTTCAACTTC | XhoI | ||
| BfGH33B | pET-21b | BfGH33BF | CGAGCATATGAAAGTTATTGTTAGACAGCCCA | NdeI |
| BfGH33BR | CGAGCTCGAGTCTCTCTTTATATGATTTACCTTT | XhoI | ||
| BfGH33C | pET-21b | BfGH33CF | CGAGGGATCCGGATACTATTTTTGTTTATGAA | BamHI |
| BfGH33CR | CGATCTCGAGTTGTATTATATCTCTCAGTCGGA | XhoI | ||
| C. perfringens ATCC 13124 | ||||
| CpGH33A | pET-21b | CpGH33AF | CGCGCATATGAAAAGTAAAAAAATTATAGC | NdeI |
| CpGH33AR | CCGCTCGAGCCTAGCAGTTCTTATAGTCTT | XhoI | ||
| CpGH33B | pET-21b | CpGH33BF | CGCGCTAGCATGAATTACAAAGGGATAAC | NheI |
| CpGH33BR | CGCGCTCGAGTTTATTAGCTCCACTCTCT | XhoI | ||
| CpGH33C | pET-28b | CpGH33CF | CGCGCTAGCATGTATAACAAAAACAACACCTT | NheI |
| CpGH33CR | CGCGCTCGAGTTGTTTATTAATTAGTGAGT | XhoI | ||
| B. bifidum JCM1254 | ||||
| SiaBb2 | pET-21b | SiaBb2F | CGACATATGGCCAGCGATGATGCTGACATG | NdeI |
| SiaBb2R | CGACTCGAGCTTGGACAGGCCGGGCTGCTC | XhoI | ||
Restriction enzyme sites are underlined.
The genomic DNAs of B. fragilis NCTC9343, C. perfringens ATCC 13124, and B. bifidum JCM1254 were extracted at room temperature with a Tianamp Bacteria DNA kit (Tiangen Biotech, Beijing, China) and used as templates for PCR. The PCR conditions were as follows: a hot start at 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 55°C to 65°C for 30 s, and 72°C for 1 kb/min, and a final step at 72°C for 10 min. The PCR products were purified, ligated into pET-21b or pET-28b vectors, and transformed into E. coli BL21(DE3). The proper transformants were grown at 37°C in LB medium containing 50 μg/ml ampicillin or 30 μg/ml kanamycin. The recombinant enzymes were induced by addition of isopropyl-1-thio-β-d-galactoside when the cell density reached 0.6 to 0.8 at 600 nm. After continuous cultivation at 16°C for 12 to 14 h, the cells were harvested and disrupted by ultrasonic treatment. The resulting lysate was centrifuged at 12,000 rpm for 25 min, and the supernatant was subjected to enzyme purification by nickel affinity chromatography.
Enzyme assays.
Standard exo-α-sialidase assays were carried out using 4MU-Neu5Ac as a substrate (23). The enzyme (10 μl) was mixed with 10 μl of 1 mM 4MU-Neu5Ac and 80 μl of 50 mM sodium acetate buffer (pH 5.0) on ice and then incubated at 37°C for 10 min. Afterward, the reaction mixture was replaced on ice, and the reaction was stopped by addition of 1.9 ml of a solution containing 85 mM glycine and 200 mM sodium carbonate (pH 10.4). The fluorescence of the liberated 4MU was quantified with excitation at 365 nm and emission at 445 nm. Assays for other 4-methylumbelliferyl or nitrophenyl glycosides were performed under the same conditions. Protein amounts were determined with a NanoDrop 2000 calibrated with the extinction coefficient predicted by ExPASy (http://web.expasy.org/protparam/).
Substrate specificities of exo-α-sialidases for hydrolysis.
