Abstract
Introduction
Due to the heterogeneity of psychiatric illnesses and overlapping mechanisms, patients with psychosis are differentially responsive to pharmaceutical drugs. In addition to having therapeutic effects for schizophrenia and bipolar disorder, antipsychotics and mood stabilizers have many clinical applications and are used unconventionally due to their direct and indirect effects on neurotransmitters. Synapsins, a family of neuronal phosphoproteins, play a key regulatory role in neurotransmitter release at synapses. In this study, we investigated the effects of mood stabilizers, lithium, and valproic acid on synapsin gene expression in the rat brain.
Methods
Intraperitoneal injections of saline, lithium, and valproic acid were administered to male Sprague Dawley rats twice daily for 14 d, corresponding to their treatment group. Following decapitation and brain tissue isolation, mRNA was extracted from various brain regions including the hippocampus, striatum, prefrontal cortex, and frontal cortex.
Results
Biochemical analysis revealed that lithium significantly increased gene expression of synapsin I in the striatum, synapsin IIa in the hippocampus and prefrontal cortex, and synapsin IIb in the hippocampus and striatum. Valproic acid significantly increased synapsin IIa in the hippocampus and prefrontal cortex, as well as synapsin IIb in the hippocampus and striatum.
Conclusion
These significant changes in synapsin I and II expression may implicate a common transcription factor, early growth response 1, in its mechanistic pathway. Overall, these results elucidate mechanisms through which lithium and valproic acid act on downstream targets compared with antipsychotics and provide deeper insight on the involvement of synaptic proteins in treating neuropsychiatric illnesses.
Keywords: lithium, valproic acid, synapsin, bipolar disorder
Significance Statement
Lithium and valproic acid (VPA) are the most commonly prescribed drugs for the treatment of bipolar disorder. However, the exact mechanisms through which these drugs exert their effects are unknown. The aim of this study was to investigate the effects of lithium and VPA on synapsin phosphoproteins in various regions of the rat brain. Synapsins play a key role in regulating synapse formation, synaptic signalling, and synaptic plasticity. In this study, rats were injected with saline, lithium, or VPA twice daily for 14 days. Several brain regions, including the hippocampus, striatum, prefrontal cortex, and frontal cortex, were analyzed for the expression of different synapsin isoforms including synapsin I, IIa, and IIb. Lithium and VPA significantly altered synapsin I, IIa, and IIb expression in different regions. The results of this study will increase our understanding of the biochemical mechanisms through which lithium and VPA exert their therapeutic effects.
Introduction
Bipolar disorder (BD) is a severely disabling psychiatric illness characterized by the occurrence of both manic and depressive episodes. The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-V) describes a manic episode as a distinct period of abnormally and persistently elevated, expansive, or irritable mood lasting at least 1 week. Depressive episodes are intense, persistent feelings of despair and hopelessness lasting over a 2-week period (APA, 2013). Mood fluctuations in BD patients can be severe enough to result in hospitalization to prevent harm to oneself or others. A higher risk of suicidal behavior is also observed in individuals with BD compared with individuals with other psychiatric conditions or the healthy population (Song et al., 2017). Furthermore, patients may find it difficult to return to their day-to-day lifestyle including work and social interactions due to the nature and prognosis of the disease. In Europe from 1999 to 2009, 70% of patients with BD were underemployed in Germany, and 63% to 67% of patients were unemployed in Italy (Fajutrao et al., 2009). BD was ranked in the top 20 causes of disability among all medical conditions worldwide by a recent global burden of disease study by the World Health Organization (WHO) (Vos et al., 2012). A greater understanding of the pathophysiology of BD may contribute to alleviating risks associated with the disorder and improving quality of life for patients.
