Abstract
Background
We compared pathological prognostic stage (PPS) with anatomic stage (AS) groups according to the updated version of breast cancer staging of the American Joint Committee on Cancer (AJCC) 8th Edition.
Material/Methods
We evaluated 353 breast cancer patients initially treated with surgery. AS and PPS were performed by evaluating the pathological data of the patients according to the AJCC 8th Edition breast cancer updated version. Stages and survival rates between the 2 staging systems were evaluated and compared. Disease-free survival (DFS) and disease-specific survival (DSS) were calculated according to both staging systems using Kaplan-Meier test. After the PPS change was made in each AS group, 10-year DFS and 10-year DSS of the changed groups were compared using the chi-square test.
Results
The median follow-up was 114 months and the median age was 48 years. In 192 (54.4%) patients the stage change. The most significant change was 1-level downstaging in 70 (22.4%) patients, and 2-levels downstaging in 78 (22.1%) patients. Five-year DFS, 10-year DFS, 5-year DSS rate, and 10-year DSS were 86.3%, 80.3%, 93.8%, and 84.1%, respectively. The PPS system was found to provide better prognostic information when the patients with AS IIB and IIIA groups were compared according to the PPS.
Conclusions
According to the updated version of the AJCC 8th Edition, half of our patients had stage change when they were evaluated according to AS and PPS system. PPS gives better information about prognosis than does AS.
MeSH Keywords: Breast Neoplasms, Neoplasm Staging, Prognosis
Background
At the end of 2016, the AJCC Cancer Staging Manual 8th Edition was published [1]. T, N, M, ER, PR, HER2, and tumor grade biomarkers were used for breast cancer staging in the prognostic stage group. Oncotype DX is required for patients who are classified as T1–T2, N0, M0, grade 1–3, HER2 negative, and ER positive. Patients with Oncotype DX recurrence score <11 are classified as pathologic prognostic stage IA. The updated breast chapter was released in December 2017. The pathologic prognostic staging group was established with patients who underwent surgery for primary treatment, without any neoadjuvant treatment [2]. There was no change in the anatomic stage group. In the present study, we compared the pathologic prognostic stage and anatomic stage of patients with breast cancer, according to the AJCC 8th Edition.
Material and Methods
We retrospectively evaluated 353 patients (stages I–III) treated with surgery as a primary treatment for breast cancer at the Institute of Oncology in Istanbul University between January 2004 and September 2006. Tumor size, lymph node involvement, distant metastasis, ER status, PR status, HER2 status, and tumor grade information were recorded. The histological grade of tumors was evaluated according to the Elston-Ellis modification of the Scarff-Bloom-Richardson grading system [3]. ER status was determined by immunohistochemistry and it was recorded as the rate of positively stained cells. The cut-off value was 1% for patients who received treatment [4]. PR status was also determined by immunohistochemistry, and if 1% or more of the cells were stained, it was considered as PR-positive. 3+ in immunohistochemistry or gene amplification demonstrated through fluorescence in situ hybridization were accepted as HER2-positive [5]. The clinicopathologic information of patients is presented in Table 1. The anatomic staging and pathologic prognostic staging according to breast cancer of the updated version of the AJCC 8th Edition were performed on the patients. Stage changes were investigated. We determined which patients were ER-positive, HER2-negative, T1–T2, N0, and who were eligible for an Oncotype DX multi-gene test, which was recommended to be used in pathological prognostic staging in the AJCC 8th Edition. Our patients did not have Oncotype DX multi-gene analysis. If the test was not performed, the appropriate stage was recorded, according to the new staging. Because biological markers are very important in the new staging system, and because they affect the survival rate, 64 patients who had not received trastuzumab in adjuvant treatment were excluded from the survival analysis, so 289 patients remained for the analysis. This study was performed in compliance with the Declaration of Helsinki.
Table 1.
