Abstract
Everolimus, an orally administered mammalian target of rapamycin inhibitor, has been widely used as an immunosuppressant and an anticancer agent. Whereas everolimus can control recurrent hypoglycemia in patients with insulinoma, possibly through tumor regression and/or the direct inhibition of insulin secretion, time-dependent changes in serum insulin levels caused by everolimus still remain unclear. Here we report a clinical case of a patient with metastatic insulinoma, in which frequent monitoring of serum insulin levels demonstrated rapid and substantial changes in insulin secretion levels, a few days after the discontinuation as well as the readministration of everolimus. To further confirm the direct effect of everolimus on β-cell function, we performed in vitro experiments using mouse insulinoma cells (MIN6) and human induced pluripotent stem cell (hiPSC)–derived insulin-producing cells and found that everolimus significantly suppressed glucose-stimulated insulin secretion in both MIN6 cells and hiPSC–derived insulin-producing cells. Thus, both a patient with metastatic insulinoma and in vitro experiments demonstrated that everolimus directly suppresses insulin secretion, independently of its tumor regression effect.
Keywords: everolimus, insulin secretion, insulinoma, hypoglycemia, mTOR inhibitor
Both a clinical case patient with metastatic insulinoma and in vitro experiments demonstrated that everolimus directly suppresses insulin secretion, independently of its tumor regression effect.
Everolimus is an orally administered mammalian target of rapamycin (mTOR) inhibitor that has been widely used as an immunosuppressant and an anticancer agent. Recently, everolimus was used as a treatment of patients with neuroendocrine tumors, including insulinoma, and was found to significantly prolong progression-free survival in patients with progressive advanced pancreatic neuroendocrine tumors [1]. In addition, everolimus can be used to control recurrent hypoglycemia in patients with malignant insulinoma [2, 3]. Thus, whereas everolimus has been proved to be effective in the management of insulinoma, it remains unclear as to whether everolimus directly suppresses insulin secretion in pancreatic β cells, independently of tumor regression.
Here we report a case of a patient with insulinoma who was treated with everolimus. Because her laboratory data were frequently measured, we were able to track rapid changes in her serum insulin levels a few days after the discontinuation as well as the readministration of everolimus, showing that everolimus directly suppresses insulin secretion, independently of its effect on tumor regression. Furthermore, our in vitro experiments using mouse insulinoma cells and human induced pluripotent stem cell (hiPSC)–derived insulin-producing cells demonstrated that everolimus inhibited glucose-stimulated insulin secretion (GSIS), even after accounting for cell growth rates.
1. Materials and Methods
A. Case Report
We encountered a 53-year-old patient with insulinoma. The detailed clinical course is described in the Results section and Fig. 1. Informed consent was obtained from the patient.
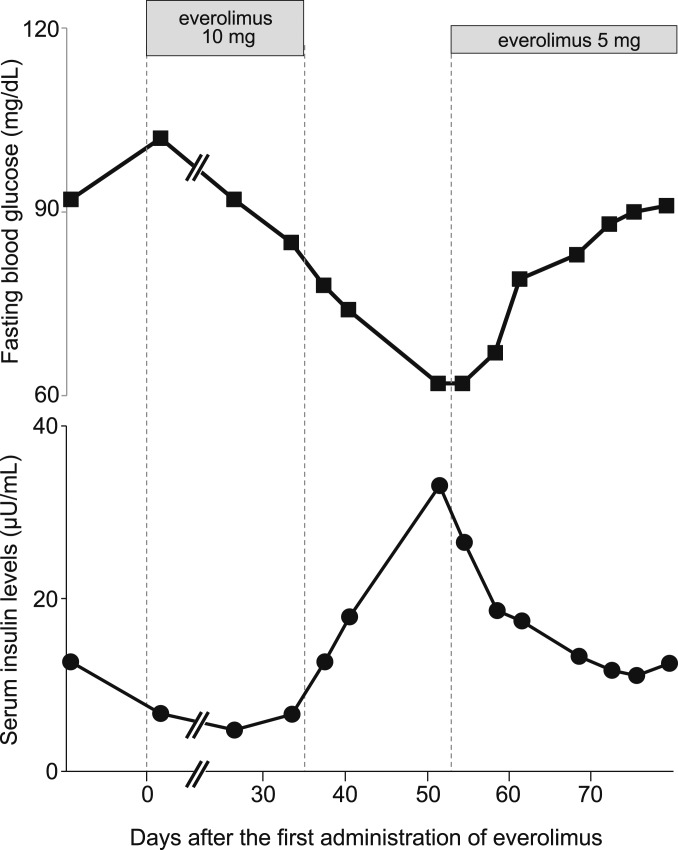
Figure 1.