Lithium and valproic acid (VPA) are the most commonly prescribed mood stabilizers for patients with BD. Lithium is the only treatment that has been shown to be effective in preventing mania and depression. It is a monovalent cation with powerful antiinflammatory, antioxidative properties (Patel and Frey, 2015). Lithium decreases suicidal risk in patients and increases the volume of brain regions associated with emotional regulation, including the amygdala, hippocampus, and prefrontal cortex (Cipriani et al., 2013; Malhi, et al., 2013). Although the mechanisms by which lithium exerts its effects are still unclear, lithium has been shown to directly inhibit glycogen synthase kinase 3B (GSK3B) through competition with Mg2+ binding as well as indirectly inhibit GSK3B through the activation of Wnt signalling pathways (Di Daniel et al., 2005; Chiu et al., 2013; Lazzara and Kim, 2015). Interestingly, lithium has been shown to suppress glutamate excitotoxicity both in vitro and in vivo (Chalecka-Franaszek et al., 1999).
Comparatively, VPA is an anticonvulsant drug used in the maintenance treatment of BD. Although an anticonvulsant drug, VPA was originally proposed to treat BD due to its effect on the enhancement of GABAergic activity and its direct effects on enzymes involved in GABA metabolism (Gould et al., 2004). It is an established histone deacetylase inhibitor that has been shown to be highly effective in attenuating manic episodes observed in BD. Similarly to lithium, VPA indirectly activates Wnt signalling pathways, but it also robustly induces the activation of the mitogen associated protein kinase pathway (Chiu et al., 2013; Lazzara and Kim, 2015). However, VPA does not show the same effects in reducing suicidal ideations and behaviors compared with lithium. Although both lithium and VPA play a role in several interconnected pathways, the exact mechanisms underlying the functionality of these drugs and their effects on various neurotransmission pathways still remain to be further investigated.
Synapsins are among the first neuronal phosphoproteins identified that play a key role in synapse regulation, including neurotransmitter release, vesicle maintenance, and synaptic plasticity (Hilfiker et al., 2005; Song and Augustine, 2015). Their functions are mediated by phosphorylation on various sites by many different kinases (Jovanovic et al., 1996; Hosaka et al., 1999; Cesca et al., 2010). The synapsin family consists of 3 functional genes, synapsin I, II, and III, and each subtype has several isoforms. Synapsin I and II are more commonly found in mature synapses, while synapsin III is often expressed during the developmental stages of neurons and synapses. The N terminus is highly conserved amongst all synapsins, and the C terminus consists of many variable domains found in different isoforms. The transcription complexity as well as distinguishing features of the synapsins may suggest key differences in functionality that are yet to be completely elucidated.
Although the structural and functional relationship of synapsins has not been specifically established, many studies focus on the functional role of synapsins in neurotransmitter signalling (Song and Augustine, 2015). Neurotransmitters, including GABA, glutamate, and dopamine, have been linked and associated with various isoforms of synapsins. These phosphoproteins are known to tether glutamatergic vesicles within the reserve pool and regulate the size of the reserve pool (Gitler et al., 2004). In triple knockout mice, glutamatergic synaptic depression is specifically rescued by the addition of synapsin IIa (Gitler et al., 2008). Synapsins regulate a late step in GABAergic synaptic vesicle trafficking that precedes the fusion of GABAergic synaptic vesicle exocytosis (Gitler et al., 2004). However, the specific isoform regulating this vesicle trafficking is unclear (Song and Augustine, 2015). Synapsin I knockout mice and synapsin III knockout mice display reduced basal inhibitory synaptic transmission, while synapsin II knockout mice display increased inhibitory transmission (Feng et al., 2002; Baldelli et al., 2007; Medrihan et al., 2013). Lack of all synapsin isoforms increases catecholamine release in chromaffin cells and is only rescued by synapsin IIa, exhibiting its function as a negative regulator of catecholamine release (Villanueva et al., 2006). Synapsin IIa appears to have opposite effects on glutamate release compared with catecholamine release. It increases glutamate release but decreases the release of catecholamines (Song and Augustine, 2015). A triple knockout of synapsins increases dopamine release from presynaptic terminals but does not affect serotonin release (Kile et al., 2010). However, it is suggested that negative regulation of dopamine release is mediated by synapsin IIa, as the same phenotype is observed in synapsin IIa knockout mice (Kile et al., 2010). Other isoforms implicated in dopaminergic and GABAergic synaptic vesicle regulation remain unclear. Expression of synapsins in bipolar disorder (BD) has previously been characterized, and studies have shown decreased levels of synapsins in the medial prefrontal cortex and hippocampus of patients with BD (Tan et al., 2014). A recent study investigating DNA modifications showed a change in CpG methylation pattern of synapsin genes in patients with BD (Cruceanu et al., 2016). The objective of this study was to investigate the effect of mood stabilizers lithium and VPA on synapsin expression to address the mechanisms involved in the action of mood stabilizers.
