Abstract
Context
Derangement of 11-β hydroxysteroid dehydrogenase type 1 and type 2 (11β-HSD1 and 11β-HSD2), which regulate intracellular cortisol production, has been suggested in both type 2 diabetes (T2D) and chronic kidney disease (CKD). However, activity of 11β-HSD enzymes in patients with T2D and CKD has never been assessed.
Objectives
To compare 11β-HSD activities between patients with T2D and healthy controls, and assess whether in T2D, renal function is associated with 11β-HSD activities.
Design
Cross-sectional analysis in the Diabetes and Lifestyle Cohort Twente (DIALECT-1).
Setting
Referral center for T2D.
Patients
Patient with T2D [n = 373, age 64 ± 9 years, 58% men, 26% of patients estimated glomerular filtration rate (eGFR) <60 mL/min·1.73 m2] and healthy controls (n = 275, age 53 ± 11 years, 48% men).
Mean Outcome Measure
We measured cortisol, cortisone, and metabolites [tetrahydrocortisol (THF), allo-THF (aTHF), and tetrahydrocortisone (THE)] in 24-hour urine samples. Whole body 11β-HSD and 11β-HSD2 activities were calculated as the urinary (THF + aTHF)/THE and cortisol/cortisone ratios, respectively.
Results
Patients with T2D had a higher (THF + aTHF)/THE ratio [1.02 (0.84 to 1.27) vs 0.94 (0.79 to 1.0), P < 0.001] and cortisol/cortisone ratio [0.70 (0.58 to 0.83) vs 0.63 (0.54 to 0.74), P < 0.001] than healthy controls. In T2D, lower eGFR was associated with a higher (THF + aTHF)/THE ratio (β = −0.35, P < 0.001), and a higher cortisol/cortisone ratio (β = −0.16, P = 0.001).
Conclusions
In this real-life secondary care setting of patients with T2D, 11β-HSD enzymes activities were shifted to higher intracellular cortisol production in T2D, which was further aggravated in patients with CKD. Prospective analyses are warranted to investigate causality of these associations.
Keywords: 11β-hydroxysteroid dehydrogenase, chronic kidney disease, cortisol metabolism, healthy volunteers, type 2 diabetes
We investigated 11β-HSD type 1 and 2 in patients with type 2 diabetes. Diabetes patients had higher intracellular cortisol production than healthy controls, especially if renal function was impaired.
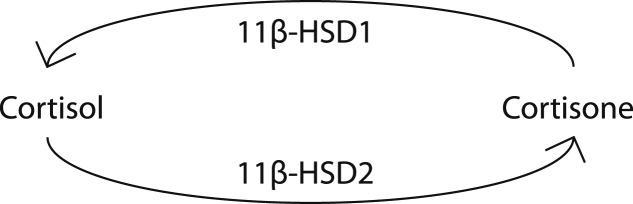
Metabolic similarities between patients with type 2 diabetes (T2D) and patients with hypercortisolism (Cushing syndrome or glucocorticoid treatment) have given rise to the hypothesis that relative hypercortisolism might occur in T2D [1]. Although overt hypercortisolism is not typical in T2D, intracellular cortisol exposure is potentially increased through upregulation of 11-β hydroxysteroid dehydrogenase type 1 (11β-HSD1), which regenerates inactive cortisone to active cortisol in the liver and in adipose tissue, or downregulation of 11-β hydroxysteroid dehydrogenase type 2 (11β-HSD2), which reduces active cortisol to inactive cortisone (Fig. 1). Sporadic studies have found signs that 11β-HSD activities are shifted toward higher intracellular cortisol production in T2D, compared with non-T2D subjects [2–4], although results are conflicting [5]. In patients with chronic kidney disease (CKD), similar shifts in 11β-HSD activities have been suggested [6, 7]. However, 11β-HSD activities in patients with T2D with renal function impairment have never been formally investigated.
Figure 1.
Effect of 11β-HSD activities on intracellular cortisol levels. 11β-HSD1 increases cortisol levels by regenerating inactive cortisone to active cortisol. 11β-HSD2 decreases cortisol levels by reducing active cortisol to inactive cortisone.
Interest in 11β-HSD pathways has recently been refueled after the development of several compounds that inhibit 11β-HSD1. Phase II preclinical trials with such agents have shown improved glycemic control, lipid profile, and blood pressure, and even demonstrated modest weight loss [8]. However, effects on each separate component of the metabolic syndrome were relatively small. If 11β-HSD1 activity is highest in T2D with renal function impairment, this could mean that pharmacological 11β-HSD1 inhibition could be a promising treatment option specifically for these patients.
In the current study, we therefore quantified total urinary excretion of cortisol, cortisone, and their metabolites [tetrahydrocortisol (THF), allo-THF (aTHF), tetrahydrocortisone (THE)] with the aim of estimating (1) whether 11β-HSD activities differ between patients with T2D and healthy controls, and (2) to investigate whether there is an association between estimated glomerular filtration rate (eGFR) and 11β-HSD activities in patients with T2D.
1. Subjects and Methods
We performed a cross-sectional analysis in baseline data from the Diabetes and Lifestyle Cohort Twente-1 (DIALECT-1). The study design was described in detail elsewhere [9]. The study was approved by the local institutional review board (METC-Twente, registration number: NL57219.044.16) and the institutional review board in the University Medical Centre Groningen (METC-Groningen registration number: 1009.68020), and is registered in the Netherlands Trial Register (NTR trial code 5855). The study was performed according to the guidelines of good clinical practice and the Declaration of Helsinki.