Immediate and robust response of insulin secretion to everolimus. Fasting blood glucose and serum insulin levels were measured at the indicated time points before and after the administration of everolimus. Insulin secretion was suppressed when everolimus was administered at a dose of 10 mg/d but was immediately increased after its discontinuation, and it was immediately suppressed again after the second administration of everolimus at a dose of 5 mg/d.
B. Cell Culture
Mouse insulinoma (MIN6) cells were cultured as previously described [4]. Cells were seeded onto 24-well plates. At 4 days after the passage, everolimus was added to the cells at a concentration of 1.0 × 10−6 M, according to a previous study [5], and insulin secretion and cell proliferation were analyzed 24 hours later.
The hiPSCs were differentiated into insulin-producing cells by using Cellartis® hiPS Beta Cells Kit (ChiPSC12; Takara Bio Europe, Göteborg, Sweden), according to the manufacturer’s instructions. At 35 days after the initiation of differentiation, everolimus was added to the cells at a concentration of 1.0 × 10−6 M, and insulin secretion was evaluated after 24-hour incubation with everolimus.
C. Static Incubation in MIN6 Cells and hiPSC-Derived Insulin-Producing Cells
Before the insulin secretion assay, MIN6 cells or hiPSC-derived insulin-producing cells were incubated for 30 minutes in HEPES-Krebs buffer containing 0.2% BSA and washed with the same buffer. Cells were then incubated with HEPES-Krebs buffer containing 2.8 or 16.7 mM glucose or 30 mM KCl and 2.8 mM glucose. Insulin concentrations in the supernatants and insulin content in MIN6 cells were measured by using the mouse insulin ELISA kit (Morinaga, Tokyo, Japan), and then normalized with total protein concentrations in whole cell lysates. The concentrations of human insulin in the supernatants of hiPSC-derived insulin-producing cells were assayed by using the human insulin ELISA kit (Mercodia AB, Uppsala, Sweden) and normalized with total protein concentrations in the whole cell lysates.
D. Cell Proliferation Assay
To assess the number of proliferating MIN6 cells, cells were incubated with 5-ethynyl-2′-deoxyuridine (EdU) for 2 hours and fixed with formaldehyde [6]. The procedures were performed by using the Click-iT Plus EdU Imaging Kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
E. RT-PCR Analysis
MIN6 cells were treated with everolimus or vehicle for 24 hours, and total RNA was extracted by using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA), and cDNA was synthesized by using SuperScript III First Strand Synthesis System (Invitrogen). RT-PCR assays were performed by using TaqMan Custom Arrays (Applied Biosystems, Foster City, CA). The expression level of each gene was presented relative to the mRNA level of β-glucuronidase (Gusb).
F. Statistical Analyses
Statistical analyses were performed by using SPSS 18.0 for Windows software (IBM, Armonk, NY). Comparisons of two samples were performed by two-tailed t tests. Multiple groups were analyzed by one-way ANOVA with a multiple comparison test. A P value <0.05 was considered to indicate a statistically significant difference between two groups. Data are presented as the mean ± SEM of three to four independent experiments carried out in duplicate.
2. Results
A. Changes in the Patient’s Insulin Secretion Levels During Everolimus Treatment
A 53-year-old female patient was hospitalized in our institution for treatment of refractory hypoglycemia. She had been diagnosed with primary pancreatic insulinoma at age 47 years, and tumor resection had been performed. Although no obvious metastatic lesions had been identified, her hypoglycemic episodes could not be prevented with octreotide and diazoxide. As an alternative treatment, we decided to administer everolimus to this patient because everolimus was approved for the treatment of advanced pancreatic neuroendocrine tumors and was reported to control hypoglycemia in patients with metastatic insulinoma.