Materials and Methods
Animal Handling/Drug Administration
Twenty-two male Sprague Dawley rats (Charles Rivers Laboratories), obtained at weights 300 to 350 g, were housed individually in the central animal facility at McMaster University, Hamilton. Animals were maintained under a reversed 12-:12- hour light cycle in a room with controlled temperature (22°C) and humidity (50% ± 5%). Animals were allowed to habituate to their homeroom for 1 week prior to animal handling and had free access to food and water. All animal procedures adhered to the policies outlined by the Canadian Council on Animal Care and McMaster University’s Animal Research and Ethics Board (AUP 14-08-28).
Both lithium and VPA were obtained from Sigma Aldrich. Weights of all animals were monitored daily to ensure the absence of large fluctuations in weight and proper dosage of drugs for all animals. All rats were randomly divided into 3 treatment groups—control (saline treated, n=6), lithium (n=8), and VPA (n=8)—and were injected via i.p. route twice daily for 2 consecutive weeks. Stock solutions of lithium (47.5 mg/mL) and VPA (200 mg/mL) were made using sterile saline. Saline, lithium, and VPA were administered to each treatment group respectively in a volume of 1 mL/kg. Rats were anaesthetized with isoflurane and killed by decapitation 5 hours after the final injection, which was consistent with the half-life of lithium in rodents (Wood et al. 1986). Rat brain regions, including cortex, striatum, PFC, and hippocampus, were dissected over ice and stored at −80°C until use.
Plasma Collection and Dosage Evaluation
Blood was collected in BD vacutainer SST blood collection tubes coated with ethylenediaminetetraacetic acid immediately following decapitation. Samples were inverted 5 times and stored at room temperature for 30 minutes. The plasma was isolated from whole blood by centrifugation of tubes at 3000 rpm for 10 minutes without a stopping break on the Eppendorf 5810R centrifuge. Plasma was stored at -80°C until processing. Lithium and VPA plasma levels were analyzed at St. Joseph’s Healthcare Hamilton (Charlton Campus) using the Easylyte machine (Medica Vendo Cypress Diagnostics Inc.) to ensure that levels fell within the therapeutic range.
RNA Extraction/cDNA Synthesis
Total RNA was extracted using TRIzol from Ambion by Life Technologies as per the manufacturer’s protocol (catalog no. 15596018). Using 1 µg of total RNA, DNAse treatment was conducted using the supplier’s protocol. Following DNAse treatment, cDNA was synthesized using an identical amount of RNA (QuantaBio qScript cDNA SuperMix) as per the manufacturer’s protocol.