The recombinant enzymes were incubated with 10 mM various substrates in 40 μl of 50 mM sodium acetate buffer (pH 5.0) at 37°C for 4 h. The tested substrates included 4MU-Neu5Ac, 6′-sialyllactose, 3′-sialyllactose, sialic acid dimer, and colominic acid. The release of sialic acid from substrate hydrolysis was detected by TLC using a Silica Gel 60 plate (Merck, Germany). The TLC plates loaded with samples were developed with 1-butanol–acetic acid–0.02% aqueous CaCl2 (2:1:1 [vol/vol/vol]). Sugars on the TLC plate were visualized by spraying with diphenylamine-aniline-phosphoric acid reagent, followed by incubation at 86°C for 10 min and analysis with ImageJ 1.34s (https://imagej.nih.gov/ij/).
Preparation of oligosialic acid.
Colominic acid was dissolved in 0.02 M HCl at 8% (wt/vol) and subjected to hydrolysis at 70°C. After 2 h, the hydrolysate was neutralized to pH 7.0 by addition of 1 M NaOH. The resulting sugars in the sample were separated by gel filtration chromatography using a Bio-Gel P2 column. The oligosaccharide fractions were detected by TLC and MS.
Screening of exo-α-sialidases for transglycosylation.
The transglycosylation activities of seven purified recombinant enzymes were examined by incubation of the enzymes with 33 mg/ml oligosialic acid as a donor and 20% (by volume) methanol or 300 mM lactose as an acceptor in 30 μl of 50 mM sodium phosphate buffer (pH 6.5) at 37°C for 1.5 h. The enzyme amounts used were as follows: BfGH33A, 265 pmol; BfGH33B, 2 nmol; BfGH33C, 8 pmol; CpGH33A, 32 pmol; CpGH33B, 33 pmol; CpGH33C, 2 nmol; SiaBb2, 3 nmol. The developing solvent for the analysis of reaction mixtures containing methanol was chloroform-methanol-0.02% aqueous CaCl2 (3:4:1 [vol/vol/vol]), while for the reactions involving lactose, it was 1-propanol–1 M ammonia water–0.02% aqueous CaCl2 (6:3:2 [vol/vol/vol]). Sugars on the TLC plate were visualized as described above. The recombinant exo-α-sialidase from B. fragilis NCTC9343, named BfGH33C, was the desired enzyme that displayed excellent properties for transglycosylation.
Molecular weight determination.
The molecular weight of BfGH33C was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as well as gel filtration chromatography. SDS-PAGE was performed in a 10% (wt/vol) gel, and the proteins in the gel were visualized by Coomassie brilliant blue R-250 staining. Gel filtration chromatography was performed with a Superdex 200 Increase 10/300 GL column (10 mm by 300 mm; GE Healthcare, United Kingdom). The standard proteins were thyroglobulin (669 kDa), ferritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), and ovalbumin (44 kDa).
Biochemical studies.
The optimal pH was determined by assaying the enzyme activity with 4MU-Neu5Ac at pH values ranging from 3.0 to 12.0 in 30 mM buffers containing citric acid, KH2PO4, boric acid, and barbitone and using NaOH to adjust the pH. The pH stability was determined by incubating the enzyme in the presence of the above-mentioned pH buffers at 4°C for 12 h and assaying the residual activity under standard reaction conditions. The optimal temperature for enzyme reaction was determined by assaying the enzyme activity with 4MU-Neu5Ac for 10 min at 20 to 70°C. The temperature stability was determined by assaying residual enzymatic activity under standard reaction conditions after incubating the enzyme for 30 min at 20 to 70°C. To determine the effects of chemicals, enzyme activities were assayed in the presence of 2 mM metal salts or additives.
Kinetic analyses of BfGH33C for 4MU-Neu5Ac and sialic acid dimer were performed by incubation of the enzyme (1 nmol) with 100 μl of 50 mM sodium acetate buffer (pH 5.0) containing each substrate at various concentrations. The reactions proceeded for 5 min at 37°C and were stopped by heating at 100°C for 20 min. The 4MU release was quantified by measuring the fluorescence intensity with excitation and emission at 365 nm and 445 nm, respectively. The release of sialic acid from sialic acid dimer was analyzed by using HPAEC with pulsed amperometric detector (PAD) as described below. The Km and kcat values of the enzyme were determined with GraphPad Prism 6 software.
