Abstract
Conscious emotional processing is characterized by a coordinated set of responses across multiple physiological systems. Although emotional stimuli can evoke certain physiological responses even when they are suppressed from awareness, it is not known whether unconscious emotional responses comprise a similar constellation or are confined to specific systems. To compare physiological responses to emotional stimuli with and without awareness, we measured a range of responses while participants viewed positive, negative and neutral images that were accompanied by noise bursts to elicit startle reflexes. We measured four responses simultaneously – skin conductance and heart rate changes in response to the images themselves; and startle eye-blink and post-auricular reflexes in response to the noise bursts that occurred during image presentation. For half of the participants, the images were masked from awareness using continuous flash suppression. The aware group showed the expected pattern of response across physiological systems: emotional images (regardless of valence) evoked larger skin conductance responses (SCRs) and greater heart rate deceleration than neutral images, negative images enhanced eye-blink reflexes and positive images enhanced post-auricular reflexes. In contrast, we found a striking dissociation between measures for the unaware group: typical modulation of SCRs and post-auricular reflexes, but no modulation of heart rate deceleration or eye-blink reflexes. Our findings suggest that although some physiological systems respond to emotional stimuli presented outside of awareness, conscious emotional processing may be characterized by a broad and coordinated set of responses across systems.
Keywords: awareness, unconscious processing, emotion, psychophysiology
Emotions are multifaceted, consisting of physiological changes, characteristic behaviours and conscious subjective feelings. Although our intuition tells us that these factors are all part of a coherent emotional response, the individual components can dissociate from each other; notably, emotional stimuli can produce physiological and behavioural responses even when we are unaware of the eliciting stimulus or any emotional feelings (Winkielman and Berridge 2004; Tamietto and de Gelder 2010). Most commonly, the physiological component of unconscious emotional processing has been indexed with the skin conductance response (SCR), a measure of sympathetic activation typically enhanced by highly arousing stimuli. But the SCR reflects activity in just one of many physiological systems that are emotionally responsive. Emotional images that are viewed with awareness evoke a characteristic set of physiological responses (Cacciopo et al. 2000; Kreibig 2010), suggesting coordinated activity across multiple systems. Do emotional images presented outside awareness evoke the same coordinated set of physiological responses? Or do they trigger only some responses, with others depending on awareness? To answer this question, we simultaneously assessed four different physiological measures while people viewed emotional images that were either perceived with awareness or made invisible through the use of continuous flash suppression (CFS; Tsuchiya and Koch 2005). We measured two autonomic responses – SCR and heart rate deceleration – and two somatic responses – startle eye-blink and post-auricular reflexes.
In a typical psychophysiological picture-viewing paradigm, physiological responses are recorded while people view emotionally positive, negative and neutral images (Bradley et al. 2001a; Bradley and Lang 2007). SCRs, which are purely sympathetic in origin, are enhanced in the presence of high arousal images of either valence (Lang et al. 1993; Codispoti et al. 2001; Bradley et al. 2001a,b). Heart rate is also sensitive to emotional arousal. The heart typically decelerates following onset of a visual stimulus, as a consequence of attentional orienting (Graham and Clifton 1966). Emotional images produce greater deceleration than neutral ones, presumably because they better engage attention (Codispoti et al. 2001; Bradley et al. 2001a,b; Codispoti et al. 2008; Fiacconi et al. 2015). Although the heart is under both sympathetic and parasympathetic control, deceleration appears to be driven primarily by parasympathetic (vagal) mechanisms (Porges 2007; Thayer and Lane 2009).
These autonomic measures, which are sensitive to emotional arousal, are complemented here by two somatic reflexes that are sensitive to emotional valence. A loud noise burst presented during picture viewing induces a set of involuntary responses, including the startle eye-blink reflex, a contraction of the orbicularis muscles around the eye and the post-auricular reflex, a vestigial reflex that contracts the post-auricular muscle behind the ear (in other mammals, this reflex pulls the ear up and back). If the noise burst is presented while the participant views a negative image, the eye-blink reflex is enhanced (Bradley et al. 1993; Bradley et al. 2001a, 2006). This enhancement is commonly interpreted as motivational priming (Lang et al. 1990): the defensive eye-blink reflex is primed by the threatening emotional context. Although the startle eye-blink reflex is sometimes attenuated while viewing positive images (Bradley et al. 2001a,b; but see Jackson et al. 2000), a response to positive images can be more reliably indexed by the post-auricular reflex, which is enhanced when a noise burst occurs in the presence of positive images such as food or erotic scenes (Benning et al. 2004; Sandt et al. 2009; Sparks and Lang 2010; Benning 2011; Hebert et al. 2015). This modulation is thought to reflect priming of the appetitive response, although the functional role of the post-auricular reflex in motivation is currently unknown (Johnson et al. 2012; Hackley 2015).
Although the two reflexes are elicited by the same noise burst, they are not correlated (Sandt et al. 2009; Sparks and Lang 2010) and they arise through independent neural mechanisms (Lang and Davis 2006; Hackley 2015). The startle eye-blink reflex is part of a broad defence network, and its modulation by threat is mediated by two interconnected pathways: one that includes the central nucleus of amygdala and mediates potentiation of startle in response to imminent and predictable threat (i.e. fear-potentiated startle) and another that includes the bed nucleus of the stria terminalis (BNST; the ‘extended’ amygdala) and mediates potentiation of startle in response to distant, contextual and unpredictable threat (i.e. anxiety-potentiated startle; Lang and Davis 2006; Grillon 2008; Davis et al. 2010; Avery et al. 2016). The post-auricular reflex, in contrast, is a disynaptic brainstem reflex. The mechanisms of emotional modulation of the post-auricular reflex are unknown, but there appears to be no limbic input into the reflex arc (Hackley 2015; Hebert et al. 2015).
Backward masking studies have shown that SCR can be modulated even without awareness of an emotional stimulus (Öhman and Soares 1994; Gläscher and Adolphs 2003; Flykt et al. 2007), although conflicting findings have been reported (Codispoti et al. 2009). Very few masking studies have assessed unconscious emotional processing across more than one physiological response, and findings are mixed (Ruiz-Padial et al. 2011; Sebastiani et al. 2011; Reagh and Knight 2013). Backward masking may not be the best methodology to address this question though, as studies have been criticized on methodological grounds for not maintaining strict control of awareness (Pessoa 2005; Grillon and Cornwell 2007; Hedger et al. 2015). But even with rigorous controls, backward masking is problematic in the study of emotion, because it requires brief stimulus presentations, which may not evoke full-blown physiological responses to emotional stimuli. In psychophysiological studies, the typical duration for picture viewing is 6 s (Bradley et al. 2001a; Bradley and Lang 2007), in part because that duration allows all physiological responses to fully emerge. In backward masking paradigms, it is impossible to equate stimulus presentation durations in masked and unmasked conditions while also presenting stimuli for long enough to assess the typical effects of emotion on slowly modulated physiological responses.
