Abstract
Adrenocorticotropic hormone (ACTH)-dependent Cushing syndrome is rarely caused by a pheochromocytoma. We present a case of a 46-year-old woman who developed severe hypertension, hypokalemia, and typical Cushingoid features. Investigations revealed extremely high metanephrine, cortisol, and ACTH levels. Imaging showed a 3.8-cm left adrenal mass. Preoperative control of hypertension and hypokalemia was very challenging. The patient was cured after surgical removal of the adrenal mass. We followed this by a review of the literature using the databases Google Scholar and PubMed. A total of 58 cases have been reported to date. In summary, ACTH-producing pheochromocytoma is a rare condition that poses a clinical challenge in the perioperative period. It is important that physicians be aware of such a condition because early recognition and treatment are crucial to decrease morbidity and mortality.
Keywords: ACTH-producing pheochromocytoma, Cushing syndrome, ectopic, pheochromocytoma
We present a case of ACTH-producing pheochromocytoma, a life-threatening condition if not recognized and treated early. We follow it with a review of all reported cases to improve physician awareness.
Ectopic adrenocorticotropic hormone (ACTH) production is a rare cause of Cushing syndrome. It is usually seen with small cell lung cancer, bronchial carcinoid, or medullary thyroid cancer. Very rarely the source of ectopic ACTH production can be a pheochromocytoma. We present a case of ACTH-producing pheochromocytoma that presented with severe symptoms of both hypercortisolism and catecholamine excess. We conducted a literature review on this rare disease to provide a better understanding of its clinical characteristics.
1. Case Presentation
A 46-year-old woman with a history of well-controlled diabetes mellitus and hypertension diagnosed a year earlier presented to the emergency department with chest pain, palpitations, tremors, headaches, and elevated blood pressure (BP). She reported that she was doing well until 10 weeks earlier when her BP had suddenly increased, with frequent systolic readings in the 180 to 200 mm Hg range, necessitating three visits to other emergency departments, during which her medications were adjusted. Magnetic resonance imaging of the head was done but showed no acute findings. Her diabetes also had become uncontrolled, with glucose readings mostly >300 mg/dL. During the same period, she had also noted irregular menstrual periods, muscle weakness, cold intolerance, thin skin, easy bruising, abdominal distention, swelling, tingling, blurred vision, shortness of breath, and 45 kg weight loss. She has no family history of endocrine disorders.
The patient was admitted for treatment of a hypertensive emergency and was found to have elevated troponins. During her hospital stay, her BP was noted to fluctuate significantly even after treatment with multiple antihypertensive medications, ranging from 91/63 to 178/100 mm Hg. There was also an orthostatic drop in BP. Her weight was 94 kg, height was 165 cm, and body mass index was 34.5 kg/m2. On examination, she had a moon face, which had significantly changed from her driver’s license picture issued only a year earlier. She also had supraclavicular and dorsocervical fat pads, wide purple abdominal striae, thin skin, multiple bruises, edema, wasting of her arms and legs, and proximal muscle weakness to the point that she started requiring a walker to ambulate. It was also noted during her stay that her mental status worsened; she became more depressed and developed mood swings.
Workup showed elevated cortisol and ACTH levels. There was loss of the expected diurnal variation in serum cortisol (51 to 107 μg/dL), and it was not suppressible by dexamethasone. Her 24-hour urine free cortisol was 100 times the upper limit of normal. Due to the palpitations, tremors, and hypertension, she was evaluated simultaneously for pheochromocytoma. Metanephrine levels were elevated in the plasma and urine samples. All results were confirmed on multiple occasions. Total testosterone and dehydroepiandrosterone sulfate were elevated, which, along with weight loss, were concerning for malignancy. She was noted to have severe hypokalemia. Aldosterone and renin levels were suppressed. Hemoglobin A1c had increased to 8.9%. Thyroid function was consistent with central hypothyroidism (Table 1).
Table 1.
Preoperative Laboratory Results
| Laboratory Test | Reference Range | Result |
|---|---|---|
| Cortisol | ||