Synthesis of 6′-sialyllactose by recombinant BfGH33C.
For preliminary determination of transglycosylation, the synthesis reaction was performed at 37°C for 20 min in a mixture (30 μl) containing 17 nmol of enzyme, 800 mM lactose, and 48 mM sialic acid dimer in 50 mM sodium phosphate buffer (pH 6.5). The reaction was stopped by heating at 100°C for 20 min. Sugars were analyzed by TLC and HPAEC-PAD as described below.
To achieve maximal glycoside production, the reaction conditions, including substrate concentration, pH, and temperature, were investigated in detail. The effects of the donor concentration were determined by incubating 17 nmol of enzyme with 800 mM lactose, pH 6.5, at 37°C for 20 min in the presence of sialic acid dimer from 10 to 60 mM. The effects of variations in the acceptor concentration were determined by incubating the enzyme with 40 mM sialic acid dimer, pH 6.5, at 37°C for 20 min in the presence of lactose ranging from 100 to 1,200 mM. The effects of pH were examined by incubating the enzyme with 1,000 mM lactose and 40 mM sialic acid dimer at 37°C for 20 min in 50 mM buffers at different pH values (acetate buffer at 4.0, 4.5, 5.0, and 5.5 and phosphate buffer at 6.0, 6.5, 7.0, 7.5, and 8.0). The effects of temperature and reaction time were investigated by incubating the enzyme with 1,000 mM lactose and 40 mM sialic acid dimer, pH 6.5, at different temperatures (30, 40, 50, and 60°C), followed by interval sampling within 60 min. The effects of concentrations of the donor oligosialic acid were tested from 10 to 50 mg/ml in the presence of 1 M lactose at pH 6.5 and 50°C, following a time course of 40 min.
All the reactions were stopped by heating at 100°C for 20 min, and the resulting products were analyzed by HPAEC. The conversion ratio of the transglycosylation product was calculated by the formula [product/(product + sialic acid)] × 100, calculated from high-performance liquid chromatography (HPLC) peak areas (43, 49). The yield represented the ratio of the amount of transglycosylation product to the amount of converted donor substrate.
Isolation of 6′-sialyllactose synthesized by the recombinant BfGH33C.
The transglycosylation product was sequentially separated by gel filtration chromatography and anion-exchange chromatography. The reaction mixture was first subjected to a Bio-Gel P2 column (1.6 by 90 cm) to remove the majority of the remaining substrates. Using distilled water as the eluent, the fractions were eluted at a flow rate of 0.2 ml/min and collected at 1 ml per tube. The resulting samples were subjected to sugar determination by TLC. The desired oligosaccharide mixture was further separated through a source Q column (1.0 by 9.5 cm; GE Healthcare) installed in an Äkta Performance fast protein liquid chromatography (FPLC) system (GE Healthcare). The fractions were eluted by a gradient of 0 to 0.5 M NaCl at a flow rate of 1 ml/min. The sialylated oligosaccharides were traced based on UV detection at 210 nm. The fractions with identical sugar compositions were combined and lyophilized to dry powder.
HPAEC-PAD analysis.