A. Participants
All patients with T2D treated in the outpatient clinic of our hospital, aged 18+ years, were eligible for the study. Exclusion criteria were inability to understand the informed consent procedure, insufficient command of the Dutch language, or dialysis dependency. As a control group reflecting the general population, we included 275 healthy subjects who participated in a screening program before kidney donation in the University Medical Centre Groningen. None of the healthy controls had a history of diabetes, cardiovascular events, or kidney disease. Hypertension, if present, was treated with a maximum of one class of antihypertensive drugs.
B. Study Procedures
Eligible patients were selected from the electronic patient file. At the clinic, sociodemographic characteristics, medical history, lifestyle behaviors, and current medications were recorded. Height, weight, waist, and hip circumference were measured. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2), and body surface area was estimated by applying the universally adopted formula of DuBois. Blood pressure was measured in a supine position by an automated device (Dinamap®; GE Medical Systems, Milwaukee, WI) for 15 minutes with a 1-minute interval. The mean systolic and diastolic pressure of the final three measurements was used for further analysis.
Blood was drawn from venepuncture for routine laboratory measurements. Serum concentration of C-reactive protein was measured routinely using immunoassay. From a 24-hour urine collection the following parameters are measured: sodium, potassium, creatinine, calcium, phosphate, and uric acid excretion. For the proper collection of the 24-hour urine sample, patients were instructed to dispose the first morning void urine, and thereafter collect all urine in the provided canister until the first morning void urine of the next day. In between voids, they were instructed to store the canister in a dark cool place, preferably in a refrigerator. Samples of blood and 24-hour urine were stored for later analysis.
C. Cortisol Measurements
Urinary cortisol, cortisone, THF, aTHF, and THE concentrations in 24-hour urine samples were measured using a validated high-performance liquid chromatography tandem mass spectrometry assay as previously described [10]. For all components, stable isotope labeled internal standards were added and the mixtures were incubated with an enzyme solution consisting of sulfatases and β-glucuronidases (Suc d’Helix Pomatia, Pall Biopharmaceuticals, Port Washington, NY), to ensure hydrolysis of cortisol and the metabolites from their sulfated and glucuronidated forms. In contrast to the more generally applied urinary free cortisol measurement, this method measures total cortisol and its metabolites. Subsequently, the analytes were extracted using a Supported Liquid Extraction technique. Finally, separation and detection were performed by use of a CSH Phenyl-Hexyl column (particle size 1.7 μm, 2.1 mm internal diameter by 100 mm; Waters, Milford, MA) and a XEVO TQ-s® tandem mass spectrometer operated in negative electrospray ionization mode (Waters), respectively. Intra- and interassay variation coefficients were <5.7% and <9.8%, respectively. Prednisone and prednisolone were chromatographically separated from cortisol and its metabolites and therefore did not interfere. Total 24-hour urinary excretions were calculated by multiplying the concentrations by 24-hour urinary volume.
The urinary ratios of (THF + aTHF)/THE and cortisol/cortisone are widely used to assess enzyme activity of 11β-HSD1 and 11β-HSD2. The urinary cortisol/cortisone ratio is considered to reflect activity of 11β-HSD2, whereas the urinary (THF + aTHF)/THE ratio is considered as an overall measure of whole body 11β-HSD activity [11–13].
D. Data Analysis and Statistics
Statistical analyses were performed using Statistical Package for the Social Sciences (IBM, Chicago, IL), version 22.0. Normality of data was assessed by visually inspecting the frequency histograms. Normally distributed data are shown as mean ± SD, skewed data are shown as median (interquartile range), and nominal data as number of patients (percentage). Differences between patients with T2D and healthy controls were tested using linear regression analyses, unadjusted and while adjusting for potential confounders such as gender, age, and BMI.
Using R software, the univariate associations between eGFR and the (THF + aTHF)/THE and cortisol/cortisone ratios were assessed with generalized additive models (mgcv package; The R-Foundation for Statistical Computing, Vienna, Austria) as described previously [14]. The model effect and nonlinearity were tested with the use of two-sided Wald tests. P-nonlinearity values were calculated by comparing restricted cubic spline terms to linear models.
To identify possible confounders, we determined the associations between clinical parameters and the (THF +aTHF)/THE and cortisol/cortisone ratios using linear regression analyses. Then we performed multivariate linear regression to determine the association between eGFR and the (THF + aTHF)/THE and cortisol/cortisone ratios, while adjusting for common confounders and for parameters with a P < 0.15 in univariate analysis. To test for gender differences, we also performed the analyses for men and women separately.
2. Results
Data on urinary cortisol excretion were available in 373 patients of DIALECT-1 and in 275 healthy controls (Table 1). In patients with T2D, the mean age was 64 ± 9 years, the majority were men (58%), and mean BMI was 32.8 ± 6.0 kg/m2. The median diabetes duration was 11 (7 to 18) years, metformin was used by 74% of patients (n = 277), and 67% (n = 250) of patients were on insulin. The majority of patients with T2D had one or more microvascular complications (70%), with nephropathy being the most prevalent (49%), and macrovascular complications were present in 39% of patients. Impaired renal function (eGFR < 60 mL/min·1.73 m2) was present in 96 (26%) patients. In the healthy controls, there were fewer men (48%; P = 0.008), participants were younger (53 ± 11; P < 0.001), had a lower BMI (25.9 ± 3.5; P < 0.001), and a higher eGFR (91 ± 24 vs 78 ± 24 mL/min·1.73 m2; P < 0.001), as compared with patients with T2D.
Table 1.