An alternative to backward masking is CFS (Tsuchiya and Koch 2005), in which an image is presented to one eye while a colourful dynamic pattern is presented to the other. Although the image is clearly visible when viewed on its own, it is suppressed from awareness by the dynamic mask. Because such interocular suppression can be effective for several seconds, CFS allows presentation of pictures for equal durations with and without masking, making it ideally suited for assessing physiological responses to emotion in the absence of awareness.
Most studies of emotional processing under CFS have used behavioural measures to show that emotional stimuli (usually faces) presented outside awareness still influence behaviour (e.g. Almeida et al. 2013; Lufityanto et al. 2016; but see Faivre et al. 2012). Fewer studies have examined physiological correlates of emotional processing under CFS. SCRs have been observed to fearful or fear-conditioned faces (Raio et al. 2012; Lapate et al. 2014), but Hedger and colleagues (2015) report no modulation of the SCR by threatening images (attacking animals). No other physiological measures of emotional processing have been studied with this paradigm, so the overall ensemble of physiological responses to emotional stimuli under CFS remains unknown.
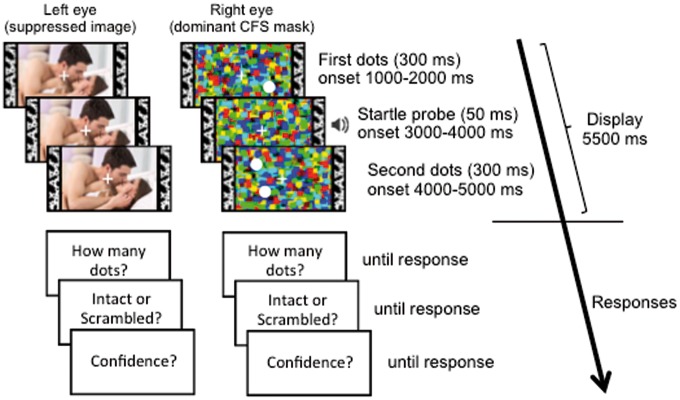
Here, we simultaneously recorded SCR, heart rate, the startle eye-blink reflex, and the post-auricular reflex while participants viewed high arousal positive (erotic) and negative (mutilation) images. They also viewed the pixel-scrambled versions of images, which served as neutral stimuli (Fig. 1). All participants viewed the images monocularly; half had a dynamic CFS mask additionally presented to their other eye. A loud burst of white noise was presented 3–4 s after image onset to trigger the reflexes. Participants performed a dot-counting task during image presentation, to ensure that their attention was engaged; the dots were superimposed on the images in the ‘aware’ condition and on the CFS mask in the ‘unaware’ condition. After each trial, participants reported the number of dots, then made a forced-choice decision to indicate whether an intact or scrambled image had been shown and finally estimated their confidence in that decision. The use of a low-level intact/scrambled judgement (instead of a higher level valence or emotion judgement) ensured that trials with even partial awareness were identified (see Yang et al. 2014). This conservative procedure allowed us to assess the efficacy of CFS suppression both objectively and subjectively on a trial-by-trial basis and verify that analysis in the ‘unaware’ condition was restricted to physiological responses that were elicited without awareness of the image.
Figure 1.
Schematic timeline of a typical trial in the ‘unaware’ condition. The image was presented to one eye (randomized from trial to trial) and the dynamic CFS mask was presented to the other. In the ‘aware’ condition, the image was still presented monocularly, but no CFS mask was presented to the other eye. The CFS mask changed dynamically at a rate of 10 Hz. A loud noise burst (50 ms; 106 dB) was presented at a random time between 3 s and 4 s after trial onset. Brief dot displays were presented before and after the noise burst. Each trial concluded with three questions that were answered using the computer keyboard: First, how many dots were shown in total? (1, 2 or 3). Second, was the picture intact or scrambled? (1–intact and 2–scrambled). Third, what is your confidence in your intact/scrambled judgement? (1–guessing 2–not sure and 3–certain). Dots have been enlarged for display clarity. Image for illustrative purposes; the experiment used images drawn from IAPS database (Lang et al. 2008). Source: Available from https://www.123rf.com/photo_29940472_stock-photo.html, ©123rf.com.
Method
Participants
Sixty-three women participated. We included only women because physiological responses to high arousal images (particularly those depicting mutilations or erotic scenes) show substantial sex differences (Bradley et al. 2001b). Participants were randomly assigned to either the ‘aware’ or ‘unaware’ conditions. To ensure a sufficient number of trials in the unaware group for obtaining stable physiological measures (see Lieberman et al. 2017), we excluded 14 participants who had fewer than 6 trials remaining in each image condition (positive, neutral and negative) after eliminating trials showing breakthrough of the image (based on high or moderate confidence combined with accurate picture/scramble judgements). One more participant was excluded, because her picture judgements were significantly better than chance (76.3%, binomial P < 0.001) despite low confidence. The remaining 48 participants (24 in each group; age range 18–27 years) had normal or corrected to normal vision, no hearing impairments, no history of neuropsychological disorder or a current/previous diagnosis of depression. The study was approved by the Victoria University of Wellington Human Ethics committee (RM20177).
Stimuli and apparatus
Participants sat in a dimly lit room. Input to the eyes was separated with a mirror stereoscope mounted 63 cm from a 23” Alienware AW2310 computer monitor, with a 120-Hz refresh rate. Images were suppressed from awareness using CFS (Tsuchiya and Koch 2005; Carmel et al. 2010). A high-contrast, dynamic CFS mask (a colourful rapidly alternating (10 Hz) ‘Mondrian-like’ images) was presented to one eye, while emotional images or their pixel-scrambled versions were presented to the other. The reflexes were elicited with a 106-dB, 50-ms white noise burst with near instantaneous rise time, delivered through Etymotic ER-4 in ear earphones.
Twenty-eight (14 positive and 14 negative) images were selected from the International Affective Picture System (IAPS) based on arousal and valence norms for women (Lang et al. 2008). Positive images (erotic couples) were more pleasant (M = 6.41, SD = 0.65) than negative images (mutilations; M = 1.71, SD = 0.34) [t(19.79) = 23.96, P < 0.001]. and did not differ in arousal (Mpositive = 6.48, SD = 0.15; Mnegative = 6.45, SD = 0.34) [t(26) < 1]. Twenty-eight neutral scrambles were created by dividing each positive and negative IAPS image into 1296 sections that were randomly reassembled. Scrambled images were used both to probe awareness (by requiring participants to make an intact/scrambled judgement after every trial) and as the neutral stimuli against which responses to emotional images were compared. Meaningless images provide an effective neutral baseline in startle probe studies (Bradley et al. 2006) and the use of the same stimuli for both functions allowed us to keep trial numbers low – a critical factor in preventing habituation of physiological responses (Bradley et al. 1993). All images were equated for luminance and reduced to 20% contrast (to increase CFS effectiveness) using the SHINE Matlab toolbox (Willenbockel et al. 2010).