The quantitative determination of sialic acid release, as well as 6′-sialyllactose formation, was carried out by HPAEC on a Dionex ICS-2500 system (Thermo Scientific) equipped with a CarboPac PA100 (4-mm by 50-mm) guard column and a CarboPac PA100 (4-mm by 250-mm) analytical column. The eluent system comprised deionized water (A), 0.5 M NaOH (B), and 0.5 M NaOAc (C), and the mobile phase was degassed in an ultrasonic bath before use. The elution started with isocratic elution at 89:10:1% (A/B/C) from 0 to 3 min, followed by a linear gradient from 89:10:1% (A/B/C) to 60:10:30% (A/B/C) from 3 to 19 min. Afterward, the column was subjected to isocratic elution at 5:10:85% (A/B/C) from 19 to 23 min. Finally, the column was reequilibrated for 5 min at 89:10:1% (A/B/C) (50). All the elution was kept at a flow rate of 1 ml/min with the temperature maintained at 30°C. Samples were diluted 50 times before being filtered through a 0.22-μm polypropylene filter. The data were analyzed with Chromeleon 6.80 SR8 build 2623 (Thermo Scientific).
MS and NMR analyses.
Mass spectra were recorded on a Shimadzu (Kyoto, Japan) LCMS-IT-TOF instrument equipped with an ESI source in negative-ion mode at a resolution of 10,000 full width at half maximum. The NMR data were collected on an Agilent DD2 600-MHz instrument at room temperature in D2O at 600 MHz for 1H and 150 MHz for 13C. Chemical shifts were given in parts per million downfield from internal tetramethyl silane (TMS) of D2O. The oligosaccharide produced by the enzyme and the commercial standard 6′-sialyllactose were both analyzed by recording the 1H NMR, 13C NMR, and heteronuclear single quantum coherence (HSQC). The data were processed using VnmrJ 4.0 and MestReNova software.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by the Major State Basic Research Development Program of China (973 Program) (no. 2012CB822102), National Natural Science Foundation of China (no. 31670062), Science and Technology Development Project of Shandong Province (no. 2016GGH4502 and 2015GSF121004), Fundamental Research Funds for the Central Universities (2018KFYYXJJ020), and Fundamental Research Funds of Shandong University (no. 2016JC028).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00071-18.
REFERENCES
- 1.Bode L. 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22:1147–1162. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt KM, Preuss J, Nissan C, Davlin CA, Williams JE, Shafii B, Richardson AD, McGuire MK, Bode L, McGuire MA. 2012. Human milk oligosaccharides promote the growth of staphylococci. Appl Environ Microbiol 78:4763–4770. doi: 10.1128/AEM.00477-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcobal A, Barboza M, Froehlich JW. 2010. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem 58:5334–5340. doi: 10.1021/jf9044205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Moyano S, Totten SM, Garrido DA, Smilowitz JT, German JB, Lebrilla CB, Mills DA. 2013. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl Environ Microbiol 79:6040–6049. doi: 10.1128/AEM.01843-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newburg DS, Ruizpalacios GM, Morrow AL. 2005. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 6.Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, Pichler J, Dehlink E, Loibichler C, Urbanek R, Szépfalusi Z. 2004. Human milk-derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res 56:536–540. doi: 10.1203/01.PDR.0000139411.35619.B4. [DOI] [PubMed] [Google Scholar]
- 7.ten Bruggencate SJ, Boveeoudenhoven IM, Feitsma AL, van Hoffen E, Schoterman MH. 2014. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr Rev 72:377–389. doi: 10.1111/nure.12106. [DOI] [PubMed] [Google Scholar]