Serum insulin levels were continuously suppressed from 3 days after the first administration of everolimus. Because the patient developed pneumonitis 35 days after the start of treatment, everolimus was discontinued according to the recommendations of the US Food and Drug Administration (reference ID: 3893024). Three days after discontinuation, her serum insulin levels were robustly increased, and her fasting blood glucose levels were subsequently reduced (Fig. 1). After her cough disappeared, everolimus was readministered at half the amount of the initial dose (5 mg/d); her serum insulin levels then immediately decreased and fasting glucose levels recovered within 3 days. Thus, the improvement of fasting hyperinsulinemia during everolimus treatment suggests that everolimus effectively suppresses insulin secretion from insulinoma cells.
Our clinical case showed that serum insulin levels were dramatically altered a few days after the administration and discontinuation of everolimus. Whereas previous studies reported that everolimus improves hypoglycemia within a few weeks [3, 7], there had been no data on serum insulin levels shortly after everolimus treatment. Therefore, we hypothesized that everolimus has a direct effect on pancreatic β-cells to inhibit insulin secretion, independently of its antiproliferative or cytotoxic effects.
B. Everolimus Suppresses Insulin Secretion in MIN6 Cells Independently of Decreased Cell Proliferation
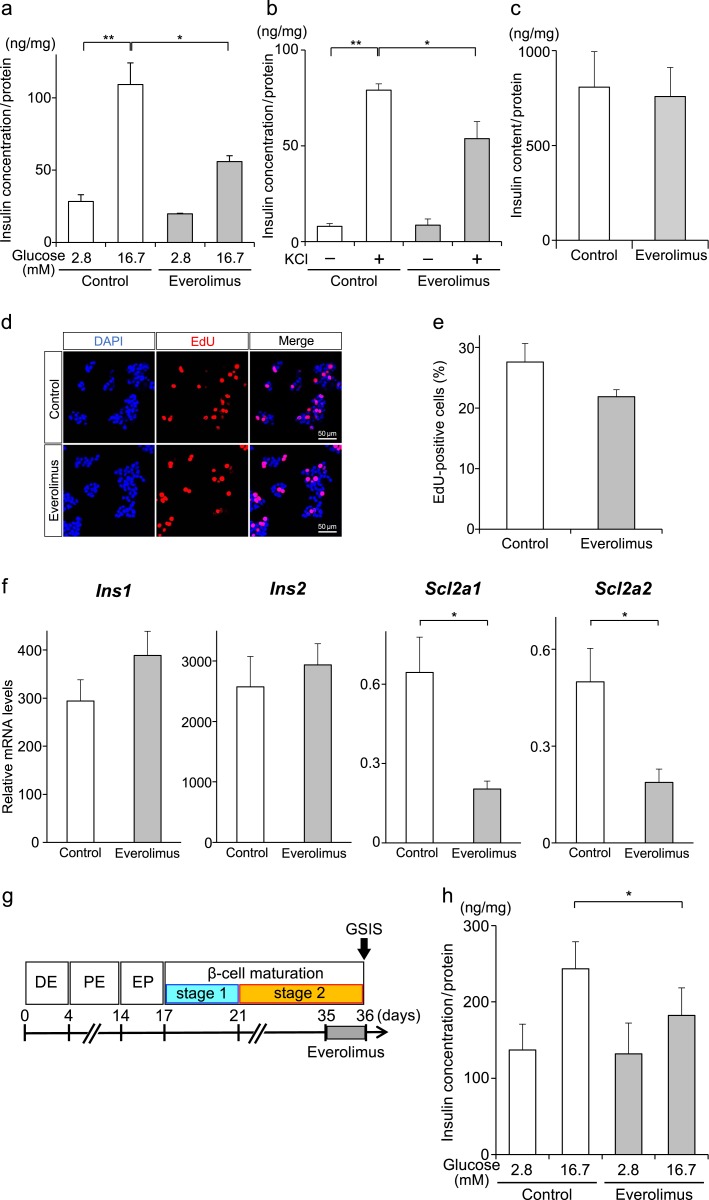
To address our hypothesis, which was based on the clinical case of insulinoma described above, we investigated the effect of everolimus on insulin secretion in MIN6 cells, which are derived from mouse insulinoma cells [4]. When MIN6 cells were incubated with everolimus at a low glucose concentration (2.8 mM), everolimus had no effect on insulin secretion (Fig. 2a). On the other hand, when MIN6 cells were incubated with a high glucose concentration (16.7 mM), insulin secretion was significantly reduced by everolimus treatment. In addition, KCl-induced insulin secretion was significantly suppressed when the cells were treated with everolimus (Fig. 2b). As insulin concentrations in Fig. 2a and 2b were normalized with protein concentrations in the whole cell lysates, this effect of everolimus is independent of the difference in MIN6 cell numbers. To further investigate the effect of everolimus on insulin biosynthesis and degradation, we measured the insulin content of whole cell lysates and observed no significant difference between everolimus and vehicle (Fig. 2c). Although everolimus has been reported to inhibit cell growth in vitro and in vivo [8], when cell proliferation rates were analyzed by using EdU staining, there was no significant difference between the two groups (Fig. 2d and 2e).