| Random | 5–25 μg/dL | 75 at 3 am |
| 107 at 10 am | ||
| 69–99 at 1–3 pm | ||
| Cortisol am (after 1 mg dexamethasone suppression) | <1.8 μg/dL | 51 |
| 24-h urine free cortisol | <50 μg/24h | 5010 |
| ACTH | 7.2–63.3 pg/mL | 339.4 |
| Catecholamines | ||
| Plasma metanephrines | ||
| Normetanephrine | 0–145 pg/mL | 233 |
| Metanephrine | 0–62 pg/mL | 351 |
| 24-h urine | ||
| Epinephrine | 0–24 μg/24 h | 154 |
| Norepinephrine | 0–135 μg/24 h | 281 |
| Dopamine | 0–510 μg/24 h | 179 |
| Metanephrine | 45–290 μg/24 h | 1449 |
| Normetanphrine | 82–500 μg/24 h | 668 |
| Thyroid function | ||
| Thyroid-stimulating hormone | 0.4–4 mIU/mL | 0.057 |
| Free thyroxine | 0.8–1.4 ng/dL | 0.67 |
| Free triiodothyronine | 1–4.2 pg/mL | 1.07 |
| Prolactin | 1.9–25 ng/mL | 5.9 |
| Follicle-stimulating hormone | 5.8–21 mIU/mL | <0.2 |
| Testosterone | 14–76 ng/dL | 255 |
| Plasma renin activity | 0.15–2.33 ng/mL/h | 0.26 |
| Aldosterone | 0–30 ng/dL | <1 |
| Chromogranin A | 0–5 nmol/L | 2 |
| Dehydroepiandrosterone sulfate | 41.2–243.7 μg/dL | 302.5 |
| Hemoglobin A1c | 4%–5.7% (20–39 mmol/mol) | 8.9 (73.8 mmol/mol) |
| Potassium | 3.3–5.1 mmol/L | 1.8 |
| Bicarbonate | 21–32 mmol/L | 42 |
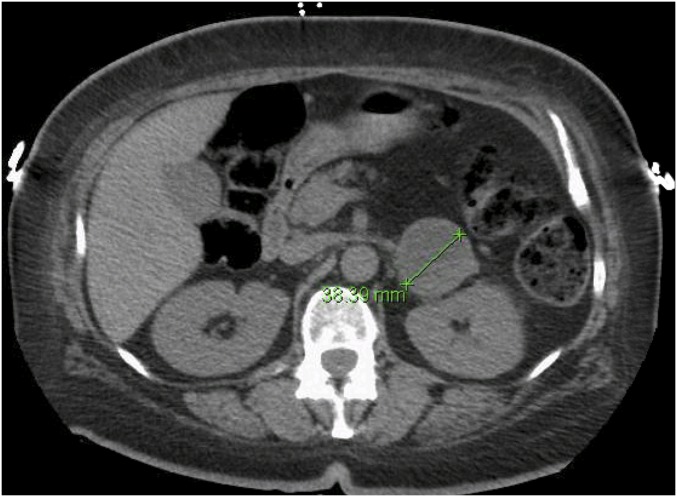
Magnetic resonance imaging revealed no mass on the brain. A computed tomography scan of the abdomen showed a well-defined mass in the left adrenal gland, measuring 3.8 × 3.6 × 3.8 cm, that was partially cystic with enhancing septations but no calcification. The contralateral adrenal gland was diffusely enlarged (Fig. 1). Echocardiogram showed no evidence of cardiomyopathy or valvular lesions.
Figure 1.
Computed tomography scan of the abdomen showing a 3.8-cm left adrenal mass that is partially cystic with enhancing septations.
A diagnosis of severe Cushing syndrome due to an ACTH-producing pheochromocytoma was made. To prepare for adrenalectomy, the signs of cortisol and catecholamine excess had to be controlled. Hypertension and potassium wasting were the most challenging. To control her BP, the patient required prazosin titrated up to 10 mg twice daily, carvedilol 50 mg twice daily, spironolactone 100 mg twice daily, losartan 100 mg daily, and nifedipine 60 mg daily. Potassium chloride 160 mEq daily was needed to keep her level in the normal range. Mifepristone was not immediately available to start prior to surgery, so ketoconazole 600 mg in divided doses was used to control severe hypercortisolism. The patient was given prophylaxis for Pneumocystisjiroveci with sulfamethoxazole/trimethoprim. She was also given prophylaxis for deep venous thrombosis and was provided vitamin D and calcium supplements. She was started on levothyroxine 50 μg daily for the hypothyroidism, which was thought to be secondary to significantly elevated cortisol. Diabetes was controlled with multiple daily injections of insulin glargine and lispro.
Four weeks after her initial presentation, the patient underwent a robotic left adrenalectomy that was uneventful. She did not require emergency intervention, and her blood pressure remained stable. Postoperatively, all medications were held, including levothyroxine, insulin, and antihypertensives. She was started on high-dose hydrocortisone replacement.
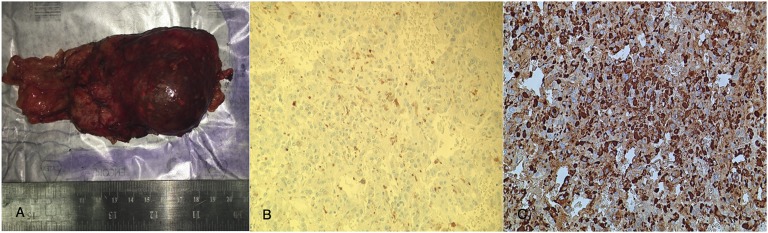
Histologic examination revealed polygonal cells with positive staining for synaptophysin, chromogranin A, and S100 (in the sustentacular cells), consistent with a pheochromocytoma. Ten percent of the cells also stained positive for ACTH. Immunostaining for SF1 was negative (Fig. 2).
Figure 2.
(A) Gross specimen, left adrenal gland. (B) Patchy light brown cytoplasmic staining for ACTH (×200). (C) Nuclear and cytoplasmic staining for chromogranin A (×200).
Cure was confirmed by a low morning serum cortisol, low ACTH, and normal plasma metanephrines 4 weeks after surgery. Hemoglobin A1c decreased to 5.5% without pharmacological treatment. The potassium level remained normal (Table 2). Over the following 3 months the patient improved significantly. Blood pressure remained well controlled, and the severe myopathy gradually improved. She was able to ambulate independently, her mental status returned to baseline, and menstrual periods became regular again. Hydrocortisone was gradually tapered and completely stopped after 3 months. There has been no recurrence for 1 year after surgery. Due to the patient’s family history of renal cell carcinoma and brain meningioma, she was tested for the VHL gene, but the result was negative for mutations.
Table 2.