- 8.Charbonneau MR, O'Donnell D, Blanton LV, Totten SM, Davis JC, Barratt MJ, Cheng J, Guruge J, Talcott M, Bain JR, Muehlbauer MJ, Ilkayeva O, Wu C, Struckmeyer T, Barile D, Mangani C, Jorgensen J, Fan YM, Maleta K, Dewey KG, Ashorn P, Newgard CB, Lebrilla C, Mills DA, Gordon JI. 2016. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 164:859–871. doi: 10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urashima T, Taufik E, Fukuda K, Asakuma S. 2013. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci Biotechnol Biochem 77:455–466. doi: 10.1271/bbb.120810. [DOI] [PubMed] [Google Scholar]
- 10.Bode L, Jantscherkrenn E. 2012. Structure-function relationships of human milk oligosaccharides. Adv Nutr 3:383S–391S. doi: 10.3945/an.111.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett CS. 2017. Selective glycosylation: synthetic methods and catalysts. Wiley VCH Verlag GmbH, Weinheim, Germany. [Google Scholar]
- 12.Chen X, Varki A. 2010. Advances in the biology and chemistry of sialic acids. ACS Chem Biol 5:163–176. doi: 10.1021/cb900266r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu C, Withers SG. 2015. Recent developments in enzymatic synthesis of modified sialic acid derivatives. Adv Synth Catal 46:1633–1654. doi: 10.1002/adsc.201500349. [DOI] [Google Scholar]
- 14.Endo T, Koizumi S, Tabata K, Ozaki A. 2000. Large-scale production of CMP-NeuAc and sialylated oligosaccharides through bacterial coupling. Appl Microbiol Biotechnol 53:257–261. doi: 10.1007/s002530050017. [DOI] [PubMed] [Google Scholar]
- 15.Taylor G. 1996. Sialidases: structures, biological significance and therapeutic potential. Curr Opin Struct Biol 6:830–837. doi: 10.1016/S0959-440X(96)80014-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Oh DB, Kwon O, Kang HA. 2010. Identification and functional characterization of the NanH extracellular sialidase from Corynebacterium diphtheriae. J Biochem 147:523–533. doi: 10.1093/jb/mvp198. [DOI] [PubMed] [Google Scholar]
- 17.Holck J, Larsen DM, Michalak M, Li H, Kjærulff L, Kirpekar F, Gotfredsen CH, Forssten S, Ouwehand AC, Mikkelsen JD, Meyer AS. 2014. Enzyme catalysed production of sialylated human milk oligosaccharides and galactooligosaccharides by Trypanosoma cruzi trans-sialidase. N Biotechnol 31:156–165. doi: 10.1016/j.nbt.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Wilbrink MH, ten Kate GA, van Leeuwen SS, Sanders P, Sallomons E, Hage JA, Dijkhuizen L, Kamerling JP. 2014. Galactosyl-lactose sialylation using Trypanosoma cruzi trans-sialidase as the biocatalyst and bovine κ-casein-derived glycomacropeptide as the donor substrate. Appl Environ Microbiol 80:5984–5991. doi: 10.1128/AEM.01465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schauer R, Kamerling JP. 2011. The chemistry and biology of trypanosomal trans-sialidases: virulence factors in Chagas disease and sleeping sickness. Chembiochem 12:2246–2264. doi: 10.1002/cbic.201100421. [DOI] [PubMed] [Google Scholar]
- 20.Paris G, Ratier L, Amaya MF, Nguyen T, Alzari PM, Frasch AC. 2005. A sialidase mutant displaying trans-sialidase activity. J Mol Biol 345:923–934. doi: 10.1016/j.jmb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Nyffenegger C, Nordvang RT, Jers C, Meyer AS, Mikkelsen JD. 2017. Design of Trypanosoma rangeli sialidase mutants with improved trans-sialidase activity. PLoS One 12:e0171585. doi: 10.1371/journal.pone.0171585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Oh DB, Kang HA, Kwon O. 2011. Features and applications of bacterial sialidases. Appl Microbiol Biotechnol 91:1–15. doi: 10.1007/s00253-011-3307-2. [DOI] [PubMed] [Google Scholar]