Patients Characteristics and Urinary Excretion of Cortisol Metabolites
|
|
Healthy Controls |
T2D |
Standardized β |
||
|---|---|---|---|---|---|
| Patient Characteristics | n = 275 | n = 373 | Model 1 | Model 2 | Model 3 |
| Men, n (%) | 132 (48) | 215 (58) | −0.10** | ||
| Age, y | 53 ± 11 | 64 ± 9 | 0.43*** | ||
| BMI, kg/m2 | 25.9 ± 3.5 | 32.8 ± 6.0 | 0.53*** | 0.58*** | |
| Systolic blood pressure, mm Hg | 125 ± 14 | 136 ± 16 | 0.33*** | 0.26*** | 0.22*** |
| Diastolic blood pressure, mm Hg | 76 ± 9 | 74 ± 10 | 0.06 | −0.02 | 0.002 |
| HbA1c, mmol/mol | 38 ± 4 | 57 ± 12 | 0.71*** | 0.70*** | 0.70*** |
| Nonfasting glucose, mmol/L | 5.4 ± 0.7 | 9.5 ± 3.4 | 0.60*** | 0.58*** | 0.58*** |
| Total cholesterol, mmol/L | 5.4 ± 1.1 | 4.0 ± 0.9 | −0.58*** | −0.60*** | −0.59*** |
| LDL cholesterol, mmol/L | 2.0 ± 0.7 | ||||
| Triglycerides, mmol/L | 1.4 ± 0.8 | 1.9 ± 1.2 | 0.24*** | 0.26*** | 0.17** |
| HDL cholesterol, mmol/L | 1.1 ± 0.3 | ||||
| eGFR, mL/min·1.73 m2 | 91 ± 14 | 78 ± 24 | −0.29*** | −0.05 | −0.009 |
| eGFR <60 mL/min·1.73 m2, n (%) | 4 (1%) | 96 (26) | |||
| eGFR <30 mL/min·1.73 m2, n (%) | 0 (0) | 14 (3) | |||
| Increased albuminuria, n (%) | 15 (6) | 136 (31) | 0.30*** | 0.25*** | 0.24*** |
| Microvascular disease, n (%) | NA | 260 (70) | |||
| Macrovascular disease, n (%) | NA | 144 (39) | |||
| Metformin use, n (%) | NA | 277 (74) | |||
| Sulfonylurea use, n (%) | NA | 87 (23) | |||
| Insulin use, n (%) | NA | 250 (67) | |||
| Urinary excretion of cortisol metabolites | |||||
| Urinary cortisol excretion, nmol/24 h | 332 (244–445) | 274 (204–400) | −0.15*** | −0.15** | −0.16** |
| Urinary cortisone excretion, nmol/24 h | 526 (418–648) | 408 (308–549) | −0.25*** | −0.24*** | −0.24*** |
| Urinary THF excretion, µmol/24 h | 6.9 (5.1–9.3) | 7.0 (5.3–9.5) | 0.02 | −0.005 | −0.11* |
| Urinary LN aTHF excretion, µmol/24 h | 4.2 (2.6–6.5) | 4.8 (2.7–7.5) | 0.07 | 0.05 | −0.07 |
| Urinary THE excretion, µmol/24 h | 12.5 (8.5–16.8) | 11.5 (8.1–15.3) | −0.06 | −0.03 | −0.16** |
| Summated urinary cortisol and metabolites excretion, µmol/24 h | 24.6 (17.4–33.7) | 24.3 (18.5–32.3) | −0.02 | −0.01 | −0.15** |
| (THF+aTHF)/THE, µmol/µmol | 0.94 (0.79–1.0) | 1.02 (0.84–1.27) | 0.19*** | 0.12** | 0.11* |
| Cortisol/cortisone, nmol/nmol | 0.63 (0.54–0.74) | 0.70 (0.58–0.83) | 0.15*** | 0.14** | 0.14* |
Differences between groups were tested via univariable and multivariable linear regression analyses of which standardized βs are presented (*P < 0.05, **P < 0.01, ***P < 0.001). Model 1 is a crude model. Model 2 was adjusted for age and gender. Model 3 was adjusted as for Model 2 and for BMI.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
A. Total 24-Hour Urinary Excretion of Cortisol and Cortisol Metabolites in Patients With T2D and Healthy Controls
Urinary excretion of cortisol and its metabolites in patients with T2D and healthy controls is demonstrated in Table 1. The median urinary excretion of cortisol and cortisone was lower in T2D than in healthy controls [cortisol 274 (204 to 400) vs 332 (244 to 445) nmol/24 hours; P < 0.001; cortisone 408 (308 to 549) vs 526 (418 to 648) nmol/24 hours; P < 0.001]. However, the ratio of cortisol/cortisone was higher in patients with T2D [1.02 (0.84 to 1.27) vs 0.94 (0.79 to 1.00), P < 0.001], also when adjusting for age, gender, and BMI. In addition, there was no difference in urinary excretion of THF, aTHF, THE, and summated cortisol and metabolites between patients with T2D and healthy controls. The (THF + aTHF)/THE ratio, however, was again higher in T2D [0.70 (0.58 to 0.83) vs 0.63 (0.54 to 0.74); P < 0.001], also after adjustment for confounders. Differences in both ratios between patients with T2D and healthy controls were similar in men and women (data not shown).
