Abstract
Background
Cryptococcus is the commonest cause of adult meningitis in Africa, with 50%–70% experiencing increased intracranial pressure. Cerebral oximetry is a noninvasive near-infrared spectroscopy technology to monitor percent regional cerebral tissue oxygenation (rSO2). We assessed if cerebral oximetry predicts meningitis mortality.
Methods
We performed cerebral oximetry within 14 days of cryptococcal meningitis diagnosis on 121 Ugandans from April 2016 to September 2017. We evaluated baseline rSO2 association with mortality by multivariable logistic regression and correlation with other clinical factors. We compared groups formed by initial rSO2 <30% vs ≥30% for longitudinal change with mixed effects models. We measured change in %rSO2 before and after lumbar puncture (LP).
Results
The median initial rSO2 (interquartile range) was 36% (29%–42%), and it was <30% in 29% (35/121). For 30-day mortality, the unadjusted odds ratio (per 5% increase in rSO2) was 0.73 (95% confidence interval [CI], 0.58 to 0.91; P = .005). Those with initial rSO2 <30% had 3.4 (95% CI, 1.5 to 8.0) higher odds of 30-day mortality than those with initial rSO2 ≥30%. Hemoglobin correlated with initial rSO2 (rho = .54; P < .001), but rSO2 did not correlate with pulse oximetry, intracranial pressure, cerebral perfusion pressure, or quantitative cerebrospinal fluid culture, and rSO2 was unchanged pre/post–lumbar punctures. The longitudinal rSO2 measurements change was 15% (95% CI, 12% to 18%) lower in the group with initial rSO2 <30%.
Conclusions
Individuals with cryptococcal meningitis and low cerebral oximetry (rSO2 < 30%) have high mortality. Cerebral oximetry may be useful as a prognostic marker of mortality. Targeted interventions to improve rSO2 should be tested in trials to try to decrease mortality in meningitis.
Keywords: cerebrovascular circulation, cryptococcal meningitis, hemodynamic monitoring, mortality, oximetry, physiologic monitoring
Globally, cryptococcal meningitis causes 15%–20% of AIDS-related deaths [1], and Cryptococcus is the most common etiology of adult meningitis in Sub-Saharan Africa [2, 3]. Even with survival, substantial reversible neurocognitive impairment and serious irreversible neurological impairments occur [4]. Elevated intracranial pressure (ICP) is a well-known complication of cryptococcal meningitis. Elevated ICP of >250 mmH20 is present in two-thirds of African cryptococcal meningitis patients and half of US patients [5]. Graybill et al. reported that those with elevated ICP at presentation had more severe clinical features and increased short-term mortality compared with those who had ICP <250 mmH20 [5]. Rolfes and colleagues further demonstrated that at least 1 therapeutic lumbar puncture during the first week of cryptococcal meningitis treatment conferred a 69% relative reduction in 10-day mortality, regardless of initial cerebrospinal fluid opening pressure [6]. The role of elevated ICP as a cause of decreased cerebral perfusion in patients with cryptococcal meningitis is unclear.
Cerebral oximetry, which conceptually is pulse oximetry for the brain, is more accurately a noninvasive technology using near-infrared spectroscopy to monitor percent regional cerebral tissue oxygenation (rSO2) [7]. Like peripheral capillary oxygen saturation (SpO2) readings by pulse oximetry, the rSO2 readings by cerebral oximetry can represent both a stable clinical status and an acute change in clinical status. Unlike pulse oximetry, near-infrared spectroscopy is not flow dependent. It penetrates several centimeters into tissue, and by measuring the percentage of oxygen bound to hemoglobin and calculating a venous weighted average, it represents the balance between oxygen supply and demand beneath the sensor. In this respect, cerebral oximetry is theoretically better than pulse oximetry at detecting when arterial oxygen level may be high but oxygen is not perfusing into tissue, more similar to a mixed venous oxygen saturation (SvO2). Low rSO2 values are associated with low cerebral perfusion, decreased oxygen-carrying capacity (eg, anemia), and poor outcomes in multiple settings. Anesthesiology commonly uses cerebral oximetry, where intraoperative cerebral oximetry changes consistently detect apneic episodes earlier than pulse oximetry [8]. Higher rSO2 levels, for example, were associated with higher rates of return of spontaneous circulation among patients with cardiac arrest [9, 10]. Two studies have shown improvements in rSO2 after blood transfusion by increasing oxygen-carrying capacity and volume status [11, 12]. Lastly, a study of patients with traumatic brain injury showed a significant association between rSO2 and invasively measured ICP, which directly relates to cerebral perfusion pressure [13]. Despite its current use in the fields of anesthesia, neonatology, neurology, and acute surgical care, cerebral oximetry is not commonly used in the management of meningitis.