Real-Time PCR
The mRNA expression of synapsins was assessed via real-time PCR using the QuantaFast SYBR Green PCR kit from Qiagen. The primers used for synapsin I, IIa, and IIb and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (internal control) are as follows: Synapsin I Fwd: GTG TCA GGG AAC TGG AAG ACC, Synapsin I Rev: AGG AGC CCA CCA CCT CAA TA, Synapsin IIa Fwd: ACT GCC ACC TTC TTC CTC, Synapsin IIa Rev: GAC TTG TTG AGC TGT GGG, Synapsin IIb Fwd: TCA GCA AGA TGA ACC AGC, Synapsin IIb Rev: GGA CCT ACT GCA ATG CC, GAPDH Fwd: CAA CTC CCT CAA GAT TGT CAG CAA, and GAPDH Rev: GGC ATG GAC TGT GGT CAT GA. All primers were used at a final concentration of 1 µM. Reagents and concentrations corresponded to the protocol as per the SYBR Green PCR kit (Qiagen) for 96-well plates (25 µL total reaction). All cDNA samples were amplified using MX3000P (Stratagene) cycler for 40 cycles. PCR amplifications began with heat activation of 95°C for 5 minutes and cycle conditions were standard for all the primers with 95°C for 10 seconds and 60°C for 30 seconds, as the annealing and extension steps were combined with the SYBR Green PCR kit. Negative controls for every plate consisted of No Reverse Transcriptase (containing DNase treated RNA without reverse transcriptase, made while synthesizing cDNA) and No Template Control (using nuclease free water in the reaction instead of cDNA). PCR specificity was confirmed when melting curve analysis of the amplified product produced a single peak for each product. All reactions were performed in duplicates, and Ct values were limited to a variability of ±0.5. For analysis purposes, average of the duplicates was used. DNA was extracted from the PCR amplicons, and the amplicon was then subjected to electrophoresis on a 1.5% agarose gel after having been resuspended in a solution with 6x loading dye and nuclease free water. The single band of interest was verified and excised for each gene. The amplicon was extracted using the Freeze N’ Squeeze Columns (BioRad) as per the manufacturer’s protocol.
Sequencing Analysis of Synapsins and Internal Control Confirmed Amplicon Sequences
Amplicons were run on a 1.5% agarose gel alongside a negative control from the same plate (supplementary Figure 1). Sequencing analysis confirmed the nucleotide sequence of the amplicons. Synapsin I had an amplicon length of 181 bp (GenBank: X04655.1). Synapsin IIa had an amplicon length of 183 bp (NCBI Ref: NM_001034020.1). Synapsin IIb had an amplicon length of 184 bp (NCBI Ref: NM_019159.1). GAPDH had an amplicon length of 118 bp (NCBI Ref: XM_017593963.1).
Statistical Analysis
GraphPad Prism 6 was used for all statistical analyses. Relative gene expression was compared using the delta-delta Ct method as described by Schmittgen and Livak (2008) using the equation ΔΔCt=2-((Ct-Gene of interest – Ct-Housekeeping) – (Ct-Avg Ctrl Gene of interest – Ct-Avg Ctrl Housekeeping)). Raw Ct values, as well as an absolute quantification method with standard curve, were used to ensure no differences in the housekeeping gene GAPDH. Two-way ANOVA with Tukey’s posthoc was used to compare differences between groups in gene expression. One-way ANOVA with Tukey’s posthoc test was used to analyze weight changes. Outliers were removed via the ROUT method developed by GraphPad using the default and conservative ROUT coefficient of 1% (Motulsky and Brown, 2006). Significance was established as P<.05.
Results
Lithium and VPA Concentrations in Plasma
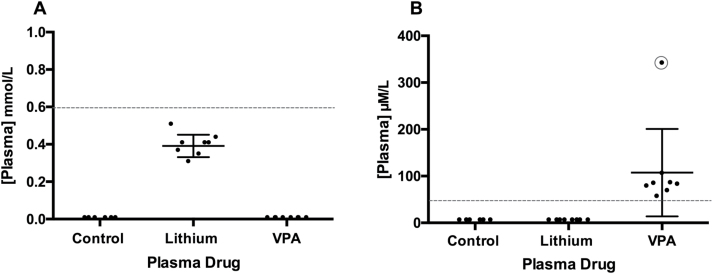
Blood plasma drug levels for both lithium and VPA were evaluated for all rats. Therapeutic concentrations for lithium range between 0.6 and 1.2 mmol/L and therapeutic concentrations for VPA range between 50 and 100 µM/mL (Sproule, 2002; Löscher, 2007; Zanni et al., 2017). Control rats showed undetectable lithium and VPA levels. Lithium-treated rats showed lithium levels between 0.3 and 0.5 mmol/L and VPA levels <50 µM/mL (Figure 1A). VPA-treated rats showed lithium levels <0.2 mmol/L and VPA levels ranging from 51 to 100 µM/mL (Figure 1B). Rat 2 (circled on Figure 1) in the VPA group had a toxic plasma concentration of 336 µM/mL along with behavioral and physiological symptoms showing toxicity (red feces, little movement, no interest in food). Therefore, that rat was excluded from all further analyses.