- 23.Russo TA, Thompson JS, Godoy VG, Malamy MH. 1990. Cloning and expression of the Bacteroides fragilis TAL2480 neuraminidase gene, nanH, in Escherichia coli. J Bacteriol 172:2594–2600. doi: 10.1128/jb.172.5.2594-2600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka H, Ito F, Iwasaki T. 1994. Two sialidases which preferentially hydrolyze sialyl α2-8 linkage from Bacteroides fragilis SBT3182. J Biochem 115:318–321. doi: 10.1093/oxfordjournals.jbchem.a124335. [DOI] [PubMed] [Google Scholar]
- 25.Kiyohara M, Tanigawa K, Chaiwangsri T, Katayama T, Ashida H, Yamamoto K. 2011. An exo-α-sialidase from bifidobacteria involved in the degradation of sialyloligosaccharides in human milk and intestinal glycoconjugates. Glycobiology 21:437–447. doi: 10.1093/glycob/cwq175. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci U S A 99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newstead SL, Potter JA, Wilson JC, Xu G, Chien CH, Watts AG, Withers SG, Taylor GL. 2008. The structure of Clostridium perfringens NanI sialidase and its catalytic intermediates. J Biol Chem 283:9080–9088. doi: 10.1074/jbc.M710247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, Gueimonde M, Margolles A, De Bellis G, O'Toole PW, van Sinderen D, Marchesi JR, Ventura M. 2012. Diversity of bifidobacteria within the infant gut microbiota. PLoS One 7:e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 30.Nagpal R, Tsuji H, Takahashi T, Nomoto K, Kawashima K, Nagata S, Yamashiro Y. 2017. Gut dysbiosis following C-section instigates higher colonisation of toxigenic Clostridium perfringens in infants. Benef Microbes 8:353–365. doi: 10.3920/BM2016.0216. [DOI] [PubMed] [Google Scholar]
- 31.Ficko-Blean E, Boraston AB. 2012. Insights into the recognition of the human glycome by microbial carbohydrate-binding modules. Curr Opin Struct Biol 22:570–577. doi: 10.1016/j.sbi.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Amaya MF, Watts AG, Damager I, Nguyen T, Buschiazzo A, Paris G, Frasch AC, Withers SG, Alzari PM. 2004. Structural insights into the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Structure 12:775–784. doi: 10.1016/j.str.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 33.Owen CD, Lukacik P, Potter JA, Sleator O, Taylor GL, Walsh MA. 2015. Streptococcus pneumoniae NanC: structural insights into the specificity and mechanism of a sialidase that produces a sialidase inhibitor. J Biol Chem 290:27736–27748. doi: 10.1074/jbc.M115.673632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roggentin P, Rothe B, Kaper JB, Lawrisuk L, Vimr ER, Schauer R. 1989. Conserved sequences in bacterial and viral sialidases. Glycoconj J 6:349–353. doi: 10.1007/BF01047853. [DOI] [PubMed] [Google Scholar]
- 35.Juge N, Tailford L, Owen CD. 2016. Sialidases from gut bacteria: a mini-review. Biochem Soc Trans 44:166–175. doi: 10.1042/BST20150226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JK, Choi DJ, Kim SM, Choi HN, Park JW, Jang SJ, Choo YK, Lee CG, Park Y. 2012. Purification and characterization of a polysialic acid-specific sialidase from Pseudomonas fluorescens JK-0412. Biotechnol Bioprocess Eng 17:526–537. doi: 10.1007/s12257-011-0495-7. [DOI] [Google Scholar]
- 37.Finne J, Mäkelä PH. 1985. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem 260:1265–1270. [PubMed] [Google Scholar]
- 38.Mizan S, Henk A, Stallings A, Maier M, Lee MD. 2000. Cloning and characterization of sialidases with 2-6 and 2-3 sialyl lactose specificity from Pasteurella multocida. J Bacteriol 182:6874–6883. doi: 10.1128/JB.182.24.6874-6883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoyer LL, Roggentin P, Schauer R, Vimr ER. 1991. Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus-typical kinetic preference for sialyl α2-3 linkages. J Biochem 110:462–467. doi: 10.1093/oxfordjournals.jbchem.a123603. [DOI] [PubMed] [Google Scholar]