Considering the high rate of death among persons with cryptococcal meningitis and the difficulty of predicting an individual’s clinical course, we hypothesized that cerebral oximetry may offer a noninvasive method to identify patients at high risk of clinical worsening who may require additional interventions to improve the chances of survival. Our primary objective was to determine if regional cerebral oxygen saturation predicted mortality in adults with cryptococcal meningitis. The secondary objectives were to determine if therapeutic lumbar punctures increased cerebral oxygen saturation when measured before and after lumbar puncture and to determine associations between regional cerebral oxygen saturation and other clinical parameters, particularly those associated with cerebral perfusion and those amenable to intervention.
METHODS
We evaluated HIV-infected adults with cryptococcal meningitis at Mulago Hospital in Kampala, Uganda, from April 2016 to September 2017 in a nested substudy of the ongoing ASTRO-CM trial (clinicaltrials.gov identifier: NCT01802385). We diagnosed cryptococcal meningitis by cerebrospinal fluid cryptococcal antigen (Immy Inc., Norman, OK). During hospitalization for induction treatment for cryptococcal meningitis, participants received amphotericin B deoxycholate 0.7–1.0 mg/kg/d, fluconazole 800 mg/d, and either sertraline 400 mg/d or placebo. All participants or their surrogates provided written informed consent to participate in the study.
From April 2016 until October 2016, one research personnel performed the monitoring with a single cerebral oximeter machine and monitored each patient for approximately 30 minutes. Left-sided and right-sided rSO2 values were collected and averaged over those 30 minutes of monitoring and then averaged together for each participant. With one cerebral oximeter and up to 20 patients hospitalized with cryptococcal meningitis at any given time, initial monitoring was sampled. Although the goal was to sample as many participants as possible, priority for cerebral oximetry monitoring was given to the most ill-appearing patients, including those with altered mental status, unstable blood pressure, or previous low rSO2 readings if previously taken. After this period, we performed an interim analysis to determine major risk factors that affected cerebral oximeter readings and to determine an appropriate cutoff for low rSO2 values requiring intervention. From November 2016 until April 2017, with an additional cerebral oximeter, we recorded cerebral oximeter readings for additional participants with a more systematic method of monitoring. The monitoring session was reduced from 30 minutes duration to the amount of time needed to register a stable reading on the monitor. The rSO2 collection and averaging were performed the same as for the earlier participants. We sampled all new participants within 48 hours of meningitis diagnosis. Over the whole course of the study, all participants were scheduled to receive lumbar punctures on days 1, 3, 7, ±10, and 14 of admission. Additional lumbar punctures were performed at the discretion of the physician if the participant had elevated opening pressure >250 mmH2O, worsening headaches, or decreasing Glasgow Coma Scale score. When study staff availability corresponded with the timing of lumbar puncture, we performed 2 cerebral oximetry measurements, one immediately before and another immediately after lumbar puncture.
Regional cerebral oxygen saturation (cerebral oximetry) recordings were performed using an INVOS 5100C Cerebral Oximeter (Medtronic, Minneapolis, MN). We performed cerebral oximetry by placing 2 sensors positioned on the forehead with the long axis parallel to the intra-aural line and the superior sensor edge adjacent to the hairline. INVOS system recording quality was assessed by inspection of the signal strength index for each channel. A continuous display of >1 out of 5 bars on the monitor represented adequate signal strength.
Other clinical data routinely collected as part of the ASTRO study included pulse oximetry, blood pressure, temperature, lumbar puncture opening pressure, hemoglobin, and Glasgow Coma Scale score. We did not, however, do cryptococcal antigen titers due to cost.
Normal rSO2 have been considered to be approximately 55%–90%, and dangerously low values to be between 30% and 40%, by the manufacturer. Prior studies, however, have been predominantly performed in light-skinned persons. Among 6 Ugandan research staff, the range of readings was 40%–57%, with a mean of 50% rSO2. In this study, rSO2 values were stratified into 2 groups: rSO2 < 30% and rSO2 ≥ 30%. Cerebral perfusion pressure was defined as mean arterial pressure minus ICP (CPP = MAP – ICP). Opening pressure from lumbar puncture was used to approximate ICP, where 1 mmH2O = 0.0736 mmHg. Mean arterial pressure was defined as 2 × diastolic blood pressure + systolic blood pressure divided by 3.