Figure 2.
Everolimus suppresses GSIS independently of insulin synthesis and cell growth inhibition. (a, b, c) MIN6 cells were treated with everolimus (gray bars) or vehicle (white bars) for 24 hours and stimulated with 2.8 or 16.7 mM glucose (a), or with/without KCl together with 16.7 mM glucose (b) (*P < 0.05; **P < 0.01; n = 3–4 for each group), and insulin content in the cells was measured (c). Insulin concentrations of each experiment were normalized with total protein concentrations in the whole cell lysates. (d, e) Cell proliferation of MIN6 cells was analyzed by EdU staining, and EdU-positive cells were counted (e). (f) The mRNA expression levels for insulin 1 (Ins1), insulin 2 (Ins2), Slc2a1, and Slc2a2 were quantified in MIN6 cells treated with everolimus or vehicle (*P < 0.05; n = 4). (g, h) Schematic protocol for the generation of hiPS-derived insulin-producing cells. The hiPSCs (ChiPSC12) were differentiated into insulin-producing cells through the following stages: definitive endoderm (DR), pancreatic endoderm (PE), and endocrine progenitor (EP). The hiPSC-derived insulin-producing cells were treated with everolimus on day 35, and GSIS was analyzed on day 36. Insulin concentrations in the supernatant were normalized with total protein concentrations in whole cell lysates (h) (*P < 0.05; n = 3). Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole. Results are shown as the mean ± standard error.
Next, we investigated the expression levels of endocrine-specific mRNAs by RT-PCR in MIN6 cells treated with everolimus or vehicle and found that the expression levels of most β cell–enriched mRNAs, such as Ins1, Ins2, Pdx1, and Abcc8, were similar between the two groups, whereas the expression levels of the glucose transporters Slc2a1 (Glut1) and Slc2a2 (Glut2) were significantly decreased in everolimus-treated MIN6 cells (Fig. 2f;Supplemental Table). Because KCl-induced insulin secretion was significantly suppressed by everolimus (Fig. 2b), the effect of everolimus on insulin secretion cannot be explained solely by the decreased expression of glucose transporters. Further investigation is required to understand the underlying mechanisms by which everolimus inhibits insulin secretion in insulinoma cells.
C. Everolimus Suppresses Insulin Secretion in hiPSC-Derived Insulin-Producing Cells
To determine whether everolimus has the same effect on hiPSCs as on mouse insulinoma cells, hiPSCs were differentiated into insulin-secreting cells (Fig. 2f) and then treated with everolimus for 24 hours. Whereas there was no significant difference in insulin secretion under a low glucose concentration (2.8 mM) between everolimus and vehicle, the everolimus treatment significantly inhibited GSIS at a high glucose concentration (16.7 mM) (Fig. 2g).