Procedure
Trial procedure is illustrated in Fig. 1. In both the ‘aware’ and ‘unaware’ conditions, a trial began with presentation of a fixation cross to each eye; each cross was flanked by two textured vertical bars (0.77° wide, 5.63° tall, positioned 4° from each side of the fixation cross) to aid stable binocular vergence. These bars remained present throughout image presentation and the inter-trial interval. Participants pressed a key upon convergence of the eyes, initiating onset of the stimulus display. An image (either intact or scrambled) was then presented to one eye, which varied randomly from trial to trial. Images subtended 7.51° by 5.63° with a 0.24° gap between image edge and each of the flanking bars. Contrast was ramped up from 0% to 20% over the initial 200 ms of the 5300 ms presentation. White noise startle probes were presented at a random point between 3000 and 4000 ms after image onset. For the ‘unaware’ condition only, the image was replaced by a grey square after 4300 ms (i.e. after the startle probe window), and the dynamic mask (which had the same dimensions as the image) was simultaneously presented to the other eye for 5500 ms, ending 200 ms after offset of the grey square. Note that although images in the ‘unaware’ condition were presented for a slightly briefer duration (to promote effective suppression), all physiological measures were taken at equivalent time points, prior to image offset, in both the ‘aware’ and ‘unaware’ conditions. The inter-trial interval was set so that the time from image onset to the convergence screen of the next trial varied from 21.5 s to 25.5 s.
During each trial, participants completed an additional task to ensure that they were attending to the display. This task also allowed us to determine, in the ‘unaware’ condition, whether participants were aware of the image or the mask. Two 300 ms displays of small white dots (15px diameter) were superimposed at random locations over the mask (in the ‘unaware’ condition) or the image (in the ‘aware’ condition). Each of the two displays contained zero, one or two dots. Participants were required to count the total number of dots they saw across the displays (always 1, 2 or 3). Dots were not presented within the window 1 s before or 0.5 s after the startle probe.
At the end of each trial, participants first indicated the number of dots (using ‘1’, ‘2’ and ‘3’ keys on the keyboard’s number pad), then whether the image presented was intact or scrambled (‘1’ or ‘2’ on the number pad, respectively) and finally how confident they were in their intact/scrambled judgement (‘1’ = high – ‘I definitely saw it’, ‘2’ = moderate – ‘I think I saw something’, ‘3’ = low – ‘I’m just guessing’; these were reverse coded during analysis so that higher values indicated greater confidence).
Prior to the main experiment, participants in both conditions were shown examples of neutral intact and scrambled images, and we ensured that participants in the ‘unaware’ condition understood the distinction between a scramble and the mask. Participants in the ‘unaware’ condition were also told that either an image or a scramble would be presented on every trial but that they might not see it; and they should guess which it was if they were not sure. They were also told (per ethical requirements) that images might be either erotic or gory scenes. All participants then completed 6 practice trials (with/without CFS masks, for the unaware/aware groups, respectively) comprising 3 neutral IAPS images and their scrambled versions (not used in the rest of the experiment), accompanied by startle probes. These trials familiarized participants with the procedure while they underwent the rapid habituation to images and probes that is typical at the beginning of a session (Blumenthal et al. 2005).
Participants then completed 66 experimental trials. Of these, 10 trials consisted of a startle probe presented without a mask or image, to reduce the predictability of startle presentation. In the 56 remaining trials images were presented; of these, 28 were intact and 28 were scrambled. The intact and scrambled categories each comprised 14 positive and 14 negative images, with 12 of each valence accompanied by a startle probe. The 66 trials (56 image trials and 10 no-image startle trials) were presented in 6 blocks of 11 trials. Image order was pseudo-randomized; each block comprised four intact (two negative and two positive) and four scrambled images with startle probes, interspersed with three atypical trials; one or two trials from the ‘no probe’ and one or two trials from the ‘no-image’ categories. Images of the same type did not appear more than twice consecutively. Each image was presented once. Participants took 1 min rest periods between blocks.
Physiological recording and data reduction
All physiological measures were collected via ADInstrument bioamps and converted from analogue to digital signals by ADInstrument’s Powerlab 16/30 and then recorded in LabChart 8.0.1. Trial and startle probe onset event markers were produced by Cedrus’ Stim-Tracker and logged in LabChart. For all analyses, responses to scrambled positive and negative images were averaged to form the neutral valence condition. All physiological measures were collected and analysed following established guidelines (Boucsein et al. 2012, for SCR; Jennings et al. 1981, for heart rate; and Blumenthal et al. 2005, for startle eye-blink and post-auricular reflex).
Skin conductance was recorded from the medial phalange of the index and ring fingers using ADInstruments bipolar dry stainless steel GSR electrodes (MLT116F) and a ML116 AC GSR Amp with a sampling rate of 1 kHz. Responses were measured in micro-Siemens (µS), operationalized as the peak activity during a window between 0.9 s and 4 s after image onset, relative to average baseline activity measured across the 1 s prior to image onset. Although the SCR recording window overlapped with the startle probe (which was presented between 3 s and 4 s after picture onset), SCR is a very slow response (Boucsein et al. 2012), and so peak activity in our window reflects response to the image and not to the probe. Responses of less than 0.02 µS were scored as zero. The resulting magnitudes were then log transformed using and SCR was calculated for each image trial (both with and without startle probes) and then averaged (including zeros) within image type for each participant. Two participants in the ‘aware’ condition were excluded as non-responders from the SCR analysis only (Boucsein et al. 2012), as they had fewer than 6 non-zero responses (<10% of trials) across the 56 image trials.
Heart rate was measured via electrocardiography (ECG) using disposable adhesive silver/silver chloride (Ag/AgCl) ECG electrodes placed on the right shoulder and lower left ribcage, referenced to the left shoulder. The ECG signal was amplified by ADInstruments ML138 Octal Bio Amp, sampled at 1 kHz and band-pass filtered offline between 1 Hz and 400 Hz. R-wave spikes were defined as peaks greater than 2 SD above mean ECG activity; heart rate was determined by the inter-beat interval between consecutive R-wave spikes, converted to beats per minute. Manual inspection for artefacts (Jennings et al. 1981) occurring between onset of the baseline period and 6 s after trial onset resulted in exclusion of 1.16% of trials (SD = 2.33). Heart rate was calculated in 0.5-s epochs beginning 1 s prior to image onset. Heart rate deceleration was defined as the maximal decrease compared to the 1-s pretrial baseline across the first 4 s following picture onset. Peak deceleration was calculated for each trial (both with and without startle probes) and then averaged within the image type for each participant. One participant (in the ‘aware’ condition) was excluded from heart rate analysis due to technical issues during recording.
Startle eye-blink and post-auricular reflexes were recorded from electromyographic (EMG) activity over the orbicularis oculi muscle below the left eye and the post-auricular muscle behind the left ear, respectively. Each was measured using two 4-mm Ag/AgCl shzielded electrodes referenced to an electrode in the centre of the forehead, sampled at 4 kHz and amplified by ADInstrument’s ML135 Dual Bio Amp. Electrode impedances were below 10 kΩ. Reflexes were measured during the 48 image trials on which noise bursts were presented.