Data were analyzed using SAS, version 9.4 (Cary, NC). Participants with an initial rSO2 measurement within 14 days of cryptococcal meningitis diagnosis were included. Groups were formed by initial rSO2 measurement (<30% or ≥30%). Descriptive summaries were expressed as proportions or medians (with interquartile range). Fisher exact and Kruskall-Wallis tests were used to compare the rSO2 groups. For the relationship between rSO2 and mortality, univariate and multivariable logistic regression tested 2-week and 30-day mortality as the dependent variable and rSO2 as the independent variable (both as a continuous variable and by comparing the rSO2 groups). The multivariable models were adjusted for Glasgow Coma Scale, temperature, cerebrospinal fluid (CSF) white blood cells counts and quantitative culture, and hemoglobin. Spearman correlation determined the association between rSO2 measurements and other continuous clinical variables (eg, hemoglobin, opening pressure, pulse oximetry, temperature, mean arterial pressure, and cerebral perfusion pressure) collected on or within 2 weeks after the initial rSO2 measurement. Longitudinal mixed models were evaluated to compare the groups formed by initial rSO2 measurement for the change in rSO2 over time. The change between pre– and post–lumbar puncture rSO2 measurements was evaluated with a paired t test.
RESULTS
We enrolled 121 participants with RSO2 measurements within 14 days of meningitis diagnosis from April 2016 through September 2017. Median (interquartile range [IQR]) initial rSO2 was 36% (29%–42%), with 29% (35/121) having an initial rSO2 <30%. Baseline characteristics were not significantly different between the initial rSO2 groups, except for hemoglobin, which was lower in the rSO2 <30% group, and temperature, which was increased in the rSO2 <30% group (Table 1).
Table 1.
Characteristics at CM Diagnosis and Mortality Outcomes for Study Participants
| rSO2 < 30% | rSO2 ≥ 30% | ||||
|---|---|---|---|---|---|
| No. With Data | No. (%) or Median (IQR) | No. With Data | No. (%) or Median (IQR) | P Valuea | |
| No. of participants | 35 | 86 | |||
| Days from diagnosis to rSO2 measurement | 3 (0–7) | 1 (0–4) | |||
| Cerebral oximetry, rSO2, % | 24 (20–27) | 39 (34–44) | |||
| Demographics | |||||
| Age, y | 35 | 35 (29–40) | 86 | 35 (29–40) | .77 |
| Men | 35 | 15 (43) | 86 | 47 (55) | .24 |
| Glasgow coma scale < 15 | 35 | 16 (46) | 86 | 49 (57) | .26 |
| Clinical data | |||||
| Months since HIV infection | 33 | 10 (1–46) | 69 | 5 (1–51) | .88 |
| Current antiretroviral use | 35 | 14 (40) | 86 | 29 (34) | .51 |
| Months on antiretroviral therapy | 14 | 17 (1–63) | 29 | 10 (3–47) | .91 |
| CD4 count, cells/μL | 30 | 16 (7–34) | 74 | 19 (8–61) | .45 |
| Weight, kg | 35 | 51 (45–55) | 80 | 50 (50–60) | .09 |
| Temperature, axillary ⁰C | 34 | 36.9 (36.1–37.8) | 85 | 36.4 (36.0–36.9) | .04 |
| Seizures present | 35 | 10 (29) | 86 | 14 (16) | .13 |
| Hemoglobin, g/dL | 31 | 10.4 (7.6–11.3) | 75 | 11.5 (10.1–13.1) | .002 |
| Mean arterial pressure,b mmHg | 35 | 94 (87–107) | 84 | 96 (87–104) | .93 |
| Pulse oximetery,c % O2 saturation | 19 | 97 (96–98) | 53 | 96 (95–98) | .61 |
| Cerebral perfusion pressure,d mmHg | 28 | 83 (72–90) | 68 | 75 (67–87) | .09 |
| CSF measures | |||||
| Opening pressure, mmH2O | 28 | 200 (110–345) | 70 | 255 (180–450) | .05 |
| Quantitative culture, log10 CFU/mL | 30 | 4.3 (2.1–5.4) | 67 | 4.8 (3.2–5.4) | .43 |
| CSF culture sterile at diagnosis | 30 | 6 (20) | 67 | 2 (3) | .005 |
| CSF white cells ≥5 cells/μL | 33 | 12 (36) | 84 | 33 (39) | .77 |
| Outcome | |||||
| Death within 14 d | 35 | 11 (31) | 86 | 11 (13) | .02 |
| Death within 30 d | 35 | 16 (46) | 86 | 17 (20) | .004 |
Abbreviations: CFU, colony-forming unit; CSF, cerebrospinal fluid; CM, cryptococcal meningitis; IQR, interquartile range; rSO2 = regional cerebral tissue oxygenation.
aData are median with 25th and 75th percentiles. Chi-square P value for categorical, Kruskall-Wallis tests for medians.
bMean arterial pressure (in mm Hg) = (2*DBP + SBP)/3.
cDay 7 measurement.
dCerebral perfusion pressure (in mm Hg) = Mean arterial pressure - CSF Opening pressure (in mm H2O)P*0.0735559.