Clinicopathological characteristics.
| N=353 | |
|---|---|
|
| |
| Median (min–max) | |
| Age (years) | 48 (24–79) |
|
| |
| Tumor size (cm) | 2.2 (0.3–14) |
|
| |
| Total excised lymph nodes | 14 (1–43) |
|
| |
| Total involved lymph nodes | 1 (0–42) |
|
| |
| Adjuvant chemotherapy cures | 6 (4–8) |
|
| |
| Radiotherapy dose (Gray) | 50 (45–54.4) |
|
| |
| N (%) | |
|
| |
| Menopausal status | |
| Premenopausal | 177 (50.1) |
| Postmenopausal | 165 (46.7) |
| Perimenopausal | 11 (3.1) |
|
| |
| Pathology | |
| IDC + ILC | 52 (14.7) |
| IDC | 255 (72.2) |
| ILC | 30 (8.5) |
| Other | 16 (5.6) |
|
| |
| Surgery type | |
| BCS+SLNB | 86 (24.4) |
| MRM | 135 (38.3) |
| Mastectomy+SLNB | 9 (2.5) |
| BCS+AD | 113 (32) |
| SSM+AD | 7 (2) |
| NSM+AD | 2 (0.6) |
| SSM+SLNB | 1 (0.3) |
|
| |
| Tumor grade | |
| 1 | 19 (5.4) |
| 2 | 154 (43.6) |
| 3 | 180 (51) |
|
| |
| ER | |
| Positive | 253 (71.7) |
| Negative | 100 (28.3) |
|
| |
| PR | |
| Positive | 265 (75.1) |
| Negative | 88 (24.9) |
|
| |
| HER2 | |
| Positive | 64 (18.1) |
| Negative | 289 (81.9) |
|
| |
| Triple negative | |
| Yes | 41 (11.6) |
| No | 312 (88.4) |
|
| |
| Adjuvant chemotherapy | |
| Yes | 282 (79.9) |
| No | 71 (20.1) |
|
| |
| HT | |
| Yes | 288 (81.6) |
| No | 65 (18.4) |
|
| |
| Radiotherapy | |
| Yes | 315 (89.2) |
| No | 38 (10.8) |
|
| |
| Loco-regional | |
| Yes | 23 (6.5) |
| No | 330 (93.5) |
|
| |
| Distant metastasis | |
| Yes | 71 (20.1) |
| No | 282 (79.9) |
|
| |
| Status | |
| Exitus (due to breast cancer) | 56 (15.9) |
| Alive | 232 (65.7) |
| Lost to follow-up | 50 (14.2) |
| Exitus (other reasons) | 15 (4.2) |
IDC – invasive ductal carcinoma; ILC – invasive lobular carcinoma; BCS – breast conserving surgery; SLNB – sentinel lymph node biopsy; MRM – modified radical mastectomy; AD – axillary dissection; SSM – skin sparing mastectomy; NSM – nipple sparing mastectomy; ER – estrogen receptor; PR – progesterone receptor.
Disease-specific survival (DSS) was calculated from the date of the operation to the date of death or the date of last control. If patients died for any other reason except breast cancer, they were censored on the date of death. Disease-free survival (DFS) was calculated from the operation date to the recurrence date of disease. SPSS software was used for statistical analysis (Version 20, IBM Corp., Armonk, NY, USA). We calculated 5-year and 10-year DFS and DSS according to both staging systems by using the Kaplan-Meier test. The Kappa test was performed by analyzing the degree of stage change, the rates of upstaging and downstaging. Each anatomic stage was re-classified according to the pathologic prognostic stage and the changed stages were compared with the chi-square test in terms of 10-year DFS and 10-year DSS. A p value <0.05 was considered statistically significant.