B. Associations Between eGFR and the (THF+aTHF)/THE Ratio and Cortisol/Cortisone Ratios in T2D
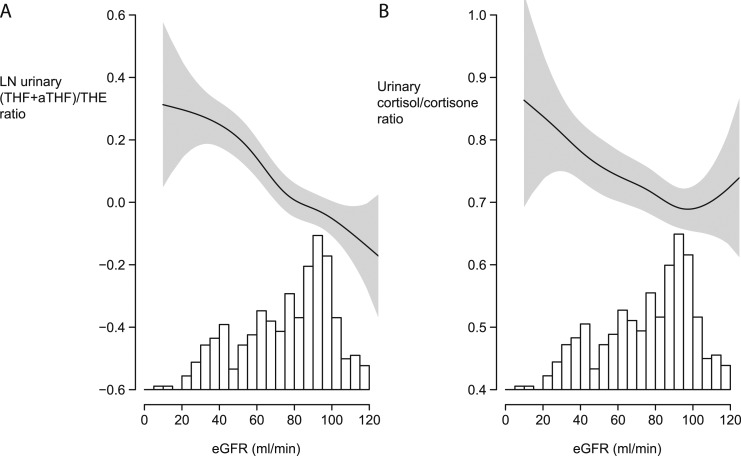
In patients with T2D, there was an inverse linear association between eGFR and the log transformed (THF + aTHF)/THE ratio (β = −0.35, P < 0.001, P nonlinearity = 0.14; Fig. 2A). In addition, eGFR was also inversely associated with the cortisol/cortisone ratio (β = −0.16, P = 0.001, P nonlinearity = 0.27; Fig. 2B).
Figure 2.
Continuous associations of eGFR with (A) the log transformed urinary (THF+aTHF)/THE and (B) the cortisol/cortisone ratios in patients with T2D (n = 373). Continuous associations were modeled via generalized additive models. Shaded areas represent the corresponding 95% CIs. The histograms illustrate distributions of eGFR in patients with T2D. For the association between eGFR and natural logarithm (THF+aTHF)/THE, P-nonlinearity was 0.14, and the β was −0.35 (P < 0.001). In case of the urinary cortisol/cortisone ratio, the P-nonlinearity was 0.27, and the β was −0.16 (P = 0.001).
As secondary analyses, we also investigated the associations of the (THF + aTHF)/THE and the cortisol/cortisone ratios with clinical characteristics (Table 2). We found that the (THF + aTHF)/THE ratio was associated with gender (β = −0.14, P = 0.006), age (β = 0.16, P = 0.002), systolic blood pressure (β = −0.13, P = 0.01), diastolic blood pressure (β = −0.14, P = 0.008), presence of coronary heart disease (β = 0.16, P = 0.002), β blocker use (β = 0.14, P = 0.005), loop diuretic use (β = 0.13, P = 0.01), and plasma LDL cholesterol (β = −0.15, P = 0.006). Insulin use was not significantly associated with the (THF + aTHF)/THE ratio, and in insulin users there was no association between cumulative daily insulin dosage and the (THF + aTHF)/THE ratio. Of note, adjustment for factors associated with the (THF+aTHF)/THE ratio did not markedly influence the association between eGFR and (THF + aTHF)/THE (fully adjusted model: β = −0.37, P < 0.001; Table 3). The association between eGFR and the (THF + aTHF)/THE ratio was similar for men and women (data not shown).
Table 2.
Unadjusted Associations Between Clinical Parameters and the (THF + aTHF)/THE and Cortisol/Cortisone Ratios in Patients With T2D
| LN (THF + aTHF)/THE |
Cortisol/Cortisone |
|||
|---|---|---|---|---|
| n = 374 | Stand β | P Value | Stand β | P Value |
| Patient characteristics | ||||
| Women | −0.14 | 0.006 | −0.01 | 0.83 |
| Age, y | 0.16 | 0.002 | 0.09 | 0.08 |
| Duration of diabetes, y | 0.07 | 0.20 | 0.02 | 0.64 |
| BMI, kg/m2 | <0.01 | 0.98 | 0.04 | 0.43 |
| Current smoker | −0.02 | 0.66 | 0.10 | 0.06 |
| Alcohol use (yes/no) | −0.02 | 0.73 | −0.02 | 0.70 |
| Systolic blood pressure, mm Hg | −0.13 | 0.01 | 0.03 | 0.62 |
| Diastolic blood pressure, mm Hg | −0.14 | 0.008 | 0.00 | 0.95 |
| Heart frequency, beats/min | −0.08 | 0.13 | 0.10 | 0.06 |
| Comorbidity | ||||
| Microvascular disease | 0.17 | 0.001 | 0.10 | 0.05 |
| Retinopathy | 0.03 | 0.58 | 0.02 | 0.74 |
| Neuropathy | 0.05 | 0.39 | 0.01 | 0.79 |
| Nephropathy | 0.12 | 0.02 | 0.10 | 0.07 |
| Macrovascular disease | 0.17 | 0.001 | 0.08 | 0.11 |
| Coronary heart disease | 0.16 | 0.002 | 0.00 | 0.96 |
| Cerebrovascular disease | 0.10 | 0.06 | 0.17 | 0.001 |
| Peripheral artery disease | 0.06 | 0.25 | 0.02 | 0.73 |
| Pharmacological treatment | ||||
| RAAS inhibition | <0.001 | 0.99 | −0.02 | 0.72 |
| β-blocker | 0.14 | 0.005 | 0.02 | 0.67 |
| Calcium antagonist | 0.05 | 0.30 | 0.02 | 0.69 |
| Thiazide diuretics | 0.02 | 0.73 | −0.05 | 0.36 |
| Loop diuretics | 0.13 | 0.01 | 0.10 | 0.05 |
| Potassium saving diuretics | 0.11 | 0.04 | 0.02 | 0.78 |
| Metformin | −0.08 | 0.13 | 0.02 | 0.77 |
| Insulin | 0.03 | 0.51 | −0.08 | 0.15 |
| Cumulative insulin dosage, units/d | −0.09 | 0.21 | −0.002 | 0.98 |
| Serum values | ||||
| Serum HbA1c, mmol/mol | −0.10 | 0.06 | 0.05 | 0.36 |
| Plasma total cholesterol, mmol/L | −0.15 | 0.004 | −0.02 | 0.75 |
| Plasma LDL cholesterol, mmol/L | −0.15 | 0.006 | 0.04 | 0.48 |
| Plasma HDL cholesterol | −0.06 | 0.24 | −0.07 | 0.20 |
| Plasma aldosterone concentration, pg/mL | 0.08 | 0.17 | 0.07 | 0.22 |
| LN serum CRP, mg/L | 0.09 | 0.09 | 0.23 | <0.001 |
| Urinary excretion | ||||
| LN Urinary albumin excretion, mg/24 h | 0.07 | 0.22 | 0.05 | 0.39 |
| Urinary Cortisol/cortison, nmol/nmol | 0.20 | <0.001 | ||
| LN (THF + aTHF)/THE, µmol/µmol | 0.20 | <0.001 | ||
| Urinary aldosterone excretion, µg/24 h | 0.02 | 0.72 | −0.04 | 0.45 |
Associations were tested using univariate linear regression of which standardized βs and P values are presented.