Postoperative Laboratory Results
| Laboratory Test | Timing Postoperative | Reference Range | Result |
|---|---|---|---|
| Thyroid function | 1 wk | ||
| Thyroid-stimulating hormone | 0.4–4 mIU/mL | 1.45 | |
| Free thyroxine | 0.8–1.4 ng/dL | 1.05 | |
| Free triiodothyronine | 1–4.2 pg/mL | 2.37 | |
| Plasma metanephrines | 2 wk | ||
| Metanephrine | 0–62 pg/mL | <10 | |
| Normetanephrine | 0–145 pg/mL | 111 | |
| Total testosterone | 2 wk | 14–76 ng/dL | <10 |
| Dehydroepiandrosterone sulfate | 2 wk | 41.2–243.7 μg/dL | 16.4 |
| ACTH | 1 wk | 7.2–63.3 pg/mL | 5.4 |
| 6 wk | 29.5 | ||
| Cortisol | 6 wk | 5–25 μg/dL | 5.613 |
| 9 wk | |||
| Hemoglobin A1c | 9 wk | 4–5.7% (20–39 mmol/mol) | 5.5 (36.6 mmol/mol) |
| Potassium | 1 wk | 3.3–5.1 mmol/L | 4.3 |
2. Discussion
Cushing syndrome is a rare disease. The incidence of endogenous Cushing syndrome is reported to be 0.7 to 2.4 per million population per year, not accounting for cases of subclinical Cushing syndrome found during workup of adrenal incidentalomas [1–4]. The majority (80%) of cases are due to oversecretion of ACTH, and the other 20% of cases originate from the adrenal glands as adenomas or, rarely, carcinomas. It is estimated that 75% to 80% of ACTH-dependent cases arise from the pituitary gland (Cushing disease), and the rest are from ectopic production of ACTH [1]. The source of ectopic ACTH syndrome is usually a small cell lung cancer, bronchial carcinoid, or medullary thyroid cancer. A pheochromocytoma has been reported as a cause of ectopic ACTH syndrome in 5.2% of cases in one review [5].
We conducted a literature review for cases of ACTH-producing pheochromocytoma. To date there have been 58 cases reported in the English literature, including our own [5–53]. Many were abstracts only lacking some details. A summary of these cases is provided in Table 3. Four cases were found in other languages (Chinese, Japanese, Korean, and Russian); these were not included in our study [54–57]. Other mechanisms for the development of ectopic Cushing syndrome from a pheochromocytoma have been reported, such as corticotropin-releasing hormone secretion [58–60] or mixed corticomedullary tumors [61, 62]. Other cases have been reported as part of the MEN 2A syndrome with bilateral masses [63, 64]. None of these entities was included in this review.
Table 3.
Characteristics of Reported Cases of ACTH-Producing Pheochromocytoma (n = 58)
| Author (Year) | Age, y | Sex | Location, Size | Duration of Symptoms |
|---|---|---|---|---|
| Berenyi et al. (1977) | 67 | M | Right, 2.5 × 3 cm | 4 mo |
| Spark et al. (1979) | 47 | F | Left, 3 cm | 8 mo |
| Forman et al. (1979) | 51 | F | Right, 8 × 5 cm | 3–5 mo |
| Fiorica et al. (1983) | 62 | F | Right, 6 × 5 cm | 2 mo |
| Lamovec et al. (1984) | 42 | F | Left, 2.8 × 2.5 cm | 3 y |
| Schroeder et al. (1984) | 47 | F | Left, 7 × 3.5 cm | 2 mo |
| Bruining et al. (1985) | 38 | F | Left, 6 cm | 3 y |
| Beaser et al. (1986) | 36 | F | Right, 5 cm | 9 mo |
| Sakurai et al. (1987) | 44 | F | Right, 8.2 × 7 cm | 4 mo |
| O’Brien et al. (1992) | 49 | F | Left, 4 cm | 1 mo |
| Terzolo et al. (1994) | 35 | F | Left, 3.3 cm | 4 mo |
| Chen et al. (1995) | 51 | F | Left, 2.5 cm | N/A |
| 38 | F | Right, 2.5 cm | N/A | |
| 26 | M | Left, 4.5 cm | N/A | |
| 57 | F | Right, 2.8 cm | N/A | |
| Loh et al. (1996) | 25 | F | Left, 2.5 ×3 cm | 6 wk |
| Sato et al. (1998) | 41 | F | Left, 6.5 cm | 3 mo |
| Khalil et al. (1999) | 44 | F | Left, 2.5 cm | N/A |
| White et al. (2000) | 44 | F | Left, 4 cm | N/A |
| van Dam et al. (2002) | 30 | M | Right, 4.5 × 3.5 cm | 1 y followed by rapid deterioration |
| Alvarez et al. (2002) | 52 | F | Left, 4.2 × 4.5 cm | 1 mo |
| Oh et al. (2003) | 28 | F | Right, 5 ×3 cm | N/A |
| Danilovic et al. (2007) | 71 | M | Bilateral (left 4.5, cm; right, 1–2.5 cm) | 4 mo |
| Brenner et al. (2008) | 53 | F | Left, 3.5 cm | 12 mo |
| Nijhoff et al. (2009) | 74 | M | Left, 3.5 cm | 4 mo |
| Fiebrich et al. (2009) | 67 | M | Left, 2 cm | N/A |
| Cohade et al. (2009) | 30 | F | Left, 2.5 × 3 cm | N/A |
| Ramasamy et al. (2010) | 30 | F | Left, 3.5 × 3 cm | N/A |
| Bernardi et al. (2011) | 63 | F | Right, 3 × 2 × 3.5 cm | N/A |
| Li et al. (2012) | 15 | F | Right multiple, 5.5 cm | N/A |
| Ballav et al. (2012) | 49 | F | Left, 4.1 × 3.8 cm | N/A |
| Negro et al. (2013) | 55 | F | Left, 3 cm | 4 mo |
| Gerber et al. (2013) | 48 | F | Right, 6.6 × 5.6 × 7.9 cm | Few months |
| Krylov et al. (2014) | 50 | F | Left, 6 cm | N/A |
| Topaloglu et al. (2014) | 48 | F | Left, 4.3 ×3.7 cm | N/A |
| Folkestad et al. (2014) | 70 | F | Bilateral (largest right, 3 × 1.8; left, 1.7 × 1.2) | N/A |
| 67 | F | Right, 3 cm | N/A | |
| Perez et al. (2014) | 27 | F | Right, size N/A | 4 y paroxysms, 8 wk rapid deterioration |
| Andreassen et al. (2015) | 75 | F | Left, 3 × 2.5 ×2 cm | 7 mo |
| 60 | F | Right, 6 × 5 ×4.5 cm | N/A | |
| 64 | M | Left, 8 ×5.5 × 4 cm | N/A | |
| Lapshina et al. (2015) | 50-63 | F | Left, 2 × 1.6 × 1 cm | 6 mo–5 y |