3. Discussion
The rapamycin analog everolimus has been used as a treatment in patients with insulinoma, resulting in rapid normalization of hypoglycemic events after its administration [3, 7, 9, 10]. In the current study we observed rapid and robust changes in serum insulin levels after both the initiation and discontinuation of everolimus treatment. Because serum insulin levels were robustly increased within 3 days after the discontinuation of everolimus, this effect is difficult to explain in terms of the inhibitory effect of everolimus on cell proliferation. Furthermore, the serum insulin levels were effectively suppressed 2 days after the readministration of everolimus. Such changes in serum insulin levels suggest that everolimus has direct effects on insulin secretion from insulinoma cells rather than anti-cell growth effects.
To demonstrate the direct effect of everolimus on insulin secretion in vitro, we treated mouse insulinoma MIN6 cells with everolimus, which resulted in the significant inhibition of GSIS. It has been reported that rapamycin, another mTOR inhibitor, suppresses insulin secretion in MIN6 cells by inhibiting cell proliferation and insulin biosynthesis [11]. Although the concentration of everolimus that we used in this study (1.0 μM) was reported to inhibit cell proliferation in INS1 cells, another insulinoma cell line [5], everolimus did not significantly affect cell proliferation in MIN6 cells (Fig. 2e). Furthermore, GSIS was significantly suppressed by everolimus even after normalization of the protein concentrations of whole cell lysates, whereas the insulin content was not significantly reduced. These data suggest that everolimus inhibits GSIS independently of cell proliferation or insulin biosynthesis, at least under the present experimental conditions.
Our in vitro experiments using mouse insulinoma cells and hiPSC-derived insulin-producing cells, which retain GSIS, showed that everolimus suppresses GSIS, but insulin secretion is not significantly changed under low glucose conditions (Fig. 2). In contrast, our clinical case showed immediate and distinct suppression of hyperinsulinemia under low glucose conditions during fasting (Fig. 1). Although there is no direct explanation for this difference, it is possible that everolimus suppressed the dysregulated hyperinsulinemia during the postprandial period, which has been reported in patients with insulinoma [12], and subsequently improved sustained hypoglycemia during fasting. Alternatively, everolimus might improve hyperinsulinemia independently of blood glucose levels in vivo, in contrast with the in vitro findings in this study.
Whereas Barlow et al. [11] have shown that rapamycin toxicity in MIN6 cells and human islets is mediated via the inhibition of mTOR complex 2 (mTORC2), another study recently demonstrated that the activity of mTOR complex 1 (mTORC1) was increased in islets from patients with type 2 diabetes and inhibition of mTORC1-S6K1 signaling improved insulin secretion [13]. These studies suggest that when diabetic patients are treated with everolimus, not only the adverse effects of everolimus on β-cell function through mTORC2 inhibition but also its reciprocal effects through mTORC1 signaling need to be taken into account.
In addition to MIN6 cells, hiPSC-derived insulin-producing cells treated with everolimus showed a significant decrease in GSIS. This finding is important for two reasons. First, the findings from our clinical case were confirmed in human cells as well as in mouse-derived insulinoma cells. Second, induced pluripotent stem cell–derived insulin-producing cells can be used as a model of human β cells and are less proliferative than MIN6 cells [14]: that is, everolimus inhibits GSIS in nontumor cells as well as in insulinoma cells. The latter point is consistent with the previous finding that rapamycin impairs insulin secretion in rat isolated islets [15]. In addition, it was recently reported that a patient who was treated with everolimus after heart transplantation developed diabetic ketoacidosis and required insulin therapy [16], whereas prolonged mTOR inhibition by rapamycin has improved metabolic profiles and insulin sensitivity [17]. Because everolimus is widely used as an immunosuppressant after organ transplantation and as an anticancer agent against many other cancers, it should be taken into account that mTOR inhibition by everolimus can result in both beneficial and detrimental effects on whole-body metabolic profiles, with consideration of patient background, duration of everolimus treatment, and other relevant factors.