Abbreviations: CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LN, natural logarithm.
Table 3.
Association Between eGFR and the Urinary (THF+aTHF)/THE Ratio in Patients With T2D After Adjustments for Possible Confounders
| LN (THF + aTHF)/THE | ||
|---|---|---|
| Stand β | P Value | |
| Model 1, eGFR, mL/min·1.73 m2 | −0.36 | <0.001 |
| Model 2, eGFR, mL/min·1.73 m2 | −0.39 | <0.001 |
| Model 3, eGFR, mL/min·1.73 m2 | −0.38 | <0.001 |
| Model 4, eGFR, mL/min·1.73 m2 | −0.38 | <0.001 |
| Model 5, eGFR, mL/min·1.73 m2 | −0.37 | <0.001 |
| Model 6, eGFR, mL/min·1.73 m2 | −0.37 | <0.001 |
Associations were tested using multivariate linear regression of which standardized βs and P values are presented. Model 1 was a crude model. Model 2 was adjusted for age and gender. Model 3 was adjusted for Model 2 + coronary artery disease (no/yes), and cerebrovascular disease (no/yes). Model 4 was adjusted for Model 3 + BMI (kg/m2), alcohol intake (none/any), and current smoking (no/yes). Model 5 was adjusted for Model 4 + systolic blood pressure (mm Hg), diastolic blood pressure (mm Hg), heart frequency (beats/min), and LDL cholesterol (mmol/L). Model 6 was adjusted for Model 5 + β blocker use (no/yes), loop diuretic use (no/yes), potassium saving diuretic use (no/yes), and metformin use (no/yes).
Abbreviation: LN, natural logarithm.
In parallel, the cortisol/cortisone ratio was associated with the presence of cerebrovascular disease (β = 0.17, P = 0.001), loop diuretic use (β = 0.10, P = 0.05), and LN serum C-reactive protein (β = 0.23, P < 0.001). Plasma aldosterone concentration and 24-hour urinary aldosterone excretion were not associated with the cortisol/cortisone ratio. Again, the association between eGFR and the cortisol/cortisone ratio was unaltered by adjustment for possible confounders (fully adjusted model: β = −0.14, P = 0.03; Table 4). Additionally, the β of the association between eGFR and the cortisol/cortisone ratio was similar in men and women, although the association only statistically significant in men (fully adjusted models: β = −0.17, P = 0.05 for men, β = −0.15, P = 0.14 for women).
Table 4.
Adjusted Associations Between Clinical Parameters and the Urinary Cortisol/Cortisone Ratio in Patients With T2D
| Cortisol/cortisone | ||
|---|---|---|
| Stand β | P Value | |
| Model 1, eGFR, mL/min·1.73 m2 | −0.17 | 0.001 |
| Model 2, eGFR, mL/min·1.73 m2 | −0.17 | 0.007 |
| Model 3, eGFR, mL/min·1.73 m2 | −0.14 | 0.02 |
| Model 4, eGFR, mL/min·1.73 m2 | −0.14 | 0.03 |
| Model 5, eGFR, mL/min·1.73 m2 | −0.16 | 0.01 |
| Model 6, eGFR, mL/min·1.73 m2 | −0.14 | 0.03 |
Associations were tested using multivariate linear regression of which standardized βs and P values are presented. Model 1 was a crude model. Model 2 was adjusted for age and gender. Model 3 was adjusted for Model 2 + cerebrovascular disease (no/yes). Model 4 was adjusted for Model 3 + BMI (kg/m2), alcohol intake (none/any), and current smoking (no/yes). Model 5 was adjusted for Model 4 + heart frequency (beats/min). Model 6 was adjusted for Model 5 + loop diuretic use (no/yes) and insulin use (no/yes).
3. Discussion
To our knowledge this is the first study on 11β-HSD activities in patients with T2D with a large subgroup of patients with renal function impairment. We found that both the urinary (THF + aTHF)/THE and cortisol/cortisone ratios were higher in patients with T2D as compared with healthy controls. Additionally, lower renal function was associated with higher urinary (THF + aTHF)/THE and cortisol/cortisone ratios in patients with T2D. These findings suggest that in T2D, intracellular cortisol exposure is increased, and that in diabetic kidney disease, there is even further derangement of 11β-HSD activity toward intracellular cortisol production.
We measured urinary excretion of cortisol and its metabolites using high-performance liquid chromatography tandem mass spectrometry, after hydrolysis from their sulfated and glucuronidated forms. Because in other literature the hydrolysis step usually is not performed, we report a higher excretion of cortisol and its metabolites, and therefore direct comparison of our data with previous literature is not possible.