Eye-blink EMG was band-pass filtered offline (24–250 Hz), rectified and smoothed using a 20-ms moving average. Eye-blink reflexes were defined as peak EMG magnitude during the 21–200 ms following startle probe onset, relative to baseline EMG activity 70 ms prior (i.e. from 50 ms prior to probe onset to 20 ms after probe onset). Trials in which EMG failed to reach more than 1.5 SDs above baseline mean activity were scored as zero responses and included in the calculation of reflex magnitude. Trials were removed if baseline activity was noisy, if blinks occurred during the baseline period or if the response was an outlier (more than 3 SD above a participant’s mean magnitude). These criteria resulted in exclusion of 1.54% of trials (SD = 1.83). Because EMG shows large variability across individuals, startle responses were standarized and converted to T-scores, which have a mean of 50 and an SD of 10 (Blumenthal et al. 2005). This transformation creates a standardized score that reflects how much each response deviates from a participant’s own mean. The resulting magnitudes were then averaged for each image type.
Post-auricular EMG was band-pass filtered offline between 10 Hz and 1000 Hz, rectified and smoothed using a 2-ms moving average. We identified post-auricular reflexes using a peak detection method (Sandt et al. 2009). Responses were defined as peak EMG magnitude occurring 8–40 ms from probe onset, relative to the mean baseline activity 50 ms prior to onset. Trials were excluded according to the same criteria used for eye-blink reflexes (M = 2.47% of trials; SD = 3.68), and responses were similarly standardized and converted to T-scores. Four participants (2 aware; 2 unaware) were excluded because of technical issues during recording.
Results
Behavioural responses
Picture judg e ments (intact/scrambled).
As expected, participants in the ‘aware’ condition were both accurate (across confidence ratings: M = 96.4%, SD = 3.7) and confident (M = 2.95 on a 3-point scale, SD = .04) in their picture judgements, indicating that they had seen the unsuppressed pictures. Participants in the ‘unaware’ condition, on the other hand, had much lower overall accuracy (M = 56.6%, SD = 8.1) and confidence (M = 1.37, SD = .34). Although overall accuracy in the ‘unaware’ condition was slightly above the 50% chance level, a breakdown of picture judgement accuracies as a function of confidence ratings (Table 1) clarifies that when confidence was low (a large majority of trials in the ‘unaware’ condition), accuracy was at chance, [t(23) = 0.58, P = .565], showing effective suppression of the images by CFS. In contrast, participants were highly accurate on the few trials when they were confident about the picture judgement, indicating that the infrequent breakthrough of the suppressed image into awareness led to an appropriate response.
Table 1.
Performance on the behavioural tasks as a function of confidence on the picture/scramble judgementa
| Aware |
Unaware |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confidence | High | Moderate | Low | High | Moderate | Low | ||||||
| Proportion of trials (SD) | 0.96 (0.03) | 0.04 (0.03) | 0.01 (0.01) | 0.12 (0.13) | 0.14 (0.14) | 0.75 (0.23) | ||||||
| Task | Picture | Dot | Picture | Dot | Picture | Dot | Picture | Dot | Picture | Dot | Picture | Dot |
| Accuracy (SD) | 0.98 (0.03) | 0.95 (0.05) | 0.62 (0.39) | 0.78 (0.32) | 0.19 (0.37) | 0.88 (0.35) | 0.92 (0.14) | 0.37 (0.32) | 0.75 (0.24) | 0.60 (0.27) | 0.49 (0.06) | 0.76 (0.15) |
aAccuracies are proportions of correct trials within each confidence rating for the picture judgement. Chance on picture task was 0.50; chance on dot task was 0.33. Proportions may sum to more than 1 due to rounding.
Dot counting task
Accuracy on this task was very high in the ‘aware’ condition (M = 94.3%, SD = 5.7) confirming that participants were attending to the display. Participants in the ‘unaware’ condition had lower accuracy on this task overall (M = 72.2%, SD = 15.6), but their performance was strongly influenced by breakthrough of the image. As Table 1 shows, dot counting performance in the ‘unaware’ condition did not differ from chance on high confidence (i.e. breakthrough) trials [t(17) = 0.59, p = .563]. This finding validates participants’ subjective reports: if participants are aware of the image, they should be unable to report the number of dots, which appear on the mask. However, performance on the dot task was well above chance (76%, compared with chance performance of 33%) when participants had low confidence about the picture judgement [t(23) = 14.18, p < .001]. This again validates the subjective reports, as participants should only be able to report the number of dots if they are aware of the mask and not the image.
Physiological responses
We compared physiological responses with and without awareness for the different image types (positive, neutral and negative). For the ‘aware’ condition, the reported analyses used all trials in which participants correctly identified the image type with high confidence. For the ‘unaware’ condition, we followed Raio et al. (2012) and used only those trials in which participants reported no awareness (lowest confidence) or reported moderate confidence but gave an incorrect response on the picture judgement. These criteria led to exclusion of an average of 6.3% (SD = 4.4) of trials from analysis for ‘aware’ participants and an average of 20.4% (SD = 17.8) of trials for ‘unaware’ participants. All participants had a minimum of six remaining trials for each image type (see Lieberman et al. 2017), which were used to extract physiological measures.
To determine whether physiological responses were modulated by the emotion of the image, and whether modulation depended on awareness, each of the four physiological measures was entered into a mixed analysis of variance (ANOVA) with the between-subject factor of awareness (aware or unaware) and within-subject factor of image type (positive, neutral and negative). A Greenhouse-Geisser correction was used when appropriate; in such cases, we report the original degrees of freedom, the corrected P-value and epsilon (Jennings 1987). Because the predicted effect for each measure involved comparison of one image type to the other two (enhancement by both types of emotional image for SCR and heart rate, enhancement by negative images for startle eye-blink reflex and enhancement by positive images for post-auricular reflex), significant main effects and interactions were followed up with planned Helmert contrasts, which first contrast one level with the average of the other two and then compare those two levels to each other (Rosenthal and Rosnow 1985). In addition, we calculated Bayes’ factors to assess emotional modulation of physiological responses in the ‘unaware’ condition. We used the R package ‘BayesFactor’ (Morey and Rouder 2015) and focused analyses on the first of the two Helmert contrasts, which is used to determine whether the response to one type of stimulus differs from the other two. We used the observed effect in the ‘aware’ condition as the prior for analysis of the ‘unaware’ condition: a half-Cauchy prior distribution was specified (Morey et al. 2016), and the scale parameter was set as Cohen’s d for the equivalent contrast in the ‘aware’ condition. We adopt Jeffrey’s (1988) convention and consider Bayes factors greater than 3 to reflect substantial evidence of the expected emotional response, and Bayes factors less than 1/3 to reflect substantial evidence for the null. Means and standard deviations for each physiological measure appear in Table 2.
Table 2.