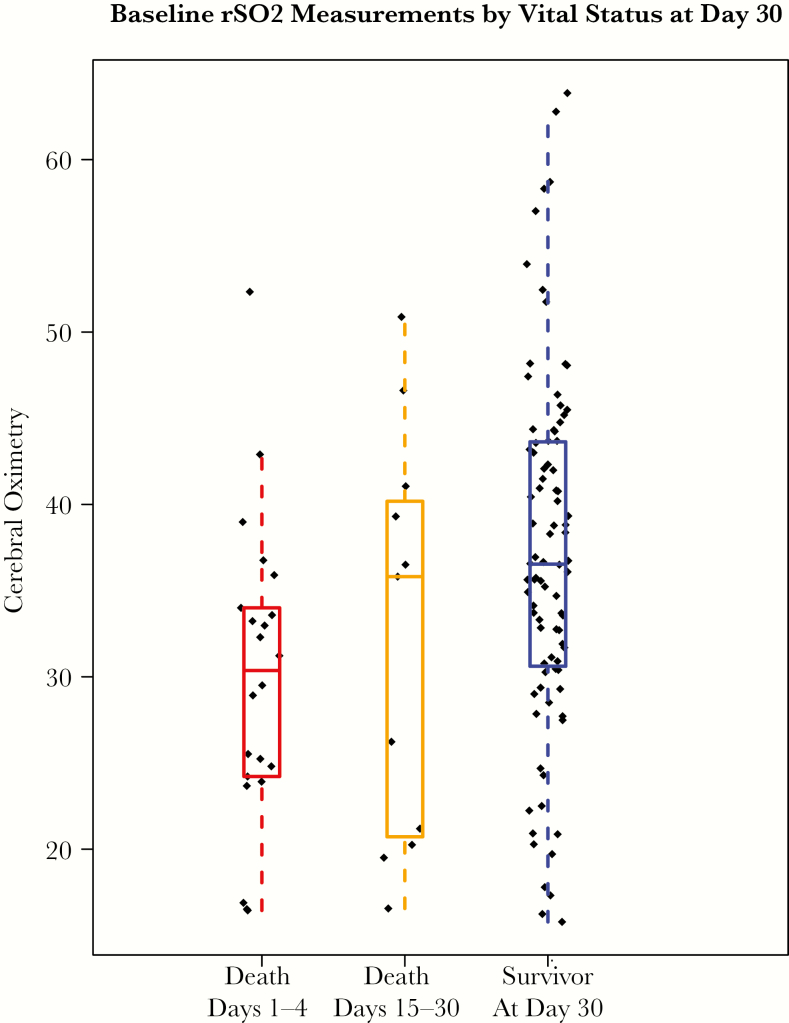
Overall, the 30-day mortality rate was 27% (33/121). Regional cerebral oxygen saturation strongly predicted both 14- and 30-day mortality (Table 2). Per 5% increase in initial rSO2, the odds ratio for 30-day mortality was 0.73 (95% confidence interval [CI], 0.59 to 0.91; P < .01). Alternately stated, there was a 27% relative reduction in 30-day mortality per 5% increase in initial cerebral oximetry. Figure 1 displays the distribution of initial rSO2 by vital status at day 30. Among the 22 people who died within 14 days of meningitis diagnosis, the median (IQR) rSO2 was 30% (24%–34%). Compared with those with initial rSO2 ≥30%, the low-rSO2 group was 3.4 (1.5–8.0) times more likely to die within 30 days (P < .01). Mortality results are consistent when adjusted for other known confounders with death. Initial rSO2 was not associated with other clinical events (Table 2).
Table 2.
Associationa Between rSO2 and Death and Clinical Outcomes
| Per 5% Increase in rSO2 | rSO2 < 30% vs rSO2 ≥ 30% | |||
|---|---|---|---|---|
| Outcome | Odds Ratio (95% CI) |
P Value | Odds Ratio (95% CI) |
P Value |
| Death within 14 d | 0.72 (0.56 to 0.93) | .01 | 3.13 (1.20 to 8.11) | .02 |
| Death within 14 d, adjustedb | 0.48 (0.29 to 0.77) | .003 | 5.79 (1.54 to 21.80) | .009 |
| Death within 30 d | 0.73 (0.58 to 0.91) | .005 | 3.42 (1.46 to 8.00) | .005 |
| Death within 30 d, adjustedb | 0.65 (0.45 to 0.93) | .02 | 3.96 (1.19 to 13.15) | .02 |
| Clinical parametersc | ||||
| CSF opening pressure > 250 mm H2O | 0.88 (0.69 to 1.11) | .28 | 1.26 (0.41 to 3.87) | .68 |
| Pulse oximetry < 94% sa02 | 0.93 (0.66 to 1.33) | .70 | 1.47 (0.33 to 6.57) | .61 |
| Mean arterial pressure < 84 mmhg | 0.86 (0.66 to 1.12) | .25 | 1.53 (0.51 to 4.60) | .45 |
| Glasgow Coma Scale < 15 | 0.98 (0.83 to 1.16) | .80 | 0.84 (0.38 to 1.85) | .67 |
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; rSO2 = regional cerebral tissue oxygenation.