In line with our findings, prior observations in small groups of patients with T2D reported a shift toward higher intracellular cortisol production in patients with T2D as compared with healthy controls [2, 3]. Studies assessing solely 11β-HSD1 activity, by measuring the conversion of labeled cortisol to cortisone, reported higher activity of 11β-HSD1 in patients with T2D than in overweight/obese controls without T2D [2, 3]. On the other hand, Valsamakis et al. [5] found no statistically significant difference in the urinary (THF + aTHF)/THE and cortisol/cortisone ratios between patients with T2D and controls. It should be noted that in the latter study, patients were in an earlier disease stage than in our study; patients were younger, there were no insulin users, and patients with renal function impairment were excluded, whereas in our study, the median diabetes duration was 11 (7 to 18) years, and approximately two-thirds of patients used insulin. Lavery et al. [4] previously reported an increased frequency of short alleles of the 11β-HSD2 gene in T1D, suggesting reduced activity of 11β-HSD2 in diabetes, however, without measurement of in vivo 11β-HSD2 activity. Our larger study adds to these findings by illustrating that in established T2D in a real-life setting, both total body 11β-HSD activity and 11β-HSD2 activity are shifted toward higher intracellular cortisol exposure in T2D, independent of age, gender, and BMI.
Although the mechanisms behind derangement of 11β-HSD activities in T2D is unknown, Anderson et al. [15] previously described that metformin may increase whole body 11β-HSD1 activity, potentially diminishing other metabolic effects of metformin. Here we found a nonstatistically significant trend toward lower 11β-HSD1 activity in patients on metformin. However, it should be noted our population represents patients with long-standing T2D [11 (7 to 18) years], complicating direct comparison of results. Prospective research is needed to further clarify the effects of metformin on 11β-HSD1 activity.
In patients with T2D, we found that the urinary (THF + aTHF)/THE and cortisol/cortisone ratios are inversely associated with eGFR. To our knowledge, the association between 11β-HSD activity and eGFR in T2D has not been reported previously. Our finding is supported by a previous study by Quinkler et al. [6], which demonstrated an inverse association between creatinine clearance and the urinary (THF + aTHF)/THE and cortisol/cortisone ratios in nondiabetic patients with renal function impairment. In line, Whitworth et al. [16] reported an association between higher plasma creatinine and lower plasma cortisone levels in nondiabetic patients with CKD, indicative of an association between lower eGFR and lower 11β-HSD2 activity. The mechanism underlying the association between lower renal function and altered 11β-HSD activities is unknown. In (diabetic) CKD, there is neuroendocrinological derangement, with increased sympathetic activity, illustrated by higher inflammatory markers, and higher cortisol and aldosterone levels in CKD [17–20]. Possibly, in CKD, alteration of 11β-HSD activities is part of this neuroendocrinological derangement. Indeed, we found that a trend between higher C-reactive protein and altered 11β-HSD activities, which is in line with previous research on 11β-HSD activities in inflamed tissues [21, 22]. However, as of now it is unknown whether a higher degree of inflammation leads to 11β-HSD dysregulation or vice versa. In case of the latter, altered 11β-HSD activities could be associated with inflammation-related complications commonly seen in CKD, such as insulin resistance, dyslipidemia, and hypertension [23–25]. Additionally, altered cortisol handling may have consequences for clinical outcomes in CKD, previously, Himmelfarb et al. [26] demonstrated that higher predialysis serum cortisol levels were associated with higher rates of hospitalization and malnutrition. It should be noted that in our cohort there were few patients with end-stage renal failure (eGFR < 15 mL/min·1.73 m2). It would be interesting to investigate whether the association between eGFR and 11β-HSD activities remains linear if the population would be expanded with patients with more severe impairment of renal function and patients approaching end-stage renal failure.
Moreover, 11β-HSD2 inactivates cortisol in the intracellular space, thus avoiding cortisol mediated mineralocorticoid receptor (MR) activation. Lower 11β-HSD2 activity in those with renal function impairment suggests increased MR activation by cortisol. MR activation has been associated with a plethora of detrimental effects on target organs, such as the kidneys, the heart, and the vasculature, which can be blocked by MR antagonism [27–34]. Therefore, lower 11β-HSD2 activity in T2D could play an important role in the development and course of diabetic nephropathy. However, it should be noted that in the current study we found no association between 11β-HSD2 and markers of MR activation such as plasma aldosterone concentration, urinary aldosterone excretion, blood pressure, and hypokalemia, although such relations might well be disturbed by unstandardized sodium intake and frequent use of antihypertensives interfering in the renin-angiotensin-aldosterone system.
Our study has several strengths. First, this is the largest study to date on 11β-HSD activities in patients with T2D and renal function impairment. Because previous assays for measuring cortisol metabolites were difficult to perform in a large group of patients, little data are available on the epidemiology of 11β-HSD activities. Second, the broad inclusion criteria of DIALECT-1 allow us to study a group of real-world patients with T2D treated in secondary care, with minimal inclusion bias. The primary limitation of the study is the cross-sectional design, which does not allow conclusions on causality. Therefore, the results of our study should be seen as predominantly hypothesis generating. In-depth prospective studies are necessary to validate our findings. Furthermore, it should be noted the urinary (THF + aTHF)/THE ratio is an indirect marker of whole body 11β-HSD enzyme activity, and therefore should be interpreted with caution, because 11β-HSD activity might differ between different tissues. Additionally, the (THF + aTHF)/THE ratio can only be used to interpret 11β-HSD1 activity if the urinary cortisol/cortisone ratios are unaltered. Therefore, additional studies are necessary to assess whether in T2D and CKD 11β-HSD1 activity specifically is altered similarly as 11β-HSD2 activity. Also, due to the fact that blood was taken in a nonfasting state, data on fasting glucose and homeostatic model assessment for insulin resistance were unavailable in DIALECT-1. Therefore, the association between 11β-HSD activities and insulin resistance could not be assessed directly. Lastly, previously it has been demonstrated that higher urinary cortisol excretion is associated with oxidative stress [35]. It would be interesting to investigate whether the 11β-HSD derangement in T2D and CKD we report here is also associated with markers of oxidative damage; however, data on markers of oxidative stress were not available in this study.