Supplementary Material
Acknowledgments
We thank Yoshio Fujitani (Gunma University) and Akiko Popiel (Tokyo Medical University) for helpful advice and criticism and Eriko Magoshi and Hiroko Hibino for their assistance with the experiments.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- EdU
5-ethynyl-2′-deoxyuridine
- GSIS
glucose-stimulated insulin secretion
- hiPSC
human induced pluripotent stem cell
- MIN6
mouse insulinoma cells
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
References and Notes
- 1. Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group . Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kulke MH, Bergsland EK, Yao JC. Glycemic control in patients with insulinoma treated with everolimus. N Engl J Med. 2009;360(2):195–197. [DOI] [PubMed] [Google Scholar]
- 3. Ong GS, Henley DE, Hurley D, Turner JH, Claringbold PG, Fegan PG. Therapies for the medical management of persistent hypoglycaemia in two cases of inoperable malignant insulinoma. Eur J Endocrinol. 2010;162(5):1001–1008. [DOI] [PubMed] [Google Scholar]
- 4. Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127(1):126–132. [DOI] [PubMed] [Google Scholar]
- 5. Grozinsky-Glasberg S, Franchi G, Teng M, Leontiou CA, Ribeiro de Oliveira A Jr, Dalino P, Salahuddin N, Korbonits M, Grossman AB. Octreotide and the mTOR inhibitor RAD001 (everolimus) block proliferation and interact with the Akt-mTOR-p70S6K pathway in a neuro-endocrine tumour cell Line. Neuroendocrinology. 2008;87(3):168–181. [DOI] [PubMed] [Google Scholar]
- 6. Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105(7):2415–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiebrich HB1 Siemerink EJ, Brouwers AH, Links TP, Remkes WS, Hospers GA, de Vries EG. Everolimus induces rapid plasma glucose normalization in insulinoma patients by effects on tumor as well as normal tissues. Oncologist. 2011;16(6):783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schuler W, Sedrani R, Cottens S, Häberlin B, Schulz M, Schuurman HJ, Zenke G, Zerwes HG, Schreier MH. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64(1):36–42. [DOI] [PubMed] [Google Scholar]
- 9. Asayama M, Yamada-Murano T, Hara H, Ooki A, Kurosumi M, Yamaguchi K. Everolimus dramatically improves glycemic control in unresectable metastatic insulinoma: a case report. Jpn J Clin Oncol. 2014;44(2):186–190. [DOI] [PubMed] [Google Scholar]
- 10. Barlow AD, Nicholson ML, Herbert TP. Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes. 2013;62(8):2674–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barlow AD, Xie J, Moore CE, Campbell SC, Shaw JA, Nicholson ML, Herbert TP. Rapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mTOR complex 2 (mTORC2). Diabetologia. 2012;55(5):1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Placzkowski KA, Vella A, Thompson GB, Grant CS, Reading CC, Charboneau JW, Andrews JC, Lloyd RV, Service FJ. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab. 2009;94(4):1069–1073. [DOI] [PubMed] [Google Scholar]
- 13. Yuan T, Rafizadeh S, Gorrepati KD, Lupse B, Oberholzer J, Maedler K, Ardestani A. Reciprocal regulation of mTOR complexes in pancreatic islets from humans with type 2 diabetes. Diabetologia. 2017;60(4):668–678. [DOI] [PubMed] [Google Scholar]
- 14. Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–1133. [DOI] [PubMed] [Google Scholar]
- 15. Shimodahira M, Fujimoto S, Mukai E, Nakamura Y, Nishi Y, Sasaki M, Sato Y, Sato H, Hosokawa M, Nagashima K, Seino Y, Inagaki N. Rapamycin impairs metabolism-secretion coupling in rat pancreatic islets by suppressing carbohydrate metabolism. J Endocrinol. 2010;204(1):37–46. [DOI] [PubMed] [Google Scholar]
- 16. Kubo F, Takahara M, Yasuda T, Shimo N, Matsuoka TA, Shimomura I. A case of diabetic ketoacidosis after everolimus treatment. Acta Diabetol. 2016;53(5):861–862. [DOI] [PubMed] [Google Scholar]
- 17. Fang Y, Westbrook R, Hill C, Boparai RK, Arum O, Spong A, Wang F, Javors MA, Chen J, Sun LY, Bartke A. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17(3):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.