aUnadjusted logistic regression models using the first rSO2 measurement in days 1–14.
bModels adjusted for Glasgow Coma Scale <15, CSF white cells >5 cells/μL, CSF quantitative culture, temperature, and anemia (<11 g/dL for men, <10.5 g/dL for women).
cClinical outcomes on or after the rSO2 measurement.
Figure 1.
Baseline regional cerebral tissue oxygenation (rSO2) measurements by vital status at day 30. Initial rSO2 measurements grouped according to early death (days 1–14), late death (days 15–30), or survivor at day 30. The median rSO2 values were 30%, 36%, and 37%, respectively.
Hemoglobin measured at or within 14 days after the initial rSO2 was strongly associated with rSO2, with higher hemoglobin associated with higher rSO2 (Spearman correlation = .54; P < .001). Hemoglobin was also independently associated with 30-day mortality. Per 1-g/dL increase in hemoglobin, 30-day mortality decreased (odds ratio, 0.84; 95% CI, 0.71 to 1.0; P = .04). This means the odds of mortality decreased by 16% for every 1-g/dL increase in hemoglobin. There was a moderate inverse correlation between rSO2 and the most closely measured temperature, with higher temperature associated with lower rSO2 (Spearman correlation = –.19; P = .04). The correlations between rSO2 and proximate measured cerebrospinal fluid opening pressure, cerebral perfusion pressure, pulse oximetry, and mean arterial pressure, were not statistically significant (Table 3).
Table 3.
Association Between rSO2 and Clinical Parameters
| Clinical Parameters | No. | Median (IQR) | Spearman Correlation | P Value |
|---|---|---|---|---|
| Cerebral oximetry, rSO2, % | 121 | 35.6 (28.9–42.1) | ||
| Hemoglobin, g/dL | 114 | 10.9 (8.8–12.6) | 0.54 | <.001 |
| Temperature, axillary ⁰C | 119 | 36.5 (36.0–37.3) | –0.19 | .04 |
| Mean arterial pressure, mmHg | 119 | 96 (87–104) | –0.02 | .86 |
| Pulse oximetry, % O2 saturation | 72 | 96.5 (95–98) | –0.16 | .19 |
| CSF opening pressure, mmH2O | 104 | 220 (155–345) | 0.11 | .27 |
| Cerebral perfusion pressure, mmHg | 103 | 78.3 (69.0–89.6) | –0.09 | .35 |
| CSF white cells/μL | 119 | <5 (<5–90) | 0.11 | .25 |
| CSF quantitative culture, log10 CFU/mL | 97 | 3.7 (1.6–4.8) | 0.13 | .19 |
Abbreviations: CFU, colony-forming unit; CSF, cerebrospinal fluid; IQR, interquartile range; rSO2 = regional cerebral tissue oxygenation.
For 28 participants with 32 pre– and post–lumbar puncture measurements, there was no significant change in rSO2 overall or by initial rSO2 group. The mean rSO2 change was 1% (95% CI, –1.4% to 3.4%) overall.