| F | Left, 2.7 × 3 × 4.6 cm | 6 mo–5 y | ||
| F | Left, two nodules, each 2.3 × 2.5 cm | 6 mo–5 y | ||
| Cho et al. (2015) | 35 | F | Left, 2.5 cm | 2 mo |
| Sood et al. (2015) | 54 | F | Left, 3.7 cm | Few months |
| Klubo-Gwiezdzinska et al. (2015) | 70 | F | Left, 3.5× 1.9 × 2.5 cm | N/A |
| Kalia-Reynolds et al. (2016) | 56 | F | Right, 5.6 cm | 3 y |
| Fukasawa et al. (2016) | 58 | M | Bilateral (right, 1.7 × 2.3 cm; left, 2.8 × 3.3 cm) | N/A |
| Flynn et al. (2016) | 63 | F | Right, two nodules 1 cm each | N/A |
| Sakuma et al. (2016) | 56 | F | Left, 5.1 × 5.4 cm | N/A |
| Gungunes et al. (2016) | 42 | M | Right, 4.3 × 7.8 × 6.2 cm | N/A |
| Samargandy and Nasser (2016) | 48 | F | Right, 2 × 3 cm | 1 y |
| Ahmad et al. (2016) | 26 | M | Right, 11 × 6.7 × 7.2 cm | 2 y |
| Chanukya et al. (2016) | 53 | F | Right, 4 × 3.8 × 3.3 cm | N/A |
| Falhammar et al. (2017) | 50 | F | Left, 3.5 cm | 3 y paroxysms, 1 mo rapid deterioration |
| Gabi et al. (2017) (present study) | 46 | F | Left, 3.8 × 3.6 cm | 3 mo |
| Boers et al. (2017) | 59 | F | Left, size N/A | 1 y |
Abbreviation: N/A, not available.
ACTH-producing pheochromocytomas have occurred at a higher frequency in women (82%). Age at diagnosis ranged from 15 to 75 years (median age, 50 years). Only three cases had bilateral masses (5%). The majority of cases were unilateral; 61% of those were found on the left adrenal gland. A previous study reported that, among all the adrenal incidentalomas reviewed, 62% occurred on the left side [65]. Two cases had multiple nodules on the same adrenal. The size of the lesions ranged from 1 to 11 cm. The Hounsfield units of the nodule was only reported in 12 cases, with values ranging from 4 to 100.
Symptoms developed rapidly in most cases. This ranged from 1 month to 5 years, but those with longer durations had a sudden decline in the last few weeks. This is unlike the insidious onset of other forms of nonectopic Cushing syndrome. The majority of patients had severe Cushingoid symptoms, likely due to the higher levels of ACTH and cortisol [5]. This also explains the high frequency of hypokalemia seen among these patients; 95% of those who reported a potassium level had hypokalemia. Only two cases reported normal potassium levels. This is much higher than what is found in Cushing disease, where, in one study, the rate of unprovoked hypokalemia was only 10% [66]. Elevated blood pressure was reported in 96% of cases, one case had hypotension, and another remained well controlled. New or worsening hyperglycemia was reported in 79% of cases. Six cases, including our own, reported weight loss. This finding, in addition to elevated androgen levels, was initially concerning for malignancy. Typical Cushingoid features were absent in three cases despite the elevated cortisol levels. Four cases reported a lack of catecholamine excess, but the pathology confirmed a pheochromocytoma. In five more cases, catecholamines were not checked preoperatively despite the presence of an adrenal mass; one of these was found elevated during adrenal venous sampling. In a sixth case, the laboratory was sent for metanephrines, but surgery was done before results were available due to the patient’s clinical decline. Severe intraoperative hypertension was reported in one of these cases. According to the most recent guidelines, pheochromocytoma should be ruled out in any adrenal mass even in the absence of the clinical signs of catecholamine excess [67].
Central hypothyroidism has only been reported in two cases, including our own (5%). Low thyroid-stimulating hormones but not the free hormones were reported in one case, which precludes us from making a diagnosis of hypothyroidism. Normalization of the thyroid hormones occurred after the adrenalectomy. Other common reported features included muscle weakness, hirsutism, and psychiatric abnormalities.
All cases were treated with α-blockers in addition to other blood pressure medications, except those not diagnosed with pheochromocytoma preoperatively. Specific treatments used for hypercortisolism preoperatively included ketoconazole in nine cases, metyrapone in seven cases, and both agents together in two cases. One case required an etomidate drip in addition to both ketoconazole and metyrapone due to the severity of hypercortisolism. Other agents used less frequently were mifepristone and mitotane. From our experience, the use of agents other than ketoconazole is limited due to the lack of immediate availability for use in hospitals and the high cost and long time needed for insurance approval in a situation where time is crucial to minimize complications.
Two cases were reported in pregnancy, which complicates the diagnosis and management. Both cases were complicated by preeclampsia and cerebrovascular accidents. One resulted in maternal death and the other in fetal demise. A third case was diagnosed in the postpartum period with a good outcome.