Results
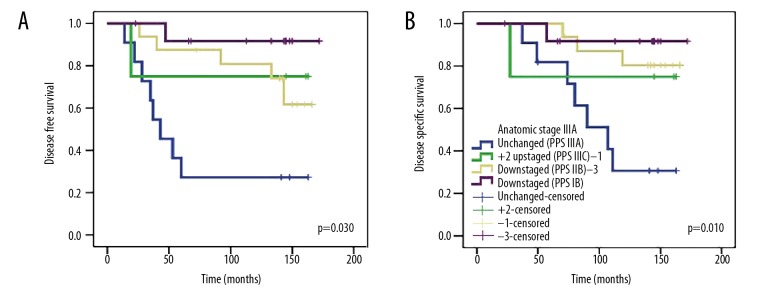
The median follow-up was 114 months (range, 6–178 months), the median age was 48 years (range, 24–79 years). The patient characteristics are presented in Table 1. Stage distribution according to the anatomical staging were: 97 (27.5%) patients stage IA, 3 (0.8%) stage IB, 100 (28.3%) IIA, 71 (20.1%) IIB, 52 (14.7%) stage IIIA, 3 (0.8%) stage IIIB, and 27 (7.6%) stage IIIB. According to the pathologic prognostic staging, 129 (36.4%) patients were stage IA, 77 (21.8%) were stage IB, 53 (15%) were stage IIA, 38 (10.8%) were stage IIB, 31 (8.8%) were stage IIIA, 17 (4.8%) were stage IIIB, and 8 (2.3%) were stage IIIC. When the stage changes were evaluated, the pathological prognostic stage remains unchanged in 161 (45.6%) patients, while 192 (54.4%) patients showed an upstaging or downstaging. The most significant change was one-level downstaging in 70 (22.4%) patients and a two-levels downstaging in 78 (22.1%) patients. The Oncotype DX assay was found to be appropriate for 97 (27.5%) patients. Stage change was generally found as downstaging (kappa=0.319, p<0.001) (Tables 2, 3). 64 (18.1%) patients who are HER2 positive, were not included in the survival analysis, because we could use trastuzumab only in 6 of these 64 patients. In the analysis of survival, the 5-year DFS, 10-year DFS, 5-year DSS, and 10-year DSS rates were 86.3%, 80.3%, 93.8%, and 84.1%, respectively. The evaluation according to the anatomical and pathologic prognostic stage groups revealed a similarity among stage IA, IIA, IIB, and IIIA groups, with relatively high numbers of patients in terms of 5-year and 10-year DFS and DSS rates. According to the pathological prognostic stage, there was obviously more downstaging. There was only 1 patient at stage IB in the anatomic stage group, while 68 (23.5%) patients were found to be classified as stage IB in the pathologic prognostic stage group due to stage change. The 5-year DFS, 10-year DFS, 5-year DSS, and 10-year DSS rates were 93.8%, 88.8%, 98.4%, and 91.5%, respectively. This result highlights the presence of patients with very good prognosis, even in the higher stages (Table 4). In addition, after the pathological prognostic stage group changes were made in each anatomic stage group, the groups were compared with the chi-square test. A statistically significant difference was found only in the pathological prognostic stage groups of the anatomic stage group IIB and IIIA. A total of 58 patients were in the anatomical stage group IIB. When they were staged according to the pathological prognostic stage group,15 patients were unchanged (pathologic prognostic stage IIB), 4 patients were +1 upstaged (pathologic prognostic stage IIIA), 19 patients were −1 downstaged (pathologic prognostic stage IIA), and 20 patients were −2 downstaged (pathologic prognostic stage IB). The 10-year DFS was 70% in patients without stage change, whereas the 10-year DFS was 94.7% in patients with −2 downstaged patients (p=0.003). A total of 44 patients were in anatomical stage group IIIA. After staging these patients according to the pathological prognostic staging, in 11 patients there was no stage change (pathologic prognostic stage IIIA), in 4 patients there was +2 upstaging (pathologic prognostic stage IIIC), in 16 patients there was −1 downstaging (pathologic prognostic stage IIB), and in 13 patients there was −3 downstaging (pathologic prognostic stage IB). The 10-year DFS rate was 27.3% in patients without stage change and 91.7% in patients with −3 downstaging (p=0.003); the 10-year DSS was 30.7% in patients without stage change and 91.7% in patients with −3 downstaging (p=0.010) (Table 5, Figures 1, 2).