Descriptive statistics for each physiological measure as a function of image type and awarenessa
| Aware |
Unaware |
|||||
|---|---|---|---|---|---|---|
| Positive | Neutral | Negative | Positive | Neutral | Negative | |
| Skin conductance | 0.282 | 0.223 | 0.294 | 0.176 | 0.091 | 0.114 |
| (0.282) | (0.282) | (0.356) | (0.184) | (0.070) | (0.108) | |
| [13.00] | [26.86] | [12.55] | [11.29] | [22.08] | [11.21] | |
| Heart rate deceleration | 6.23 | 4.84 | 6.64 | 5.69 | 5.16 | 5.09 |
| (2.48) | (2.25) | (3.14) | (2.61) | (2.02) | (2.56) | |
| [13.09] | [26.74] | [12.43] | [10.83] | [21.79] | [10.96] | |
| Startle eye-blink magnitude | 48.97 | 49.42 | 52.45 | 49.45 | 50.39 | 49.84 |
| (2.11) | (1.39) | (2.62) | (3.53) | (2.29) | (2.44) | |
| [11.17] | [22.67] | [10.38] | [9.50] | [18.67] | [9.58] | |
| Post-auricular reflex magnitude | 50.98 | 49.94 | 48.95 | 51.29 | 49.54 | 49.38 |
| (2.63) | (0.83) | (2.36) | (2.45) | (1.60) | (2.54) | |
| [10.55] | [22.45] | [10.23] | [8.91] | [18.59] | [9.64] | |
aSkin conductance response measured in log(x + 1) µS. Heart rate deceleration in beats per minute. Startle eye-blink and post-auricular reflex are T-scores, standardized within participants and scaled to have a mean of 50. Standard deviations appear in parentheses. Mean number of trials per condition (after exclusions for breakthrough and artefacts) appear in [brackets]. Skin conductance and heart rate measures had 14 trials with positive and negative images and 28 trials with neutral images; startle eye-blink and post-auricular reflex measures had 12 trials with positive and negative images and 24 trials with neutral images.
Skin conductance response
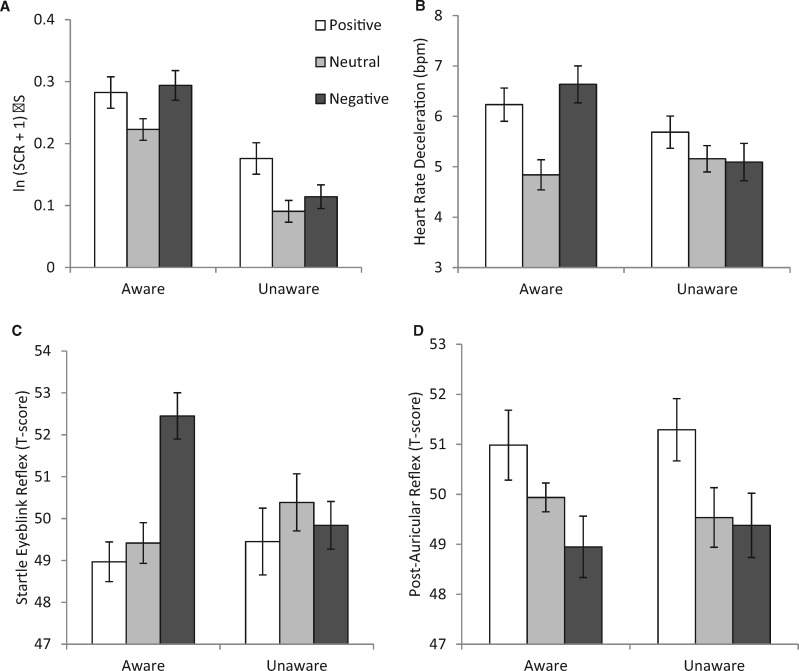
A main effect of group showed that aware participants produced larger SCRs overall [F(1, 44) = 4.75, P = 0.035, = 0.10] (Fig. 2A). There was also a main effect of image type [F(2, 88) = 5.75, P = 0.007, = 0.12, ɛ = 0.853], reflecting typical SCR enhancement by high arousal images. Helmert contrasts showed that SCRs were larger when viewing emotional relative to neutral images [F(1, 44) = 15.56, P < 0.001, = 0.26], while responses to positive and negative images did not differ [F(1, 44) = 1.00, P = 0.324, = 0.02]. Importantly, there was no interaction between awareness and image type [F(2, 88) = 1.46, P = 0.239, = 0.03, ɛ = 0.870], indicating that emotional modulation of the SCR did not significantly differ between groups. Although the interaction was not significant, we conducted follow-up contrasts in each group separately to determine whether emotional modulation was observed in each condition and to ensure that the absence of an interaction was not simply due to lack of statistical power. In each group, SCRs were larger when viewing emotional, relative to neutral images [aware: F(1, 21) = 9.45, P = 0.006, = 0.31; unaware: F(1, 23) = 6.37, P = 0.019, = 0.22], but responses to positive and negative images did not differ [aware: F(1, 21) = 0.09, P = 0.766, < 0.01; unaware: F(1, 23) = 3.35, P = 0.080, = 0.13]. The Bayes factor for comparison of responses to emotional vs. neutral images in the unaware condition was 5.770, providing substantial evidence in support of typical emotional modulation of SCR outside awareness. Thus, even though the SCR was attenuated in the ‘unaware’ condition, it was still modulated by emotion. These conclusions were unchanged when the two non-responders were included (see Supplementary Material), meaning that effects were not driven by our exclusion criteria.
Figure 2.
Physiological responses to positive, neutral and negative images, as a function of awareness. (A) SCRs (n = 22 aware, 24 unaware); (B) heart rate deceleration (n = 23 aware, 24 unaware); (C) startle eye-blink magnitude (n = 24 aware, 24 unaware) and (D) post-auricular reflex magnitude (n = 22 aware, 22 unaware). SCRs and post-auricular reflexes show main effects of emotion that do not interact with awareness. Heart rate deceleration and startle eye-blink magnitudes differ by awareness: expected emotion effects are observed in the aware condition but not the unaware condition. Error bars are standard errors for within-subject comparisons (Morey, 2008).
Heart rate
Both aware and unaware participants showed heart rate deceleration (Fig. 2B). There was no main effect of awareness on deceleration [F(1, 45) = 0.86, P = 0.359, = 0.02], but there was a significant effect of image type [F(2, 90) = 5.26, P = 0.007, = 0.11] that interacted with awareness [F(2, 90) = 4.07, P = 0.020, = 0.08]. To investigate the interaction, we ran separate follow-up ANOVAs with one factor (image type) for each group. The aware group showed the expected main effect of image type [F(2, 44) = 8.01, P = 0.001, = 0.27], with follow-up contrasts confirming greater deceleration while viewing emotional, compared to neutral images [F(1, 22) = 18.99, P < 0.001, = 0.46], but no difference between negative and positive images [F(1, 22) = 0.61, P = 0.443, = 0.03]. In the unaware group, a similar ANOVA showed that heart rate deceleration was not modulated by image type [F(2, 46) = 1.03, P = 0.364, = 0.04]. The Bayes factor for comparison of emotional with neutral images in the unaware group was 0.332, providing substantial support for the conclusion that there was no emotional modulation of heart rate deceleration outside awareness.