Figure 1.
Blood plasma concentrations of lithium and valproic acid (VPA) treatment drugs. Plasma concentrations of lithium and VPA measured 5 hours after administration in (A) lithium-treated rats and (B) VPA-treated rats. Lower limit of the therapeutic concentration range is shown by red dotted line. Rat 2 in the VPA-treated rats (circled in red) showed symptoms of toxicity including very high plasma concentrations and therefore was not used in further analyses.
Both Lithium and VPA Increase Synapsin I Gene Expression in the Striatum
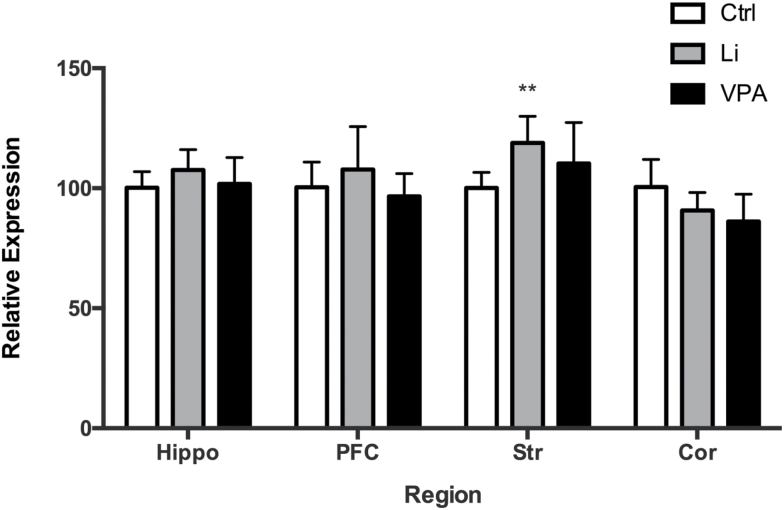
To ascertain whether mood stabilizers affect gene expression of synapsins in the brain, we quantified the expression of synapsin I in brain regions of rats following treatment with the mood stabilizers. There was a significant effect of lithium and VPA treatment on synapsin I expression (F(DFn, DFd): F(2, 72)=3.619, P=.0318), and region (F(DFn, DFd): F(3, 72)=8.035, P=.0001); an interaction between the two was not significant (F(DFn, DFd): F(6, 72)=2.094, P=.0643). Tukey’s posthoc analysis revealed a significant increase of synapsin I in the striatum by lithium treatment (P=.0096) compared to saline treatment. There were no significant changes in other regions (Figure 2).
Figure 2.
Lithium and valproic acid (VPA) increase synapsin I gene expression. Synapsin I gene mRNA expression increased post lithium and VPA treatment (P=.0318). Specifically, a significant increase in the striatum (Str) by lithium treatment was observed through Tukey’s test. **P<.01.