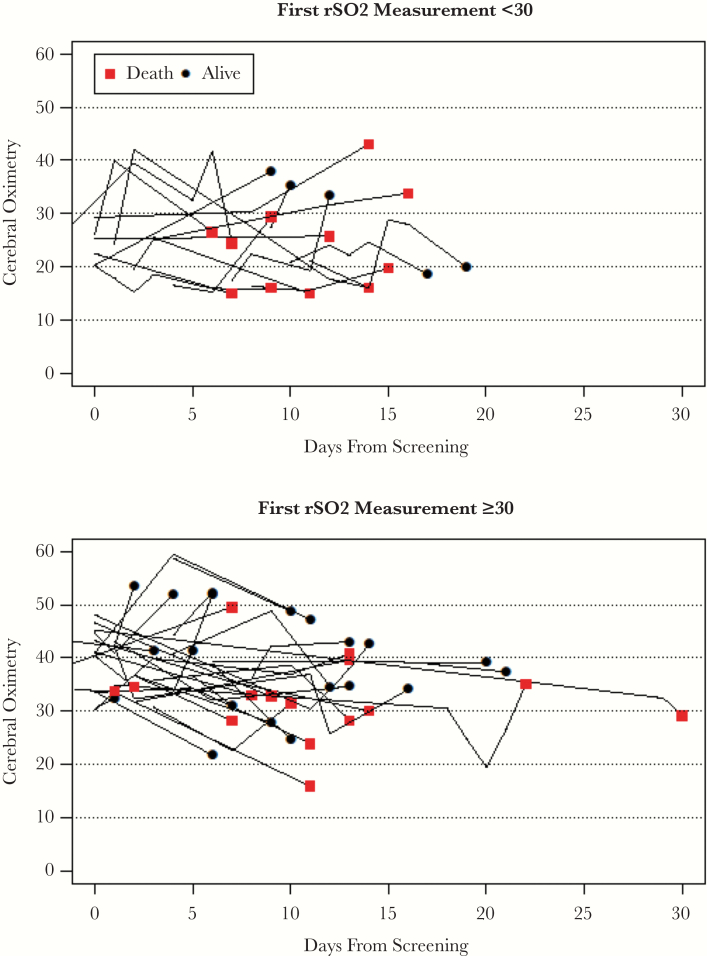
Figure 2 presents the rSO2 measurement over time for 51 participants with rSO2 measurements over the first 30 days; 31% (16/51) had initial rSO2 <30%. From longitudinal mixed models, the change over time in rSO2 was 15% (95% CI, 12% to 18%) lower in the group with initial rSO2 <30%.
Figure 2.
Longitudinal changes in regional cerebral tissue oxygenation (rSO2) readings over time for 51 participants who had multiple cerebral oximetry measurements during the first 30 days after meningitis diagnosis. Generally, survivors improved their cerebral oximetry readings over time. The red squares represent patients who died after the last measurement. The top graph shows 16 of 51 participants with initial rSO2 <30%, and the bottom graph includes 35 participants with initial rSO2 ≥30%. From longitudinal mixed models, the change over time in rSO2 was 15% (95% confidence interval, 12% to 18%) lower in the group with initial rSO2 <30%.
DISCUSSION
We determined that regional cerebral oxygen saturation of the brain was associated with both 2-week and 30-day mortality in Ugandan adults with cryptococcal meningitis, with a 27% relative reduction in 30-day mortality per 5% increase in initial cerebral oximetry. Low rSO2 (<30%) was associated with 3-fold higher mortality compared with those with higher rSO2 (≥30%). Cerebral oximetry remained independently associated with mortality even after controlling for known mortality risk factors such as altered mental status, CSF quantitative Cryptococcus culture, CSF white cell count, and anemia [14, 15]. Cryptococcal antigen titers in CSF were not done, though high titers have been associated with mortality [16–18]
Our main hypothesis was that mortality in cryptococcal meningitis would be driven by elevated ICP, leading to reduced cerebral perfusion, cerebral ischemia, and eventually death. Our results, however, demonstrated no relationship between rSO2 and ICP, mean arterial pressure, pulse oximetry, or the calculated cerebral perfusion pressure. We also found that therapeutic lumbar punctures to reduce ICP did not acutely improve rSO2. The lack of an association between ICP and rSO2 was surprising, especially considering the survival benefits of therapeutic lumbar punctures [6]. In studies of traumatic brain injury, elevated ICP has been associated with reduced rSO2 [13, 19, 20]. Our findings suggest that additional mechanisms beyond ICP likely influence cerebral oxygenation (and mortality) in addition to previously identified prognostic markers in cryptococcosis. The association between rSO2 and mortality may be due to multiple factors influencing cerebral oxygen delivery and/or oxygen consumption.
The studies on early goal-directed therapy have highlighted the importance of early rapid hemodynamic stabilization and the early correction of organ perfusion in the prevention of organ failure and death. One hypothesis states that patients without signs of clinical shock (eg, hypotension, tachycardia, oliguria) can still have hypoperfusion at the cellular level and are at risk for complications if this hypoperfusion is not rapidly corrected [21, 22]. Cerebral oximetry could allow identification of patients who may have subclinical hypoperfusion, early intervention to improve cerebral oxygenation, and monitoring response to interventions.
The other factor related to cerebral oxygen delivery is hemoglobin. We found a strong positive association between rSO2 and hemoglobin levels. In multiple other cryptococcal meningitis cohorts, anemia has been consistently associated with mortality [14, 23]. This study herein suggests that the probable mechanism of mortality with anemia is decreased oxygen-carrying capacity leading to cerebral hypoxia. With blood transfusions, others have demonstrated increases in rSO2 [11, 12]. Conversely, CSF white cell counts did not correlate with rSO2, nor was there any statistical difference in CSF white cells between the low- and high-rSO2 groups. Perivascular edema, or blood–brain barrier breakdown from inflammation, which disrupts oxygen diffusion and extraction, can contribute to cerebral hypoxia [24]. There was a weak negative correlation between rSO2 and increasing temperature, whereby temperature was higher in the low-rSO2 group. This concurs with multiple previous studies reporting hyperthermia associated with elevated ICP, increased cerebral metabolism, and decreased cerebral blood flow [25–27].