Among cases with reported outcomes, five deaths occurred; one preoperatively due to severe sepsis and the other four, including the pregnant case, after the adrenalectomy. Sepsis was the most common complication. Staphylococcus, Pneumocystis, Nocardia, Aspergillus, Cytomegalovirus, and Epstein Barr virus have all been reported. Thrombosis of the deep veins, acute limb ischemia, myocardial infarction, cerebrovascular accidents, and gastrointestinal bleeding are some of the other reported complications.
For the rest of the cases, adrenalectomy resulted in cure. Of those who included follow-up results, only one case reported not requiring steroids from the start. Nine were able to come off steroids within 4 weeks to 1 year. Six cases reported chronic steroid need; two of these had unilateral adrenalectomy, three had bilateral adrenalectomies, and one had a right total and left subtotal adrenalectomy. Other cases reported improvement and cure of the hormonal abnormalities but no specific mention of steroid management.
All cases of pheochromocytoma were confirmed by histology. Only one case (1.7%) was reported to be a malignant pheochromocytoma with metastasis, and three more were suspicious for malignancy. ACTH staining was done in the majority of cases, whereas others used the cure as a means for diagnosis.
Genetic testing should be considered in all cases of pheochromocytoma according to the Endocrine Society guidelines [67], especially if there is a concerning family history, as in our case. Of all the cases reviewed, only five mentioned genetic testing. Three were negative, including our own; one had a missense mutation in VHL that is nonpathogenic; and in the fifth case the patient refused testing.
3. Conclusion
Early diagnosis is key to preventing the morbidity and mortality of an ACTH-producing pheochromocytoma. It should be suspected in any patient with ACTH-dependent Cushing syndrome when workup is suggestive of an ectopic source along with an adrenal mass on imaging. Pheochromocytoma should be ruled out in any adrenal mass even in the absence of typical symptoms of catecholamine excess [67]. Proceeding to surgery with an undiagnosed pheochromocytoma can result in a hypertensive crisis and increase mortality [68]. Optimizing BP control by maximum α-blockade in addition to other medications can prevent such a crisis. Adrenalectomy is usually curative in benign ACTH-secreting pheochromocytomas. Genetic testing should be considered in any patient with pheochromocytoma because 40% of patients have an underlying genetic mutation, especially if there are other suggestive features, such as a family history or young age [67].
Acknowledgments
We thank Dr. James Jensen, the urologist on the case, for his work on our patient and for contributing the gross adrenal images and Dr. Teresa Limjoco and the pathology department for contributing the histology pictures.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACTH
adrenocorticotropic hormone
- BP
blood pressure
References and Notes
- 1. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367(9522):1605–1617. [DOI] [PubMed] [Google Scholar]
- 2. Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol. 2015;7:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lindholm J, Juul S, Jørgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jørgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J. Incidence and late prognosis of cushing’s syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86(1):117–123. [DOI] [PubMed] [Google Scholar]
- 4. Etxabe J, Vazquez JA. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf). 1994;40(4):479–484. [DOI] [PubMed] [Google Scholar]
- 5. Ballav C, Naziat A, Mihai R, Karavitaki N, Ansorge O, Grossman AB. Mini-review: pheochromocytomas causing the ectopic ACTH syndrome. Endocrine. 2012;42(1):69–73. [DOI] [PubMed] [Google Scholar]
- 6. Berenyi MR, Singh G, Gloster ES, Davidson MI, Woldenberg DH. ACTH-producing pheochromocytoma. Arch Pathol Lab Med. 1977;101(1):31–35. [PubMed] [Google Scholar]
- 7. Spark RF, Connolly PB, Gluckin DS, White R, Sacks B, Landsberg L. ACTH secretion from a functioning pheochromocytoma. N Engl J Med. 1979;301(8):416–418. [DOI] [PubMed] [Google Scholar]
- 8. Forman BH, Marban E, Kayne RD, Passarelli NM, Bobrow SN, Livolsi VA, Merino M, Minor M, Farber LR. Ectopic ACTH syndrome due to pheochromocytoma: case report and review of the literature. Yale J Biol Med. 1979;52(2):181–189. [PMC free article] [PubMed] [Google Scholar]
- 9. Fiorica V, Males JL, Kem DC, DeBault LE, Knickerbocker FM. Cushing’s syndrome from an ACTH-secreting pheochromocytoma. J Okla State Med Assoc. 1983;76(2):45–50. [PubMed] [Google Scholar]
- 10. Lamovec J, Memoli VA, Terzakis JA, Sommers SC, Gould VE. Pheochromocytoma producing immunoreactive ACTH with Cushing’s syndrome. Ultrastruct Pathol. 1984;7(1):41–48. [DOI] [PubMed] [Google Scholar]
- 11. Schroeder JO, Asa SL, Kovacs K, Killinger D, Hadley GL, Volpé R. Report of a case of pheochromocytoma producing immunoreactive ACTH and beta-endorphin. J Endocrinol Invest. 1984;7(2):117–121. [DOI] [PubMed] [Google Scholar]
- 12. Bruining HA, Ong EG, Gershuny AR, Lamberts SW. Cushing’s syndrome and pheochromocytoma caused by an adrenal tumor, also containing met-enkephalin and somatostatin: a case report. World J Surg. 1985;9(4):639–642. [DOI] [PubMed] [Google Scholar]