Mood Stabilizers Increase Expression of Synapsin IIa in Rat Brain Regions
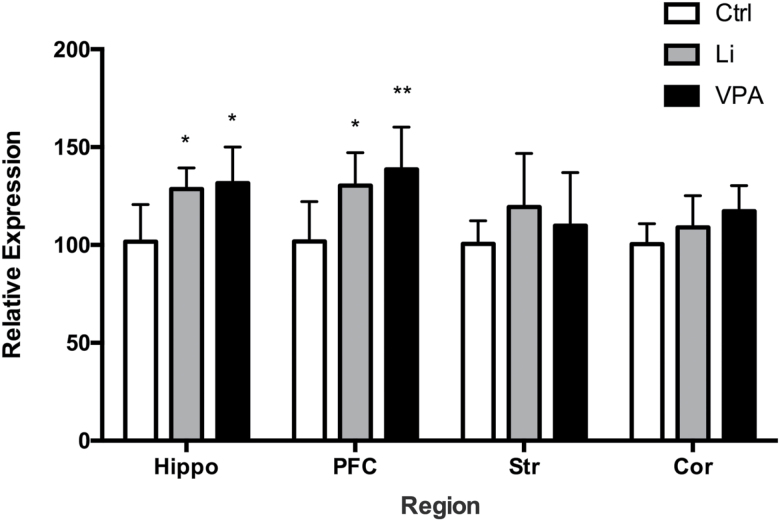
In order to determine whether mood stabilizers such as lithium and VPA have selective effects on synapsins, we also quantified the gene expression for synapsin IIa and IIb. A significant effect of treatment was observed on synapsin IIa mRNA expression (F(2,71)=11.99, P<.0001) and region (F(3,71)=3.284, P=.0257); an interaction between the two was not significant (F(6,71)=0.9864, P=.4411). Tukey’s post hoc analysis revealed that there was a significant increase of synapsin IIa in the hippocampus and PFC following lithium treatment (P=.0250, .0157 respectively), compared to saline treatment. There was also a significant increase in the hippocampus and PFC upon VPA treatment (P=.0142, .0019, respectively) compared to saline treatment. There were no significant changes in the other brain regions (Figure 3).
Figure 3.
Lithium and valproic acid (VPA) increase synapsin IIa gene expression. Synapsin IIa gene mRNA expression increased post lithium and VPA treatment (P<.0001). A significant increase in gene expression was observed in the hippocampus (Hippo) and prefrontal cortex (PFC) by lithium and VPA treatment individually. *P<.05, **P<.01.
Mood Stabilizers Increase Expression of Synapsin IIb in Rat Brain Regions
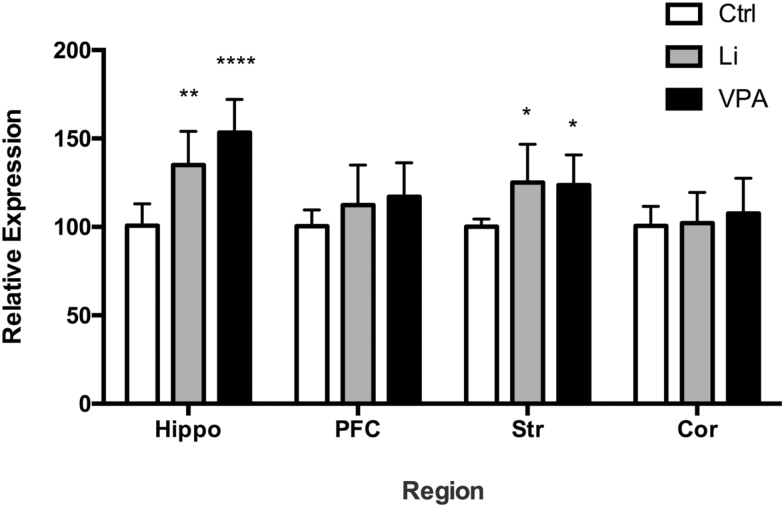
There was a significant effect of treatment on synapsin IIb mRNA expression (F(2,72)=14.02, P<.0001) and region (F(3,72)=8.562, P<.0001); an interaction between the two was also significant (F(6, 72)=2.35, P=.0396). Tukey’s posthoc analysis revealed that synapsin IIb mRNA expression significantly increased in the hippocampus and striatum upon lithium treatment (P=.0014, .0260, respectively) compared with saline treatment. Similarly, VPA treatment also significantly increased synapsin IIb expression in the hippocampus and striatum (P<.0001, .0463, respectively). There were no significant changes in other regions (Figure 4).