Our findings have several potential clinical implications. The shift toward higher intracellular cortisol production by 11β-HSD enzymes in T2D with renal function impairment could indicate that 11β-HSD1 inhibitors might have an increased beneficial effect in patients with diabetic nephropathy. Additionally, the lower 11β-HSD2 activity in T2D and renal function impairment might have implications for treatment with MR antagonists, especially in diabetic nephropathy. Future prospective studies are necessary to validate our findings, and assess the association between 11β-HSDs derangements and clinical adverse outcomes. Additionally, future studies should be performed to evaluate whether alterations between 11β-HSD in T2D patients with CKD are similar to CKD patients without T2D.
4. Conclusion
This is the largest study to date on 11β-HSD activities in a real-life secondary care setting of patients with T2D. We found that activity of 11β-HSDs is shifted toward higher intracellular cortisol production in T2D, and especially in those with T2D and renal function impairment. This could have important implications for the use of both 11β-HSD1 inhibitors and MR antagonists in T2D and renal function impairment.
Acknowledgments
We thank Else van den Berg, Willeke van Kampen, Sanne van Huizen, Anne Davina, Manon Harmelink, and Jolien Jaspers for their contribution to patient inclusion.
Clinical Trial Information: Netherland trial register code 5855 (registered 19 April 2016).
Author Contributions: G.N., S.J.L.B., and G.D.L. designed the study. C.M.G and S.H.B. included the patients. I.M. performed the cortisol measurements. I.P.K. contributed materials. C.M.G. performed the analyses and wrote the paper. I.M., L.dV, A.P.vB, G.N., S.J.L.B., and G.D.L. reviewed the paper.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 11β-HSD
11-β hydroxysteroid dehydrogenase
- aTHF
allo-tetrahydrocortisol
- BMI
body mass index
- CKD
chronic kidney disease
- DIALECT-1
Diabetes and Lifestyle Cohort Twente
- eGFR
estimated glomerular filtration rate
- MR
mineralocorticoid receptor
- T2D
type 2 diabetes
- THE
tetrahydrocortisone
- THF
tetrahydrocortisol
References and Notes
- 1. Tirabassi G, Boscaro M, Arnaldi G. Harmful effects of functional hypercortisolism: a working hypothesis. Endocrine. 2014;46(3):370–386. [DOI] [PubMed] [Google Scholar]
- 2. Dube S, Norby BJ, Pattan V, Carter RE, Basu A, Basu R. 11β-hydroxysteroid dehydrogenase types 1 and 2 activity in subcutaneous adipose tissue in humans: implications in obesity and diabetes. J Clin Endocrinol Metab. 2015;100(1):E70–E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stimson RH, Andrew R, McAvoy NC, Tripathi D, Hayes PC, Walker BR. Increased whole-body and sustained liver cortisol regeneration by 11beta-hydroxysteroid dehydrogenase type 1 in obese men with type 2 diabetes provides a target for enzyme inhibition. Diabetes. 2011;60(3):720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lavery GG, McTernan CL, Bain SC, Chowdhury TA, Hewison M, Stewart PM. Association studies between the HSD11B2 gene (encoding human 11beta-hydroxysteroid dehydrogenase type 2), type 1 diabetes mellitus and diabetic nephropathy. Eur J Endocrinol. 2002;146(4):553–558. [DOI] [PubMed] [Google Scholar]
- 5. Valsamakis G, Anwar A, Tomlinson JW, Shackleton CH, McTernan PG, Chetty R, Wood PJ, Banerjee AK, Holder G, Barnett AH, Stewart PM, Kumar S. 11beta-hydroxysteroid dehydrogenase type 1 activity in lean and obese males with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89(9):4755–4761. [DOI] [PubMed] [Google Scholar]
- 6. Quinkler M, Zehnder D, Lepenies J, Petrelli MD, Moore JS, Hughes SV, Cockwell P, Hewison M, Stewart PM. Expression of renal 11beta-hydroxysteroid dehydrogenase type 2 is decreased in patients with impaired renal function. Eur J Endocrinol. 2005;153(2):291–299. [DOI] [PubMed] [Google Scholar]
- 7. Mongia A, Vecker R, George M, Pandey A, Tawadrous H, Schoeneman M, Muneyyirci-Delale O, Nacharaju V, Ten S, Bhangoo A. Role of 11βHSD type 2 enzyme activity in essential hypertension and children with chronic kidney disease (CKD). J Clin Endocrinol Metab. 2012;97(10):3622–3629. [DOI] [PubMed] [Google Scholar]
- 8. Anderson A, Walker BR. 11β-HSD1 inhibitors for the treatment of type 2 diabetes and cardiovascular disease. Drugs. 2013;73(13):1385–1393. [DOI] [PubMed] [Google Scholar]
- 9. Gant CM, Binnenmars SH, Berg EVD, Bakker SJL, Navis G, Laverman GD. Integrated assessment of pharmacological and nutritional cardiovascular risk management: blood pressure control in the Diabetes and Lifestyle Cohort Twente (DIALECT). Nutrients. 2017;9(7):E709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cuzzola A, Mazzini F, Petri A. A comprehensive study for the validation of a LC-MS/MS method for the determination of free and total forms of urinary cortisol and its metabolites. J Pharm Biomed Anal. 2014;94:203–209. [DOI] [PubMed] [Google Scholar]