- 13. Beaser RS, Guay AT, Lee AK, Silverman ML, Flint LD. An adrenocorticotropic hormone-producing pheochromocytoma: diagnostic and immunohistochemical studies. J Urol. 1986;135(1):10–13. [DOI] [PubMed] [Google Scholar]
- 14. Sakurai H, Yoshiike Y, Isahaya S, Matsushita H, Yamanaka K, Okubo T, Kanamori H, Takahashi H, Fu T. A case of ACTH-producing pheochromocytoma. Am J Med Sci. 1987;294(4):258–261. [DOI] [PubMed] [Google Scholar]
- 15. O’Brien T, Young WF Jr, Davila DG, Scheithauer BW, Kovacs K, Horvath E, Vale W, van Heerden JA. Cushing’s syndrome associated with ectopic production of corticotrophin-releasing hormone, corticotrophin and vasopressin by a phaeochromocytoma. Clin Endocrinol (Oxf). 1992;37(5):460–467. [DOI] [PubMed] [Google Scholar]
- 16. Terzolo M, Alì A, Pia A, Bollito E, Reimondo G, Paccotti P, Scardapane R, Angeli A. Cyclic Cushing’s syndrome due to ectopic ACTH secretion by an adrenal pheochromocytoma. J Endocrinol Invest. 1994;17(11):869–874. [DOI] [PubMed] [Google Scholar]
- 17. Chen H, Doppman JL, Chrousos GP, Norton JA, Nieman LK, Udelsman R. Adrenocorticotropic hormone-secreting pheochromocytomas: the exception to the rule. Surgery. 1995;118(6):988–994, discussion 994–995. [DOI] [PubMed] [Google Scholar]
- 18. Loh KC, Gupta R, Shlossberg AH. Spontaneous remission of ectopic Cushing’s syndrome due to pheochromocytoma: a case report. Eur J Endocrinol. 1996;135(4):440–443. [DOI] [PubMed] [Google Scholar]
- 19. Sato M, Watanabe T, Ashikaga T, Taneda T, Yamawake N, Nishizaki M, Arimura A, Azegami N, Arita M, Fukuoka H, Kitamura H. Adrenocorticotropic hormone-secreting pheochromocytoma. Intern Med. 1998;37(4):403–406. [DOI] [PubMed] [Google Scholar]
- 20. Khalil WK, Vadasz J, Rigo E, Kardos L, Tiszlavicz L, Gaspar L. Pheochromocytoma combined with unusual form of Cushing’s syndrome and pituitary microadenoma. Eur J Endocrinol. 1999;141(6):653–654. [DOI] [PubMed] [Google Scholar]
- 21. White A, Ray DW, Talbot A, Abraham P, Thody AJ, Bevan JS. Cushing’s syndrome due to phaeochromocytoma secreting the precursors of adrenocorticotropin. J Clin Endocrinol Metab. 2000;85(12):4771–4775. [DOI] [PubMed] [Google Scholar]
- 22. van Dam PS, van Gils A, Canninga-van Dijk MR, de Koning EJP, Hofland LJ, de Herder WW. Sequential ACTH and catecholamine secretion in a phaeochromocytoma. Eur J Endocrinol. 2002;147(2):201–206. [DOI] [PubMed] [Google Scholar]
- 23. Alvarez P, Isidro L, González-Martín M, Loidi L, Arnal F, Cordido F. Ectopic adrenocorticotropic hormone production by a noncatecholamine secreting pheochromocytoma. J Urol. 2002;167(6):2514–2515. [PubMed] [Google Scholar]
- 24. Oh HC, Koh JM, Kim MS, Park JY, Shong YK, Lee KU, Kim GS, Hong SJ, Koo HL, Kim WB. A case of ACTH-producing pheochromocytoma associated with pregnancy. Endocr J. 2003;50(6):739–744. [DOI] [PubMed] [Google Scholar]
- 25. Danilovic DL, Brandão Neto RA, D’Abronzo H, Menezes MR, Lucon AM, Mendonca BB. Ectopic ACTH syndrome caused by pheochromocytoma: computed tomography-guided percutaneous ethanol injection as an alternative treatment. J Endocrinol Invest. 2007;30(9):780–786. [DOI] [PubMed] [Google Scholar]
- 26. Brenner N, Kopetschke R, Ventz M, Strasburger CJ, Quinkler M, Gerl H. Cushing’s syndrome due to ACTH-secreting pheochromocytoma. Can J Urol. 2008;15(1):3924–3927. [PubMed] [Google Scholar]
- 27. Nijhoff MF, Dekkers OM, Vleming LJ, Smit JWA, Romijn JA, Pereira AM. ACTH-producing pheochromocytoma: clinical considerations and concise review of the literature. Eur J Intern Med. 2009;20(7):682–685. [DOI] [PubMed] [Google Scholar]
- 28. Fiebrich HB, Brouwers AH, van Bergeijk L, van den Berg G. Image in endocrinology. Localization of an adrenocorticotropin-producing pheochromocytoma using 18F-dihydroxyphenylalanine positron emission tomography. J Clin Endocrinol Metab. 2009;94(3):748–749. [DOI] [PubMed] [Google Scholar]
- 29. Cohade C, Broussaud S, Louiset E, Bennet A, Huyghe E, Caron P. Ectopic Cushing’s syndrome due to a pheochromocytoma: a new case in the post-partum and review of literature. Gynecol Endocrinol. 2009;25(9):624–627. [DOI] [PubMed] [Google Scholar]
- 30. Ramasamy MM, Thiagarajan R, Dass PS. Adrenocorticotrophic hormone secreting pheochromocytoma. Indian J Urol. 2010;26(1):123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernardi S, Grimaldi F, Finato N, De Marchi S, Proclemer A, Sabato N, Bertolotto M, Fabris B. A pheochromocytoma with high adrenocorticotropic hormone and a silent lung nodule. Am J Med Sci. 2011;342(5):429–432. [DOI] [PubMed] [Google Scholar]
- 32. Li XG, Zhang DX, Li X, Cui XG, Xu DF, Li Y, Gao Y, Yin L, Ren JZ. Adrenocorticotropic hormone-producing pheochromocytoma: a case report and review of the literature. Chin Med J (Engl). 2012;125(6):1193–1196. [PubMed] [Google Scholar]
- 33. Negro A, Manicardi E, Grasselli C, Babini M, Santi R, Pugni V, Spaggiari L, Tagliavini E.. Severe ectopic Cushing’s syndrome due to ACTH-secreting pheochromocytoma. Int J Clin Med. 2013;4(04):228–231. [Google Scholar]
- 34. Gerber SM, McCullen MK, Jabbour SA. Ectopic Cushing’s syndrome from ACTH-producing pheochromocytoma. Program of the 95th Annual Meeting of the Endocrine Society; June 15–18, 2013; San Francisco, CA. Abstract SUN-40.