Startle eye-blink reflex
The eye-blink measure was standardized within individuals (see Method section), so by design, there could be no main effect of awareness (Fig. 2C). There was a main effect of image type [F(2, 92) = 5.27, P = 0.007, = 0.10], but its interaction with awareness [F(2, 92) = 5.16, P = 0.007, = 0.10] indicated different patterns of emotional modulation in aware and unaware participants. A follow-up ANOVA on the ‘aware’ condition showed the typical effect of image type [F(2, 46) = 14.05, P < 0.001, = 0.38]; follow-up contrasts revealed that the eye-blink reflex was enhanced during negative compared with neutral and positive images [F(1, 23) = 23.18, P < 0.001, = 0.50], while responses during positive and neutral images did not differ [F(1, 23) = 0.49, P = 0.493, = 0.02]. In contrast, the unaware group showed no effect of image type on startle eye-blink reflexes [F(2, 46) = 0.46, P = 0.632, = 0.02]. The Bayes factor for comparison of negative to the other image types in the unaware group was 0.146, providing substantial support for the conclusion that there was no modulation of the startle eye-blink reflex outside awareness.
Post-auricular reflex
Similar to the startle eye-blink, post-auricular reflexes were standardized within individuals, so there could be no main effect of awareness; however, there was an effect of image type [F(2, 84) = 6.64, P = 0.004, = 0.14, ɛ = 0.847], reflecting the expected effect of emotion on the post-auricular reflex (Fig. 2D). Follow-up contrasts showed that the response was larger for positive compared with negative and neutral images [F(1, 42) = 9.80, P = 0.003, = 0.19], which did not differ [F(1, 42) = 1.41, P = 0.242, = 0.002]. There was no image type by awareness interaction [F(2, 84) = 0.33, P = 0.720, = 0.01, ε = 0.847], indicating that enhancement of the post-auricular reflex by positive images did not depend on awareness. Despite the absence of an interaction, we examined emotional modulation in each condition separately. Each group showed a main effect of image type [aware: F(2, 42) = 3.54, P = 0.038, = 0.14; unaware: F(2, 42) = 3.43, P = 0.042, = 0.14]. Follow-up contrasts showed that potentiation by positive relative to neutral and negative images approached significance in the ‘aware’ condition [F(1, 21) = 3.56, P = 0.073, = 0.14] and was significant in the ‘unaware’ condition [F(1, 21) = 6.79, P = 0.017, = 0.244] . The Bayes factor for comparison of positive to other image types in the unaware group was 7.079, providing substantial support for emotional modulation of the post-auricular reflex outside awareness.
Discussion
Importantly, when images were viewed with awareness, all four physiological measures showed expected responses. Autonomic responses (SCR and heart rate deceleration) were enhanced by arousing images regardless of valence; reflexes showed the expected modulation by valence, with startle eye-blink responses enhanced by negative images and post-auricular reflexes enhanced by positive images. In contrast, dissociations occurred across physiological systems when images were suppressed from awareness. SCRs and post-auricular reflexes were still modulated by the emotional nature of the image, but effects on heart rate deceleration and startle eye-blink reflexes were abolished. We established these dissociations using combined objective and subjective trial-by-trial judgements of awareness to ensure we included only trials in which images were effectively suppressed. Our findings indicate that the ensemble of physiological components that characterize an emotional response is not triggered in an all-or-none fashion; rather, some physiological systems respond in the absence of awareness, but others do not.
Within the autonomic system, emotional modulation of the SCR without awareness is consistent with several backward masking studies (Öhman and Soares 1994; Gläscher and Adolphs 2003; Flykt et al. 2007), and two CFS studies using fearful faces (Raio et al. 2012; Lapate et al. 2014), which all point to emotional modulation of sympathetic activation as a response that does not require awareness of an eliciting stimulus. Our finding is at odds, though, with a CFS study comparing negative (threatening animals) and neutral images (Hedger et al. 2015) that found no modulation of SCR outside awareness. Our stringent criteria for judging awareness on a trial-by-trial basis (including the use of a low-level intact/scrambled judgement as our objective measure), combined with the fact that other physiological responses (heart rate and startle eye-blink) were abolished under CFS, suggests that our SCR finding does not reflect emotional modulation associated with residual awareness. There are some notable differences between studies, including our use of erotic and mutilation images (instead of threatening animals), a longer stimulus presentation (4500 ms instead of 800 ms, allowing for the unfolding of a full emotional response), a longer inter-trial interval (21 instead of 8 s, allowing skin conductance level to return to baseline) and fewer trials (56 images trials instead of 128, reducing the likelihood of habituation). It is therefore possible that our experimental parameters were optimized for the detection of an SCR to emotional stimuli viewed without awareness. It is perhaps notable as well that the response to positive images was (numerically, though not significantly) larger than that to negative images in the ‘unaware’ condition. Combined with the finding that positive images enhance the post-auricular reflex under suppression (described below), this sensitivity leads us to speculate that positive (or specifically erotic) images may be preferentially processed outside awareness.
We must consider the possibility, however, that SCR was modulated by meaningfulness rather than emotion (Bradley 2009), because our neutral images were meaningless scrambles. However, we think this is unlikely: physiological responses to meaningless stimuli closely resemble those to neutral images (Bradley et al. 2006), and we found effects of just one emotional valence on each of the somatic reflex measures (negative for startle eye-blink; positive for post-auricular reflex), indicating that our other physiological effects cannot be attributed to meaningfulness.
Our finding that modulation of heart rate deceleration does require awareness of the emotional stimulus is consistent with studies using backward masking (Ruiz-Padial et al. 2011; Sebastiani et al. 2011). However, heart rate effects in backward masking studies are difficult to interpret because briefly presented images do not modulate cardiac deceleration even with awareness (Codispoti et al. 2001). Here, we recorded 4 s of heart rate data during presentation of suppressed images – sufficient time to produce a typical cardiac effect when participants are aware of the image. Cardiac deceleration is thought to reflect activity in prefrontal networks that support emotion regulation, mediated predominantly via parasympathetic activation (Thayer and Lane 2009). Such regulation may rely on conscious appraisal processes.
A dissociation was also observed between the two reflexes. The failure of our masked negative images to enhance startle eye-blink responses stands in contrast to two studies using backward masking paradigms (Reagh and Knight 2013; Ruiz-Padial et al. 2011). Although null effects can always arise through low statistical power that explanation is unlikely here, given that our paradigm produced typical startle eye-blink modulation in the ‘aware’ condition and the Bayesian analysis provided substantial evidence in support of the null hypothesis.
The discrepancy between our findings and those of previous masking studies more likely reflects our more stringent criteria for awareness. Both backward masking studies used post-experiment recognition tests to assess awareness; such tests have been criticized as being overly liberal in attributing lack of awareness (Pessoa 2005; Grillon and Cornwell 2007). Alternatively, findings from backward masking might reflect atypical startle effects associated with brief stimulus presentation (Codispoti et al. 2001). Notably, Ruiz-Padial and colleagues (2011) observed enhanced startle by both positive and negative masked images, suggesting an arousal-based mechanism instead of the typically observed motivational priming effect. Finally, the discrepant findings might reflect differences in the mechanisms by which CFS and backward masking affect awareness (Faivre et al. 2012; Breitmeyer 2015). Direct comparison of the two methods using the same criteria for awareness would distinguish between these explanations. Although emotional modulation of the startle eye-blink reflex occurs sub-cortically via either central amygdala or BNST pathways (Davis et al. 2010), it can be modulated by conscious reappraisal strategies (Jackson et al. 2000; Eippert et al. 2007). Our finding demonstrates that awareness of an emotional stimulus is required for modulation of eye-blink responses, suggesting that such modulation may rely on processes downstream from those that mediate selection into awareness.