Table 2.
Distribution of anatomic stage and pathologic prognostic stage.
| Anatomic stage | Pathologic Prognostic Stage | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IA | IB | IIA | IIB | IIIA | IIIB | IIIC | Total | |
| IA | 77 | 13 | 0 | 0 | 0 | 0 | 0 | 90 |
| %31.1 | ||||||||
|
| ||||||||
| IB | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| %0.3 | ||||||||
|
| ||||||||
| IIA | 30 | 22 | 24 | 1 | 0 | 0 | 0 | 77 |
| %26.6 | ||||||||
|
| ||||||||
| IIB | 0 | 20 | 19 | 15 | 4 | 0 | 0 | 58 |
| %20.1 | ||||||||
|
| ||||||||
| IIIA | 0 | 13 | 0 | 16 | 11 | 0 | 4 | 44 |
| %15.2 | ||||||||
|
| ||||||||
| IIIB | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 |
| %0.7 | ||||||||
|
| ||||||||
| IIIC | 0 | 0 | 0 | 0 | 5 | 9 | 3 | 17 |
| %5.9 | ||||||||
|
| ||||||||
| Total | 108 | 68 | 43 | 32 | 21 | 9 | 8 | 289 |
| % | %37.4 | %23.5 | %14.9 | %11.1 | %7.3 | %3.1 | %2.8 | %100 |
Table 3.
Stage changes according to pathologic prognostic staging of previous anatomic stages in AJCC 8th Edition.
| Variables | n=353 n (%) |
|---|---|
| Stage unchanged | 161 (45.6) |
|
| |
| Stage changed | 192 (54.4) |
|
| |
| Degree of stage changed | |
| +1 upstaged | 18 (5.1) |
| +2 upstaged | 4 (1.1) |
| −1 downstaged | 70 (22.4) |
| −2 downstaged | 78 (22.1) |
| −3 downstaged | 13 (3.7) |
|
| |
| Oncotype Dx assay | |
| Indicated | 97 (27.5) |
| Not indicated | 256 (72.5) |
Table 4.
Patient distribution according to anatomic-and pathologic prognostic stages and 5 & 10-year DFS & DSS rates.
| AJCC 8th Edition update Anatomic stage |
AJCC 8th Edition update Pathologic prognostic stage |
||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n=289 | n=289 | ||||||
|
| |||||||
| Stage | n (%) | DFS | DSS | Stage | n (%) | DFS | DSS |
| IA | 90 (31.1) | 5-year 95.4% | 5-year 98.9% | IA | 108 (37.4) | 5-year 96.2% | 5-year 98.1% |
| 10-year 90.3% | 10-year 95.15 | 10-year 89.8% | 10-year 96% | ||||
|
| |||||||
| IB | 1 (0.3) | Lost to follow-up | Lost to follow-up | IB | 68 (23.5) | 5-year 93.8% | 5-year 98.4% |
| 10-year 88.8% | 10-year 91.5% | ||||||
|
| |||||||
| IIA | 77 (26.6) | 5-year 92% | 5-year 94.3% | IIA | 43 (14.9) | 5-year 81% | 5-year 90.4% |
| 10-year 85.6% | 10-year 91.1% | 10-year 70% | 10-year 77.3% | ||||
|
| |||||||
| IIB | 58 (20.1) | 5-year 85.9% | 5-year 92.9% | IIB | 32 (11.1) | 5-year 84% | 5-year 93.3% |
| 10-year 74.4% | 10-year 79.5% | 10-year 67% | 10-year 74.9% | ||||
|
| |||||||
| IIIA | 44 (15.2) | 5-year 72.2% | 5-year 90.6% | IIIA | 21 (7.3) | 5-year 45.1% | 5-year 90% |
| 10-year 69.6% | 10-year 69.9% | 10-year 45.1% | 10-year 47.6% | ||||
|
| |||||||
| IIIB | 2 (0.7) | No recurrence | Alive | IIIB | 9 (3.1) | 5-year 50% | 5-year 62.5% |
| 10-year 50% | 10-year 50% | ||||||
|
| |||||||
| IIIC | 17 (5.9) | 5-year 43.1% | 5-year 71.4% | IIIC | 8 (2.8) | 5-year 71% | 5-year 71% |
| 10-year 43.1% | 10-year 41.7% | 10-year 71% | 10-year 71% | ||||
DFS – disease free survival; DSS – disease specific survival.