Although cerebral oximetry was associated with mortality, rSO2 is not a fixed characteristic. rSO2 is a dynamic process that is amenable to interventions and may be more valuable as a noninvasive monitoring tool to guide patient management in a neuro–critical care setting. Considering the strong association between rSO2 and hemoglobin, oxygen status could be optimized by monitoring individual response to interventions such as increasing volume status, supplemental oxygen, and lowered threshold for blood transfusion.
Limitations to this study included convenience sampling early in the study, which oversampled more ill patients. This oversampling skews the median descriptive results but does not affect the conclusion that lower rSO2 was associated with higher mortality. A second limitation was that our study included people with darker skin than the previous studies in high-income countries. Darker skin pigmentation may have lowered the range of measured “normal” rSO2 within the population. As rSO2 is a continuous measure, focusing on an exact 30% rSO2 threshold is not warranted. We chose <30% as a cutpoint for analysis as this was the lowest quartile. The relative reading is likely more generalizable across populations as there was a 27% relative reduction in 30-day mortality per 5% increase in initial rSO2. A third limitation is the inclusion of only one type of meningitis, cryptococcal. Our results in cryptococcosis are not immediately applicable to other types of meningitis caused by bacteria, tuberculosis, or aseptic meningitis but are worthy of future study. A fourth possible limitation to consider is the effect of temperature. Patient hyperthermia has been previously associated with decreased cerebral blood flow and decreased cerebral oximetry measurements [25–27]. These trends are most significant with temperatures >38°C. On the other hand, measurements by older cerebral oximeter models in the past have been shown to increase in response to cutaneous applied heat [28, 29], implying that the cutaneous tissue oxygenation was largely contributing to the measurements, rather than solely cerebral tissue oxygenation. We used a newer model cerebral oximeter that has been updated to measure more deeply into tissue without being affected by cutaneous tissue oxygenation status.
Our data have shown that cerebral oximetry is associated with mortality in cryptococcal meningitis and may have prognostic utility for patients. Having a noninvasive prognostic marker, rSO2, gives us not only a way to focus on identified high-risk patients who may need closer observation but also a method to continually evaluate the efficacy of interventions. Further research with this technology should investigate its use to guide interventions, such as supplemental oxygen, blood transfusion, fluid bolus therapy, hypertonic saline, and mannitol therapy, with the ultimate goal of improving survival of patients with infectious meningitis.
Acknowledgments
ASTRO-CM Kampala Team Members. Edward Mpoza, Reuben Kiggundu, Lillian Tugume, Kenneth Ssebambulidde, Andrew Akampurira, Paul Kirumira, Darlisha A. Williams, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Tadeo Kiiza Kandole, Tony Luggya, Julian Kaboggoza, Eva Laker, Alice Namudde, Sarah Lofgren, Richard Kwizera, Kirsten Nielsen, Anna Stadelman, and Ananta S. Bangdiwala.
Financial support. This research was supported by the National Institute of Neurologic Diseases and Stroke (NINDS), National Institutes of Health through the Fogarty International Center (FIC; grant number 1K43TW010718-01), and National Institute of Allergy and Infectious Diseases (NIAID; R01NS086312, R25TW009345, T32AI055433); United Kingdom Medical Research Council and Wellcome Trust (MR/M007413/1); and the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. John W. Diehl is a Doris Duke International Clinical Research Fellow. We thank Steven Bushey for supporting this project.