- 11. Tomlinson JW, Stewart PM. Cortisol metabolism and the role of 11beta-hydroxysteroid dehydrogenase. Best Pract Res Clin Endocrinol Metab. 2001;15(1):61–78. [DOI] [PubMed] [Google Scholar]
- 12. Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25(5):831–866. [DOI] [PubMed] [Google Scholar]
- 13. Chapman K, Holmes M, Seckl J. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93(3):1139–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minović I, van der Veen A, van Faassen M, Riphagen IJ, van den Berg E, van der Ley C, Gomes-Neto AW, Geleijnse JM, Eggersdorfer M, Navis GJ, Kema IP, Bakker SJ. Functional vitamin B-6 status and long-term mortality in renal transplant recipients. Am J Clin Nutr. 2017;106(6):1366–1374. [DOI] [PubMed] [Google Scholar]
- 15. Anderson AJ, Andrew R, Homer NZ, Jones GC, Smith K, Livingstone DE, Walker BR, Stimson RH. Metformin increases cortisol regeneration by 11βHSD1 in obese men with and without type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101(10):3787–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitworth JA, Stewart PM, Burt D, Atherden SM, Edwards CR. The kidney is the major site of cortisone production in man. Clin Endocrinol (Oxf). 1989;31(3):355–361. [DOI] [PubMed] [Google Scholar]
- 17. Meuwese CL, Carrero JJ. Chronic kidney disease and hypothalamic-pituitary axis dysfunction: the chicken or the egg? Arch Med Res. 2013;44(8):591–600. [DOI] [PubMed] [Google Scholar]
- 18. Asao T, Oki K, Yoneda M, Tanaka J, Kohno N. Hypothalamic-pituitary-adrenal axis activity is associated with the prevalence of chronic kidney disease in diabetic patients. Endocr J. 2016;63(2):119–126. [DOI] [PubMed] [Google Scholar]
- 19. Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Parati G, Rossignol P, Wiecek A, London G; European Renal and Cardiovascular Medicine (EURECA-m) Working Group of the European Renal Association – European Dialysis Transplantation Association (ERA-EDTA) . The systemic nature of CKD. Nat Rev Nephrol. 2017;13(6):344–358. [DOI] [PubMed] [Google Scholar]
- 20. Gant CM, Laverman GD, Vogt L, Slagman MCJ, Heerspink HJL, Waanders F, Hemmelder MH, Navis G; Holland Nephrology Study (HONEST) Network . Renoprotective RAAS inhibition does not affect the association between worse renal function and higher plasma aldosterone levels. BMC Nephrol. 2017;18(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooper MS, Bujalska I, Rabbitt E, Walker EA, Bland R, Sheppard MC, Hewison M, Stewart PM. Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res. 2001;16(6):1037–1044. [DOI] [PubMed] [Google Scholar]
- 22. Stegk JP, Ebert B, Martin HJ, Maser E. Expression profiles of human 11beta-hydroxysteroid dehydrogenases type 1 and type 2 in inflammatory bowel diseases. Mol Cell Endocrinol. 2009;301(1-2):104–108. [DOI] [PubMed] [Google Scholar]
- 23. Chan DT, Watts GF, Irish AB, Dogra GK. Insulin resistance and vascular dysfunction in chronic kidney disease: mechanisms and therapeutic interventions. Nephrol Dial Transplant. 2017;32(8):1274–1281. [DOI] [PubMed] [Google Scholar]
- 24. Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. 2010;267(6):543–560. [DOI] [PubMed] [Google Scholar]
- 25. Chapagain A, Caton PW, Kieswich J, Andrikopoulos P, Nayuni N, Long JH, Harwood SM, Webster SP, Raftery MJ, Thiemermann C, Walker BR, Seckl JR, Corder R, Yaqoob MM. Elevated hepatic 11β-hydroxysteroid dehydrogenase type 1 induces insulin resistance in uremia. Proc Natl Acad Sci USA. 2014;111(10):3817–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Himmelfarb J, Holbrook D, McMonagle E, Robinson R, Nye L, Spratt D. Kt/V, nutritional parameters, serum cortisol, and insulin growth factor-1 levels and patient outcome in hemodialysis. Am J Kidney Dis. 1994;24(3):473–479. [DOI] [PubMed] [Google Scholar]
- 27. Gant CM, Laverman GD, Navis GJ. MRA inhibition in CKD: more than salt and water In: Goldsmith D, Covic A, Spaak J, eds. Cardio-Renal Clinical Challenges. Cham, Switzerland: Springer International Publishing; 2015:41–50. [Google Scholar]
- 28. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6(5):261–273. [DOI] [PubMed] [Google Scholar]
- 30. Mavrakanas TA, Gariani K, Martin PY. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy: a systematic review. Eur J Intern Med. 2014;25(2):173–176. [DOI] [PubMed] [Google Scholar]
- 31. Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009;(3). [DOI] [PubMed]
- 32. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J; Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709–717. [DOI] [PubMed] [Google Scholar]
- 33. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–1321. [DOI] [PubMed] [Google Scholar]
- 34. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. [DOI] [PubMed] [Google Scholar]
- 35. Joergensen A, Broedbaek K, Weimann A, Semba RD, Ferrucci L, Joergensen MB, Poulsen HE. Association between urinary excretion of cortisol and markers of oxidatively damaged DNA and RNA in humans. PLoS One. 2011;6(6):e20795. [DOI] [PMC free article] [PubMed] [Google Scholar]