- 35.Krylov V, Dobreva E, Kuznetsov N, Latkina N, Marova E. ACTH-secreting pheochromocytoma: case report. In: Program of the 16th European Congress of Endocrinology; May 3–7, 2014; Wroclaw, Poland. Abstract P4. doi:10.1530/endoabs.35.P4. [Google Scholar]
- 36.Topaloglu O, Polat SB, Ogmen BE, Baser H, Dirikoc M, Tokac M, Sak SD, Reyhan Ersoy1 R, Cakir B. A difficult case of ectopic Cushing's syndrome due to ACTH-producing pheochromocytoma presented with normal fractionated urinary catecholamines and metanephrines. In: Program of the 16th European Congress of Endocrinology; May 3–7, 2014; Wroclaw, Poland. Abstract P61. doi:10.1530/endoabs.35.P61. [Google Scholar]
- 37. Folkestad L, Andersen MS, Nielsen AL, Glintborg D. A rare cause of Cushing’s syndrome: an ACTH-secreting phaeochromocytoma. BMJ Case Rep. 2014;2014:bcr2014205487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perez C, Ott M, Ehrenkranz JRL. Postpartum pheochromocytoma and ectopic ACTH secretion: the first report of a case in which both mother and child survived. In: Program of the 96th Annual Meeting of the Endocrine Society; June 21–24, 2015; Chicago, IL. Abstract SUN-0791.
- 39. Andreassen M, Toft BG, Feltoft CL, Hitz MF, Rasmussen AK, Feldt-Rasmussen U. Three cases of ACTH-Producing pheochromocytoma: full hormonal workup in patients with an adrenal mass may be crucial for correct management. AACE Clin Case Rep. 2015;1(3):e170–e174. [Google Scholar]
- 40. Lapshina AM, Marova EI, Voronkova IA, Kuznetsov NS, Latkina NV, Kats LE, Remisov OV, Kolesnikova GS, Arapova SD, Krylov V, Rozhinskaya LY.. ACTH-ectopic syndrome in 4 patients, caused by pheochromocytoma. In: Program of the 97th Annual Meeting of the Endocrine Society; March 5–8, 2015; San Diego, CA. Abstract FRI-388.
- 41. Cho JH, Jeong DE, Lee JY, Jang JG, Moon JS, Kim MJ, Yoon JS, Won KC, Lee HW. Adrenocorticotropic hormone (ACTH)-producing pheochromocytoma presented as Cushing syndrome and complicated by invasive aspergillosis. Yeungnam Univ J Med. 2015;32(2):132–137. [Google Scholar]
- 42. Sood SC, Leinung M, Lin MT, Jennings T, Lee D Sudden clinical development of ectopic Cushing’s syndrome due to a non-catecholamine producing pheochromocytoma. https://www.amc.edu/academic/gme/programs/Endocrinology/documents/comley.pdf. Accessed 17 December 2017.
- 43. Klubo-Gwiezdzinska J, Zilbermint M, Demidowich AP, Nambuba J, Glanville J, Kebebew E, Sharma ST, Nieman LK, Stratakis CA, Pacak K.. ACTH-secreting pheochromocytoma leading to respiratory failure secondary to multiple opportunistic infections: a unique presentation of ectopic Cushing syndrome. In: Program of the 97th Annual Meeting of the Endocrine Society; March 5–8, 2015; San Diego, CA. Abstract FRI-382.
- 44.Kalia-Reynolds M, Giordano J, Khanna I, Houston C. ACTH-secreting pheochromocytoma. In: Program of the AACE 24th Annual Scientific and Clinical Congress; May 13–17, 2015; Nashville, TN. Abstract 100.
- 45. Fukasawa M, Sawada N, Miyamoto T, Kira S, Aoki T, Zakoji H, Mitsui T, Takeda M. Laparoscopic unilateral total and contralateral subtotal adrenalectomy for bilateral adrenocorticotropin hormone-secreting pheochromocytoma: report of a rare case. J Endourol Case Rep. 2016;2(1):232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flynn E, Baqar S, Liu D, Ekinci EI, Farrell S, Zajac JD, De Luise M, Seeman E. Bowel perforation complicating an ACTH-secreting phaeochromocytoma. Endocrinol Diabetes Metab Case Rep. 2016;2016:16–0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sakuma I, Higuchi S, Fujimoto M, Takiguchi T, Nakayama A, Tamura A, Kohno T, Komai E, Shiga A, Nagano H, Hashimoto N, Suzuki S, Mayama T, Koide H, Ono K, Sasano H, Tatsuno I, Yokote K, Tanaka T. Cushing syndrome due to ACTH-secreting pheochromocytoma, aggravated by glucocorticoid-driven positive-feedback loop. J Clin Endocrinol Metab. 2016;101(3):841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gungunes A, Durmaz SA, Cifci A, Erden E, Tas M, Yalcin S. A case of ectopic Cushing syndrome due to pheocromositoma. Endocrine Abstracts. 2016;41:90. [Google Scholar]
- 49. Ahmad S, Lee JH, Park E, Leinung M, Joseph J. Ectopic ACTH co-secretion and adrenal pheochromocytoma from an 11 cm adrenal mass. In: Program of the 98th Annual Meeting of the Endocrine Society; April 1–4, 2016; Boston, MA. Abstract FRI-419.