Table 5.
The effects of alterations on survival after reclassification of anatomic stages to pathologic prognostic stages.
| Anatomic stage group | Pathologic prognostic stage group | 10-year DFS | 10-year DSS | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Stage | n | Alteration | n | % | χ2 | p | % | χ2 | p |
| IA | 90 | Unchanged (IA) | 77 | 91.4 | 0.319 | 0.572 | 97.5 | 0.382 | 0.536 |
| +1 upstaged (IB) | 13 | 83.3 | 91.7 | ||||||
|
| |||||||||
| IB | 1 | – | – | – | – | – | – | – | – |
|
| |||||||||
| IIA | 77 | Unchanged (IIA) | 24 | 87.5 | 0.101 | 0.951 | 86.7 | 0.166 | 0.920 |
| −1 downstaged (IB) | 23 | 84 | 88.2 | ||||||
| −2 downstaged (IA) | 30 | 85.4 | 96.6 | ||||||
|
| |||||||||
| IIB | 58 | Unchanged (IIB) | 15 | 70 | 14.242 | 0.03 | 67.7 | 6.927 | 0.074 |
| +1 upstaged (IIIA) | 4 | – | – | ||||||
| −1 downstaged (IIA) | 19 | 49.3 | 66.1 | ||||||
| −2 downstaged (IB) | 20 | 94.7 | 94.7 | ||||||
|
| |||||||||
| IIIA | 44 | Unchanged (IIIA) | 11 | 27.3 | 14.223 | 0.003 | 30.7 | 11.259 | 0.010 |
| +2 upstaged (IIIC) | 4 | – | – | ||||||
| −1 downstaged (IIB) | 16 | 80.8 | 80.4 | ||||||
| −3 downstaged (IB) | 13 | 91.7 | 91.7 | ||||||
|
| |||||||||
| IIIB | 2 | – | – | – | – | – | – | – | – |
|
| |||||||||
| IIIC | 17 | Unchanged (IIIC) | 3 | – | 0.130 | 0.937 | – | 0.037 | 0.982 |
| −1 downstaged (IIIB) | 9 | – | – | ||||||
| −2 downstaged (IIIA) | 5 | – | – | ||||||
DFS – disease free survival; DSS – disease specific survival; χ2 – chi-square test.
Figure 1.
Survival analysis of anatomic stage IIB patients after reclassification according to pathologic prognostic stages: (A) Disease-free survival and (B) disease-specific survival.
Figure 2.
Survival analysis of anatomic stage IIIA patients after reclassification according to pathologic prognostic stages: (A) Disease-free survival and (B) disease-specific survival.