In contrast, the post-auricular reflex was enhanced in the presence of positive images, regardless of awareness. The reflex is vestigial in humans and its circuitry is not well understood nor is its functional role in appetitive responding. Importantly, there appears to be no limbic input into the reflex arc (Hackley 2015; Hebert et al. 2015). It is commonly observed in mammal species in relation to nursing behaviour, and one hypothesis links it to anticipation of food or sexual rewards (Johnson et al. 2012). Our finding that modulation of the reflex does not depend on awareness shows that such anticipation need not be consciously mediated. The fact that the post-auricular reflex can be primed by activation of facial muscles (e.g. those involved in smiling; Hebert et al. 2015) suggests that appetitive modulation of the reflex could occur peripherally. Further studies will be important in establishing the mechanism of post-auricular modulation and may well shed light on its currently mysterious role in appetitive motivation. Ours is the first study to assess post-auricular responses to emotional stimuli presented outside awareness, and our findings encourage its further study as an index of unconscious emotional processing.
Identifying neurological, physiological and behavioural responses that can arise without awareness is a central theme in consciousness research (Block et al. 2014). However, a complete understanding of the role of consciousness in emotion requires that we know both sides of the coin – which responses don’t require awareness but also which ones do. The dissociations we report here have several important implications. First, we demonstrate the value of using multiple physiological measures when studying unconscious emotion. SCR is commonly used to show ‘physiological’ responses in the absence of awareness, but our findings indicate that the relationship between consciousness and physiological responding is more complex and that SCR should not be taken as a proxy for physiological responding in toto. Methodologically, the use of multiple measures also strengthens conclusions based on null effects, because dissociations across physiological responses increase confidence that some responses are not modulated by unconscious emotion while others are. Of course, we assessed responses to images drawn from only two categories and recorded only four physiological measures, so we still don’t know whether similar dissociations exist for other emotional categories (such as anger, sadness or joy) or for other physiological systems (such as blood pressure, pupil responses or pilo-erection) that are also sensitive to emotion. Rather than a complete analysis of the physiology of unconscious emotional processing, our study is a demonstration that physiological responses can dissociate outside awareness.
Second, our findings raise questions about the relationship between physiological responses and emotional experience. In this study, participants reported their visual awareness but not their emotional experience. We intentionally had participants judge the images on low-level visual characteristics (the intact/scrambled judgement) and not higher level characteristics, such as valence, so as to provide a stringent criterion for awareness (Yang et al. 2014). However, we do think that, in future studies, a valence judgement might provide an interesting extension to the findings reported here, because it may induce interoceptive monitoring. The consensus from studies that have probed emotional experience in the absence of visual awareness is that physiological and behavioural responses can be observed without subjective emotional feeling (e.g. Winkielman et al. 2005). However, feedback from the body figures prominently in many theories of emotional experience (James 1894; Schacter and Singer 1962; Critchley et al. 2004; Prinz 2004; Damasio and Carvalho 2013; Seth 2013), and so one might expect that the physiological responses examined here could give rise to subtle feelings (e.g. arousal or approach/avoid tendencies) that remain undifferentiated until awareness of a trigger produces a specific emotional experience. Alternatively, it may be the case that subjective emotional feelings are triggered only by a coherent physiological response or that feelings may depend critically on specific modulations (e.g. in cardiac response) that require awareness of the stimulus.
Conclusions
The respective roles of different physiological systems that contribute to the emotional response are poorly understood at present. Our findings provide an important step towards disentangling the dissociable physiological systems that underlie emotional responses as a function of awareness and demonstrate that there is much to be learned about both consciousness and emotion through a deeper understanding of the intimate relationship between mind and body.
Data Availability Statement
All experimental materials, raw and processed data and analyses are available online at https://osf.io/5p2mj/.
Supplementary Material
Acknowledgments
We thank Will Brown and Samora O’Neill for assistance in preparing stimuli and piloting the CFS procedure, and we also thank Al Abenoja for programming assistance.
Supplementary data
Supplementary data is available at NCONSC Journal online.
Conflict of interest statement. None declared.
Funding
This project was supported by a grant from the Royal Society of New Zealand Marsden Fund (VUW1307) to G.G. and D.C. D.C. was supported by the European Research Council (ERC Advanced Grant XSPECT - DLV-692739, awarded to Andy Clark).
References
- Almeida J, Pajtas P, Mahon BZ. et al. Affect of the unconscious: visually suppressed angry faces modulate our decisions. Cogn Affect Behav Neurosci 2013;13:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU.. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 2016;41:126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD. Postauricular and superior auricular reflex modulation during emotional pictures and sounds. Psychophysiology 2011;48:410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Lang AR.. Emotional modulation of the post-auricular reflex. Psychophysiology 2004;41:426–32. [DOI] [PubMed] [Google Scholar]
- Block N, Carmel D, Fleming SM. et al. Consciousness science: real progress and lingering misconceptions. Trends Cogn Sci 2014;18:556–7. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL. et al. Committee report: guidelines for human startle eyeblink electromyography studies. Psychophysiology 2005;42:1–15. [DOI] [PubMed] [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S. et al. Publication recommendations for electrodermal measures. Psychophysiology 2012;49:1017–34. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology 2009;46:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN. et al. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion 2001a;1:276–98. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ.. A multi-process account of startle modulation during affective perception. Psychophysiology 2006;43:486–97. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D. et al. Emotion and motivation II: sex differences in picture processing. Emotion 2001b;1:300–19. [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang AR.. Pictures as prepulse: attention and emotion in startle modulation. Psychophysiology 1993;30:541–5. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ.. The International Affective Picture System (IAPS) in the study of emotion and attention In: Coan JA, Allen JB (eds.), Handbook of Emotion Elicitation and Assessment. Oxford, UK: Oxford University Press, 2007, 29–46. [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN.. Emotion, novelty, and the startle reflex: habituation in humans. Behav Neurosci 1993;107:970–80. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG. Psychophysical ‘blinding’ methods reveal a functional hierarchy of unconscious visual processing. Conscious Cogn 2015;35:234–50. [DOI] [PubMed] [Google Scholar]
- Cacciopo JT, Berntson GG, Larsen JT. et al. The psychophysiology of emotion In: Lewis M., Haviland-Jones JM (eds.), Handbook of Emotions. New York: Guilford Press, 2000, 119–42. [Google Scholar]
- Carmel D, Arcaro M, Kastner S. et al. How to create and use binocular rivalry. J Vis Exp 2010;45:e2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codispoti M, Bradley MM, Lang PJ.. Affective reactions to briefly presented pictures. Psychophysiology 2001;38:474–8. [PubMed] [Google Scholar]