- 50. Samargandy SH, Nasser T. Pheochromcytoma co-secreting ectopic ACTH: a rare combination. In: Program of the 98th Annual Meeting of the Endocrine Society; April 1–4, 2016; Boston, MA. Abstract FRI-416.
- 51. Falhammar H, Calissendorff J, Höybye C. Frequency of Cushing’s syndrome due to ACTH-secreting adrenal medullary lesions: a retrospective study over 10 years from a single center. Endocrine. 2017;55(1):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chanukya GV, Mengade M, Reddy B. Cushing’s syndrome due to adrenocorticotropic hormone-secreting pheochromocytoma. CHRISMED J Health Res. 2016;3(4):291–294. [Google Scholar]
- 53.Boers J, van Treijen MJ, Zelissen PMJ, Oldenburg-Lightenberg PC. ACTH-producing pheochromocytoma, initially presenting with hypokalemia. In: Program of the Annual Meeting of the Netherlands Association of Internal Medicine; 19–21 April 2017; Maastricht, The Netherlands. Abstract P51.
- 54. Hu ZL, Yang GY, Zhang WL. A case of ACTH-producing pheochromocytoma. Chin J Urol. 1997;11:650. [Google Scholar]
- 55. Kuznetsov NS, Marova EI, Latkina NV, Dobreva EA, Krylov VV, Kats LE, Remizov OV, Voronkova IA. ACTH-secreting pheochromocytoma: case report. Endocr Surg. 2012;6(4):43–50. [Google Scholar]
- 56. Shigekawa T, Sato T, Mizobuchi S, Nakatsuka H, Yokoyama M, Morita K. Perioperative treatment of a patient with ectopic ACTH-producing pheochromocytoma [in Japanese]. Masui. 2007;56(4):442–445. [PubMed] [Google Scholar]
- 57. Hong YS, Kim HJ, Sung YA, Kyung NH, Kim HJ, Kim SS. A case of pheochromocytoma associated with ectopic ACTH syndrome. J Korean Soc Endocrinol. 1997;12:99–104. [Google Scholar]
- 58. Eng PH, Tan LH, Wong KS, Cheng CW, Fok AC, Khoo DH. Cushing’s syndrome in a patient with a corticotropin-releasing hormone-producing pheochromocytoma. Endocr Pract. 1999;5(2):84–87. [DOI] [PubMed] [Google Scholar]
- 59. Bayraktar F, Kebapcilar L, Kocdor MA, Asa SL, Yesil S, Canda S, Demir T, Saklamaz A, Seçil M, Akinci B, Yener S, Comlekci A. Cushing’s syndrome due to ectopic CRH secretion by adrenal pheochromocytoma accompanied by renal infarction. Exp Clin Endocrinol Diabetes. 2006;114(8):444–447. [DOI] [PubMed] [Google Scholar]
- 60. Kageyama K, Sakihara S, Yamashita M, Takahashi K, Kawashima S, Tanabe J, Tsutaya S, Yasujima M, Suda T. A case of multiple endocrine neoplasia type II accompanied by thyroid medullary carcinoma and pheochromocytomas expressing corticotropin-releasing factor and urocortins. Am J Med Sci. 2008;335(5):398–402. [DOI] [PubMed] [Google Scholar]
- 61. Mathison DA, Waterhouse CA. Cushing’s syndrome with hypertensive crisis and mixed adrenal cortical adenoma-pheochromocytoma (corticomedullary adenoma). Am J Med. 1969;47(4):635–641. [DOI] [PubMed] [Google Scholar]
- 62. Lwin TM, Galal N, Gera S, Marti JL. Adrenal Cushing syndrome with detectable ACTH from an unexpected source. BMJ Case Rep. 2016;2016:bcr2016216965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moon JM, Kim YJ, Seo YJ, Choi HY, Kim JH, Park JR, Lee YL, Kim HY, Kim SG, Choi DS. Ectopic ACTH syndrome with bilateral pheochromocytoma in multiple endocrine neoplasia type 2A. J Korean Endocr Soc. 2009;24(4):265–271. [Google Scholar]
- 64. Mendonça BB, Arnhold IJ, Nicolau W, Avancini VA, Boise W. Cushing’s syndrome due to ectopic ACTH secretion by bilateral pheochromocytomas in multiple endocrine neoplasia type 2A. N Engl J Med. 1988;319(24):1610–1611. [DOI] [PubMed] [Google Scholar]
- 65. Kim J, Bae KH, Choi YK, Jeong JY, Park KG, Kim JG, Lee IK. Clinical characteristics for 348 patients with adrenal incidentaloma. Endocrinol Metab (Seoul). 2013;28(1):20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Howlett TA, Drury PL, Perry L, Doniach I, Rees LH, Besser GM. Diagnosis and management of ACTH-dependent Cushing’s syndrome: comparison of the features in ectopic and pituitary ACTH production. Clin Endocrinol (Oxf). 1986;24(6):699–713. [DOI] [PubMed] [Google Scholar]
- 67. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society . Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 68. O’Riordan JA. Pheochromocytomas and anesthesia. Int Anesthesiol Clin. 1997;35(4):99–127. [PubMed] [Google Scholar]