Discussion
Breast cancer treatment is based on both anatomical and biologic characteristics [6]. Greater understanding of cancer biology has been accompanied by identification and validation of biological markers of the treatment benefits and prognosis [7]. The aim of cancer staging is to identify the prevalence of the disease and to help to develop the treatment plan, as well as to provide information about the prognosis [8]. At the end of 2016, the AJCC Cancer Staging Manual 8th Edition was published [1,9]. In this staging system, the prognostic stage was defined by using T, N, M, ER, PR, HER2 status, and grade information. After Oncotype DX multi-gene analysis was applied in the group of those with T1–T2, N0, M0, ER-positive, and HER2-negative, if the recurrence score was <11, it was classified as IA. After the publication of the AJCC 8th Edition staging manual, this prognostic staging was validated in additional cohorts [8,10–15]. In some of these studies, anatomical and prognostic stage survival analysis of the AJCC 8th Edition was performed in breast cancer subgroups, and in some studies the general group of patients with non-metastatic invasive breast cancer was analyzed [8,10–15]. Hu et al. evaluated the stage changes in the AJCC 8th Edition according to the anatomical and prognostic stage system [16]. Ye et al. aimed to make a prognostic evaluation in luminal-A breast cancer by using AJCC 8th Edition [11]. They found 412 luminal-A patients DFS and overall survival (OS) rates were 98.3% and 99.3%, respectively. There was a significant difference in the 5-year DFS rate of different anatomic stages. No difference was found in OS but there was a significant difference between different prognostic stages in 5-year DFS and OS rates. They reported stage changes in 41.6% of the patients through the prognostic staging system. Zhou et al. analyzed the prognostic value of the anatomical stage and prognostic stage groups in 170 HER2-positive breast cancer patients [15]. They showed that both anatomic stage and prognostic stage have prognostic value in HER2-positive breast cancer. Both anatomic stage and prognostic stage were significant prognostic markers for DFS and OS. In multivariate analysis, both groups were independent predictors of OS. Wang et al., in their prognostic evaluation study of the AJCC 8th Edition, reported that the prognostic stage provided more correct information in locally advanced breast cancer compared with the anatomic TNM stage [12]. Abdel-Rahman reported in 209 304 patients with non-metastatic breast cancer that AJCC 8th Edition prognostic stage system showed an improvement in determination of prognosis when compared with the anatomic staging system [14]. Finally, the prognostic stage gave better prognostic information than the anatomical stage in the single-institution cohort and a large population database study carried out with 3327 patients with stage I–IIIC breast cancer and 54 727 patients with stage I to IV by Weiss et al. [8]. In all of these studies, the breast cancer prognostic staging in the AJCC Cancer Staging Manual, 8th Edition published at the end of 2016 was used [1]. However, in December 2017, the AJCC 8th Edition was updated with the new data. The new staging system offers a pathologic prognostic staging for patients who underwent surgery as the initial treatment.
While staging our study population according to the updated version of the AJCC 8th Edition updated staging system, we evaluated the distribution of stages in the new staging system according to anatomic stage and DFS-DSS rates. Our work has some limitations. It is primarily a retrospective study and the number of patients is relatively limited. However, it appears to be the first study comparing the anatomical stage and the pathological prognostic stage groups. We showed that the re-evaluation of anatomical stage as the pathologic prognostic stage was mostly presented as downstaging. After pathologic prognostic staging in anatomical stage IIIA group, 4 different groups were established (unchanged, +1 upstaged, −1 downstaged, and −3 downstaged). The comparison among these groups showed statistically significant differences in survival in 10-year DFS and DSS rates. After pathologic prognostic staging in the anatomical stage IIB group, 4 different groups were established (unchanged, +1 upstaged, −1 downstaged, and −2 downstaged). There was a significant difference in 10-year DFS among groups, whereas no significant difference was found among groups in terms of 10-year DSS (which was nearly statistically significant). Studies with larger series are needed to verify our results. According to our findings, the breast cancer patients seem to have been shifted to an earlier stage by use of the pathologic prognostic staging system.
Conclusions
Pathologic prognostic staging caused stage changes in 54.4% of our patients. One patient was in stage IB according to anatomic staging, whereas 68 patients were in stage IB according to pathologic prognostic staging due to stage change, and the 5-and 10-year DFS and DSS rates were over 90%. In addition, within the IIB group staged according to the anatomical stage system, 10-year DFS was 94.7% according to the pathologic prognostic staging system, the 10-year DSS rate of the −2 downstaged group (pathological prognostic stage group IA) was 94.7%, and the 10-year DSS and DFS rates of the −3 downstaged group (pathological prognostic stage group IB) was 91.7%. Pathologic prognostic staging provides better prognostic information than does anatomic staging. Validation studies should be done with extended series.