- 40.Robinson LS, Lewis WG, Lewis AL. 2017. The sialate O-acetylesterase EstA from gut Bacteroidetes species enables sialidase-mediated cross-species foraging of 9-O-acetylated sialoglycans. J Biol Chem 292:11861–11872. doi: 10.1074/jbc.M116.769232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis AL, Lewis WG. 2012. Host sialoglycans and bacterial sialidases: a mucosal perspective. Cell Microbiol 14:1174–1182. doi: 10.1111/j.1462-5822.2012.01807.x. [DOI] [PubMed] [Google Scholar]
- 42.Nishiyama K, Yamamoto Y, Sugiyama M, Takaki T, Urashima T, Fukiya S, Yokota A, Okada N, Mukai T. 2017. Bifidobacterium bifidum extracellular sialidase enhances adhesion to the mucosal surface and supports carbohydrate assimilation. mBio 8:e00928-17. doi: 10.1128/mBio.00928-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ajisaka K, Fujimoto H, Isomura M. 1994. Regioselective transglycosylation in the synthesis of oligosaccharides: comparison of β-galactosidases and sialidases of various origins. Carbohydr Res 259:103–115. doi: 10.1016/0008-6215(94)84201-9. [DOI] [PubMed] [Google Scholar]
- 44.Zeuner B, Jers C, Mikkelsen JD, Meyer AS. 2014. Methods for improving enzymatic trans-glycosylation for synthesis of human milk oligosaccharide biomimetics. J Agric Food Chem 62:9615–9613. doi: 10.1021/jf502619p. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Xu L, Jin L, Sun B, Gu G, Lu L, Xiao M. 2016. Efficient and regioselective synthesis of β-GalNAc/GlcNAc-lactose by a bifunctional transglycosylating β-N-acetylhexosaminidase from Bifidobacterium bifidum. Appl Environ Microbiol 82:5642–5652. doi: 10.1128/AEM.01325-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makimura Y, Ishida H, Kondo A, Hasegawa A, Kiso M. 1998. Regioselective α2-3-sialylation of LeX and LeA by sialidase-catalyzed transglycosylation. J Carbohydr Chem 17:975–979. doi: 10.1080/07328309808007467. [DOI] [Google Scholar]
- 47.Tanaka H, Ito F, Iwasaki T. 1995. A system for sialic acid transfer by colominic acid and a sialidase that preferentially hydrolyzes sialyl α2-8 linkages. Biosci Biotechnol Biochem 59:638–643. doi: 10.1271/bbb.59.638. [DOI] [PubMed] [Google Scholar]
- 48.Maru I, Ohta Y, Okamoto K, Suzuki S, Kakehi K, Tsukada Y. 1992. Synthesis of sialyllactose from N-acetylneuraminic acid and lactose by a neuraminidase from Arthrobacter ureafaciens. Biosci Biotechnol Biochem 56:1557–1561. doi: 10.1271/bbb.56.1557. [DOI] [Google Scholar]
- 49.Kim S, Oh DB, Kwon O, Kang HA. 2010. Construction of an in vitro trans-sialylation system: surface display of Corynebacterium diphtheria sialidase on Saccharomyces cerevisiae. Appl Microbiol Biotechnol 88:893–903. doi: 10.1007/s00253-010-2812-z. [DOI] [PubMed] [Google Scholar]
- 50.Zeuner B, Luo J, Nyffenegger C, Aumala V, Mikkelsen JD, Meyer AS. 2014. Optimizing the biocatalytic productivity of an engineered sialidase from Trypanosoma rangeli for 3′-sialyllactose production. Enzyme Microb Technol 55:85–93. doi: 10.1016/j.enzmictec.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y, Youn HS, Lee JG, An JY, Park KR, Kang JY, Ryu YB, Jin MS, Park KH, Eom SH. 2017. Crystal structure of the catalytic domain of Clostridium perfringens neuraminidase in complex with a non-carbohydrate-based inhibitor, 2-(cyclohexylamino)ethanesulfonic acid. Biochem Biophys Res Commun 486:470–475. doi: 10.1016/j.bbrc.2017.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.