Contributor Information
ASTRO-CM Trial Team:
Edward Mpoza, Reuben Kiggundu, Lillian Tugume, Kenneth Ssebambulidde, Andrew Akampurira, Paul Kirumira, Darlisha A Williams, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Tadeo Kiiza Kandole, Tony Luggya, Julian Kaboggoza, Eva Laker, Alice Namudde, Sarah Lofgren, Richard Kwizera, Kirsten Nielsen, Anna Stadelman, and Ananta S Bangdiwala
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Durski KN, Kuntz KM, Yasukawa K, et al. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr 2013; 63:e101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adeyemi B, Ross A. Profile and mortality outcome of patients admitted with cryptococcal meningitis to an urban district hospital in KwaZulu-Natal, South Africa. J Int AIDS Soc 2014; 17:19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlson RD, Rolfes MA, Birkenkamp KE, et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab Brain Dis 2014; 29:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis 2000; 30:47–54. [DOI] [PubMed] [Google Scholar]
- 6. Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 2014; 59:1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol 1999; 58:541–60. [DOI] [PubMed] [Google Scholar]
- 8. Tobias JD. Cerebral oximetry monitoring with near infrared spectroscopy detects alterations in oxygenation before pulse oximetry. J Intensive Care Med 2008; 23:384–8. [DOI] [PubMed] [Google Scholar]
- 9. Parnia S, Yang J, Nguyen R, et al. Cerebral oximetry during cardiac arrest: a multicenter study of neurologic outcomes and survival. Crit Care Med 2016; 44:1663–74. [DOI] [PubMed] [Google Scholar]
- 10. Singer AJ, Ahn A, Inigo-Santiago LA, et al. Cerebral oximetry levels during CPR are associated with return of spontaneous circulation following cardiac arrest: an observational study. Emerg Med J 2015; 32:353–6. [DOI] [PubMed] [Google Scholar]
- 11. Nahavandi M, Tavakkoli F, Hasan SP, et al. Cerebral oximetry in patients with sickle cell disease. Eur J Clin Invest 2004; 34:143–8. [DOI] [PubMed] [Google Scholar]
- 12. Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Cerebral oximetry in Ugandan children with severe anemia: clinical categories and response to transfusion. JAMA Pediatr 2016; 170:995–1002. [DOI] [PubMed] [Google Scholar]
- 13. Dunham CM, Sosnowski C, Porter JM, et al. Correlation of noninvasive cerebral oximetry with cerebral perfusion in the severe head injured patient: a pilot study. J Trauma 2002; 52:40–6. [DOI] [PubMed] [Google Scholar]
- 14. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis 2008; 46:1694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antinori S, Galimberti L, Magni C, et al. Cryptococcus neoformans infection in a cohort of Italian AIDS patients: natural history, early prognostic parameters, and autopsy findings. Eur J Clin Microbiol Infect Dis 2001; 20:711–7. [DOI] [PubMed] [Google Scholar]
- 17. Tseng HK, Liu CP, Ho MW, et al. Microbiological, epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997–2010. PloS One 2013; 8:e61921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Antinori S, Radice A, Galimberti L, et al. The role of cryptococcal antigen assay in diagnosis and monitoring of cryptococcal meningitis. J Clin Microbiol 2005; 43:5828–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mousa WF, Mowafi HA, Al-Metwalli RR, et al. Preoperative mannitol infusion improves perioperative cerebral oxygen saturation and enhances postoperative recovery after laparoscopic cholecystectomy. Saudi Med J 2015; 36:1199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kampfl A, Pfausler B, Denchev D, et al. Near infrared spectroscopy (NIRS) in patients with severe brain injury and elevated intracranial pressure. A pilot study. Acta Neurochir Suppl 1997; 70:112–4. [DOI] [PubMed] [Google Scholar]
- 21. Blow O, Magliore L, Claridge JA, et al. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma 1999; 47:964–9. [DOI] [PubMed] [Google Scholar]
- 22. Simpson SQ, Gaines M, Hussein Y, Badgett RG. Early goal-directed therapy for severe sepsis and septic shock: a living systematic review. J Crit Care 2016; 36:43–8. [DOI] [PubMed] [Google Scholar]
- 23. Tugume L, Morawski BM, Abassi M, et al. Prognostic implications of baseline anaemia and changes in haemoglobin concentrations with amphotericin B therapy for cryptococcal meningitis. HIV Med 2017; 18:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korbakis G, Vespa PM. Multimodal neurologic monitoring. Handb Clin Neurol 2017; 140:91–105. [DOI] [PubMed] [Google Scholar]
- 25. Bain AR, Morrison SA, Ainslie PN. Cerebral oxygenation and hyperthermia. Front Physiol 2014; 5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nyholm L, Howells T, Lewén A, et al. The influence of hyperthermia on intracranial pressure, cerebral oximetry and cerebral metabolism in traumatic brain injury. Ups J Med Sci 2017; 122:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nemoto EM, Frankel HM. Cerebral oxygenation and metabolism during progressive hyperthermia. Am J Physiol 1970; 219:1784–8. [DOI] [PubMed] [Google Scholar]
- 28. Davis SL, Fadel PJ, Cui J, et al. Skin blood flow influences near-infrared spectroscopy-derived measurements of tissue oxygenation during heat stress. J Appl Physiol (1985) 2006; 100:221–4. [DOI] [PubMed] [Google Scholar]
- 29. Miyazawa T, Horiuchi M, Komine H, et al. Skin blood flow influences cerebral oxygenation measured by near-infrared spectroscopy during dynamic exercise. Eur J Appl Physiol 2013; 113:2841–8. [DOI] [PubMed] [Google Scholar]