Acknowledgements
We would like to thank Prof. Dr. Rian Disci for his extraordinary support in this project.
Footnotes
Source of support: Departmental sources
Conflicts of interest.
None.
References
- 1.Hortobagyi GN, Connolly JL, Edge SB, et al. Breast. In: Amin MB, Edge S, Greene G, et al., editors. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016. [Google Scholar]
- 2.Cancerstaging.org Chicago. American Joint Committee on Cancer. Updated Breast Chapter for 8th Edition Available from: https://cancerstaging.org/references-tools/deskreferences/Pages/Breast-Cancer-Staging.aspx.
- 3.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a larger study with long-term follow-up. Histopathology. 2002;41:154–61. [PubMed] [Google Scholar]
- 4.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (published correction appears in J Clin Oncol, 2010; 28(21): 3543) J Clin Oncol. 2010;28(16):2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff AC, Hammond ME, Hicks DG, et al. American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 6.Jackisch C, Lammers P, Jacobs I. Evolving landscape of human epidermal growth factor receptor 2-positive breast cancer treatment and the future of biosimilars. Breast (Edinburgh, Scotland) 2017;32:199–216. doi: 10.1016/j.breast.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:1134–50. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss A, Chavez-MacGregor M, Lichtensztajn DY, et al. Validation study of the American Joint Committee on Cancer 8th edition prognostic stage compared with the anatomic stage in breast cancer. Jama Oncol. 2018;4(2):203–9. doi: 10.1001/jamaoncol.2017.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winchester DJ, Edge SB, Giuliano AE, et al. Important 8th edition changes for the AJCC breast cancer staging system. Ann Surg Oncol. 2017;24:S61. [Google Scholar]
- 10.Xu L, Li JH, Ye JM, et al. A retrospective survival analysis of anatomic and prognostic stage group based on the American Joint Committee on Cancer 8th edition cancer staging manual in luminal B human epidermal growth factor receptor 2-negative breast cancer. Chin Med J (Engl) 2017;130(16):1945–52. doi: 10.4103/0366-6999.211896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye J, Wang W, Xu L, et al. A retrospective prognostic evaluation analysis using the 8th edition of American Joint Committee on Cancer (AJCC) cancer staging system for luminal A breast cancer. Chin J Cancer Res. 2017;29(4):351–60. doi: 10.21147/j.issn.1000-9604.2017.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Chen H, Wu K, et al. Evaluation of the prognostic stage in the 8th edition of the American Joint Committee on Cancer in locally advanced breast cancer: An analysis based on SEER 18 database. Breast. 2018;37:56–63. doi: 10.1016/j.breast.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Lee SB, Shon G, Kim J, et al. A retrospective prognostic evaluation analysis using the 8th edition of the American Joint Committee on Cancer staging for breast cancer. Breast Cancer Res Treat. 2018;169(2):257–66. doi: 10.1007/s10549-018-4682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Rahman O. Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res Treat. 2018;168(1):269–75. doi: 10.1007/s10549-017-4577-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou B, Xu L, Ye J, et al. The prognostic value of the 8th edition of the American Joint Committee on Cancer (AJCC) staging system in HER2-enriched subtype breast cancer, a retrospective analysis. Anticancer Res. 2017;37(8):4615–21. doi: 10.21873/anticanres.11862. [DOI] [PubMed] [Google Scholar]
- 16.Hu H, Wei W, Yi X, et al. A retrospective analysis of clinical utility of AJCC 0 edition cancer staging system for breast cancer. World J Oncol. 2017;8(3):71–75. doi: 10.14740/wjon1039e. [DOI] [PMC free article] [PubMed] [Google Scholar]