Figure 4.
Lithium and valproic acid (VPA) increase synapsin IIb gene expression. Synapsin IIb gene mRNA expression increased post lithium and VPA treatment (P<.0001). A significant increase in gene expression was observed in the hippocampus (Hippo) and striatum (Str) by lithium and VPA treatment individually. *P<.05, **P<.01, ****P<.0001.
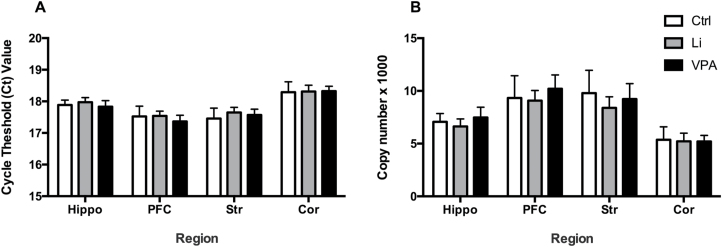
No Changes in Expression of the Housekeeping Gene, GAPDH, by Mood Stabilizers Lithium and VPA
Two-way ANOVA and posthoc analysis indicated no significant changes in raw cycle threshold (Ct) values of GAPDH upon treatment with lithium and VPA (F(2,72)=1.68, P=.1936) (Figure 5A). A significant effect with respect to region (F(3,72)=64.73, P<.0001) was detected; however, this was just indicative of different levels of GAPDH in different regions of the rat brain. Posthoc analysis revealed no significant difference comparing lithium and VPA-treated rats to saline-treated rats in the cortex (P=.9856, .9624), hippocampus (P=.7371, .8893), striatum (P=.2315, .6249), and PFC (P=.9918, .3871). Raw Ct values were used for this analysis as GAPDH was intended to be used as the internal control. Absolute quantification of raw Ct values using a standard curve also showed no significant changes with respect to treatment or interaction (F(2,76)= 2.844, P=.0644; F(6, 76)=0.7629, P=.6013) (Figure 5B). A significant effect of region was observed (F(3,76)=56.21, P<.0001), indicating differential expression amongst regions.
Figure 5.
Lithium and valproic acid (VPA) do not change GAPDH expression. GAPDH gene expression did not change in brain regions, including striatum (Str), PFC, hippocampus (Hippo), and cortex (Cor) of rats treated with lithium and VPA by (A) analysis of raw cycle threshold (Ct) values or by (B) absolute analysis using a standard curve.
Inhibition of Weight Gain by Lithium and VPA
Rats were weighed daily prior to the morning i.p. injections for the 14-day treatment period. Weight gain was calculated by subtracting the weight before the first treatment from the weight before the final injection. Weight gained during the 14-day treatment period was analyzed (supplementary Figure 2A). One-way ANOVA and Tukey’s posthoc analysis showed a significant effect of treatment on weight gain (P<.01). Lithium and VPA significantly inhibited the weight increase observed in the control group (P=.0095, P=.0014, respectively). Averaged weights through time were also graphed (supplementary Figure 2B).
Discussion
In the present study, we describe the effects of lithium and VPA, two commonly prescribed mood stabilizers, on synapsin I and II expression in different rat brain regions. There was an overall effect of treatment on synapsin I, IIa, and IIb mRNA expression. Specifically, treatment with lithium resulted in a robust increase of synapsin I in the striatum. Both lithium and VPA caused a significant increase in synapsin IIa in the PFC and hippocampus, and synapsin IIb in the striatum and hippocampus. In vivo results from this study are consistent with the in vitro results of lithium treatment in lymphoblastoid cell lines from patients with bipolar disorder (Cruceanu et al., 2012).