- Codispoti M, Mazzetti M, Bradley MM.. Unmasking emotion: exposure duration and emotional engagement. Psychophysiology 2009;46:731–8. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Surcinelli P, Baldaro B.. Watching emotional movies: affective reactions and gender differences. Int J Psychophysiol 2008;69:90–5. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P. et al. Neural systems supporting interoceptive awareness. Nat Neurosci 2004;7:189–95. [DOI] [PubMed] [Google Scholar]
- Damasio A, Carvalho GB.. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci 2013;14:143–52. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker KL, Miles L. et al. Phasic vs. sustained fear in rats and humans: role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology 2010;35:105–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N. et al. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp 2007;28:409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre N, Berthet V, Kouider S.. Nonconscious influences from emotional faces: a comparison of visual crowding, masking, and continuous flash suppression. Front Psychol 2012;3:129.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacconi CM, Dekraker J, Köhler S.. Psychophysiological evidence for the role of emotion in adaptive memory. J Exp Psychol Gen 2015;144:925–33. [DOI] [PubMed] [Google Scholar]
- Flykt A, Esteves F, Öhman A.. Skin conductance responses to masked conditioned stimuli: phylogenetic/ontogenetic factors versus direction of threat? Biol Psychol 2007;74:328–36. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R.. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci 2003;23:10274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FK, Clifton RK.. Heart-rate change as a component of the orienting response. Psychol Bull 1966;65:305–20. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology 2008;199:421–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Cornwell B.. Comments on ‘Fearful and sexual pictures not consciously seen modulate the startle reflex in human beings’. Biol Psychol 2007;62:541.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackley SA. Evidence for a vestigial pinna-orienting system in humans. Psychophysiology 2015;52:1263–70. [DOI] [PubMed] [Google Scholar]
- Hedger N, Adams WJ, Garner M.. Autonomic arousal and attentional orienting to visual threat are predicted by awareness. J Exp Psychol Hum Percept Perform 2015;41:798–806. [DOI] [PubMed] [Google Scholar]
- Hebert KR, Valle-Inclán F, Hackley SA.. Modulation of eyeblink and postauricular reflexes during the anticipation and viewing of food images. Psychophysiology 2015;52:509–17. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL. et al. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology 2000;37:515–22. [PubMed] [Google Scholar]
- James W. The physical basis of emotion. Psychol Rev 1894;1:516–29. [DOI] [PubMed] [Google Scholar]
- Jeffreys H. Theory of Probability. Oxfordshire: Oxford University Press, 1998. [Google Scholar]
- Jennings JR. Editorial policy on analyses of variance with repeated measures. Psychophysiology 1987;24:474–5. [Google Scholar]
- Jennings JR, Berg WK, Hutcheson JS. et al. Publication guidelines for heart rate studies in man. Psychophysiology 1981;18:226–31. [DOI] [PubMed] [Google Scholar]
- Johnson GM, Valle-Inclán F, Geary DC. et al. The nursing hypothesis: an evolutionary account of emotional modulation of the postauricular reflex. Psychophysiology 2012;49:178–85. [DOI] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: a review. Biol Psychol 2010;84:394–421. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN.. Emotion, attention, and the startle reflex. Psychol Rev 1990;97:377–95. [PubMed] [Google Scholar]
- Lang PJ., Bradley MM., Cuthbert BN. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL, 2008.
- Lang PJ, Davis M.. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res 2006;156:3–29. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM. et al. Looking at pictures: affective, facial, visceral and behavioral reactions. Psychophysiology 1993;30:261–73. [DOI] [PubMed] [Google Scholar]
- Lapate RC, Rokers B, Li T. et al. Nonconscious emotional activation colors first impressions: a regulatory role for conscious awareness. Psychol Sci 2014;25:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Stevens ES, Funkhouser CJ. et al. How many blinks are necessary for a reliable startle response? A test using the NPU-threat task. Int J Psychophysiol 2017;114:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufityanto G, Donkin C, Pearson J.. Measuring intuition: nonconscious emotional information boosts decision accuracy and confidence. Psychol Sci 2016;27:622–34. [DOI] [PubMed] [Google Scholar]
- Morey RD. Confidence intervals from normalized data: a correction to Cousineau (2005). Tutor Quant Methods Psychol 2008;4:61–4. [Google Scholar]
- Morey RD, Romeijn J, Rouder JN.. The philosophy of Bayes factors and the quanification of statistical evidence. J Math Psychol 2016;72:6–18. [Google Scholar]
- Morey RD, Rouder JN.. BayesFactor: Computation of Bayes Factors for Common Designs R package version 0.9.12-2, 2015. https://cran.r-project.org/web/packages/BayesFactor/BayesFactor.pdf
- Öhman A, Soares JJ.. ‘Unconscious anxiety’: phobic responses to masked stimuli. J Abnorm Psychol 1994;103:231–40. [DOI] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Curr Opin Neurobiol 2005;15:188–96. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory. Biol Psychol 2007;74:116–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz J. Emotions embodied. In: Solomon R. (ed.), Thinking About Feeling. New York: Oxford University Press, 2004, pp. 44–60. [Google Scholar]
- Raio CM, Carmel D, Carrasco M. et al. Nonconscious fear is quickly acquired but swiftly forgotten. Curr Biol 2012;22:R477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Knight DC.. Negative, but not positive emotional images modulate the startle response independent of conscious awareness. Emotion 2013;13:782–91. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL.. Contrast Analysis: Focused Comparisons in the Analysis of Variance. Cambridge, UK: Cambridge University Press, 1985. [Google Scholar]
- Ruiz-Padial E, Vila J, Thayer JF.. The effect of conscious and non-conscious presentation of biologically relevant emotion pictures on the emotion modulated startle and phasic heart rate. Int J Psychophysiol 2011;79:341–6. [DOI] [PubMed] [Google Scholar]
- Sandt AR, Sloan DM, Johnson KJ.. Measuring appetitive responding with the postauricular reflex. Psychophysiology 2009;46:491–7. [DOI] [PubMed] [Google Scholar]
- Schacter S, Singer J.. Cognitive, social, and physiological determinants of emotional state. Psychol Rev 1962;69:379–99. [DOI] [PubMed] [Google Scholar]
- Sebastiani L, Castellani E, D’Alessandro L.. Emotion processing without awareness: features detection or significance evaluation? Int J Psychophysiol 2011;80:150–6. [DOI] [PubMed] [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 2013;17:565–73. [DOI] [PubMed] [Google Scholar]
- Sparks JV, Lang A.. An initial examination of the post-auricular reflex as a physiological indicator of appetitive activation during television viewing. Commun Methods Meas 2010;4:311–30. [Google Scholar]
- Tamietto M, de Gelder B.. Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci 2010;11:697–709. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD.. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 2009;33:81–8. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C.. Continuous flash suppression reduces negative afterimages. Nat Neurosci 2005;8:1096–101. [DOI] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC.. Unconscious emotion. Curr Dir Psychol Sci 2004;13:120–3. [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL.. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull 2005;31:121–35. [DOI] [PubMed] [Google Scholar]
- Willenbockel V, Sadr J, Fiset D. et al. Controlling low-level image properties: the SHINE toolbox. Behavior Res Methods 2010;42:671–84. [DOI] [PubMed] [Google Scholar]
- Yang E, Brascamp J, Kang M-S. et al. On the use of continuous flash suppression for the study of visual processing outside of awareness. Front Psychol 2014;5:724.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All experimental materials, raw and processed data and analyses are available online at https://osf.io/5p2mj/.