The mechanisms involved in upregulating synapsin gene expression may involve the transcription factor sites on promoter regions for synapsin I and II. There are some similarities and differences in these transcription factor sites on the promoter regions for synapsin I and II genes. Both synapsin I and II contain early growth response factor 1 (EGR-1) as a common transcription factor site, whereas activating protein-2α (AP2α) and polyoma enhancer activator 3 (PEA-3) are absent on the synapsin I promoter. Similarly, neural-restrictive silencing element (NRSE) and cAMP response element (CRE) are specifically localized to the synapsin I promoter (Chong et al., 2002; Skoblenick et al., 2010). To summarize, synapsin I regulation can involve EGR1, NRSE, and CRE transcription factors, while synapsin II regulation can involve EGR1, AP2α, and PEA-3 transcription factors solely based on their promoter regions. Previous studies from our laboratory have established the role of AP2α transcription factor in increasing synapsin IIa and IIb expression with the treatment of antipsychotic drugs such as haloperidol, a dopamine D2 receptor antagonist (Chong et al., 2002, 2006; Skoblenick et al., 2010). Dopaminergic agents regulate synapsin II involving the AP2a transcription factor through cyclic AMP (cAMP)-mediated mechanisms. However, lithium and VPA likely involve EGR-1, which is common to both synapsin I and II promoters, to upregulate gene expression of both phosphoproteins. In this regard, other studies have shown increases in EGR-1 expression by lithium in various brain regions (Lamprecht and Dudai, 1995) and by VPA in PC12 cells (Almutawaa et al., 2014). These findings are consistent with the hypothesis that the EGR-1 transcription factor is involved in the increase in synapsin I and II gene expression by mood stabilizers. Future directions may involve assessing the role of EGR-1 in the mechanistic pathway involving upregulation of synapsins by lithium and VPA. Additionally, it has been reported that treatments with mood stabilizers induce epigenetic changes in rodents and humans (Ookubo et al, 2013; Lee et al., 2015; Cruceanu et al., 2016). Postmortem studies on human brains of patients with BD revealed epigenetic changes of the synapsin gene (Cruceanu et al., 2016). This may be another mechanism through which synapsin gene expression is altered by these mood stabilizers. However, the mechanisms by which mood stabilizers alter synapsin I and II expression may not be limited to the ones hypothesized here. Furthermore, there was no common effect on all brain regions, as synapsin I and II were altered differently in the various brain regions. This finding may suggest that transcriptional regulation varies with different brain regions and that mood stabilizers act at various levels in the expression of synapsins.
In this study, we have shown that lithium and VPA change the expression of synapsin I and II mRNA levels in the brain. Synaptic proteins play key regulatory roles in the cell involving the maintenance of synapses and neurotransmitters. Both synapsin I and II have also been widely implicated in mood disorders as well as neurodegenerative disorders such as Huntington’s disease and Alzheimer’s disease (Bernardo et al., 2017). The upregulation of synapsins by lithium and VPA may point to their effects on synaptic plasticity in the brain, which have been extensively researched (Monti et al., 2009; Gray and McEwen, 2013). Some limitations of our study include the treatment regimens and subjects chosen. In a clinical setting, lithium and VPA are often given in combination to patients with BD for a period of time that extends much beyond 2 weeks. In our study, these drugs were administered individually to healthy rats as a longer and combination treatment of drugs would have been toxic for the rats. Additionally, as no current rodent models of BD have been well established, these treatments were given to healthy, drug naïve rats. To see the effects of combination treatments, cellular in vitro models can be used. Lastly, the lithium plasma levels detected were just below the therapeutic range. Therefore, although significant differences were detected upon treatment with lithium in both synapsin I and II, the level of significance may be different or other effects of lithium may not be observed. An explanation for the observed plasma concentrations is that levels were measured approximately 5 hours after the final injection, and the half-life of lithium in rats is approximately 6 hours (Wood et al., 1986). Thus, the plasma levels may be lower than the therapeutic range due to the time frame between blood collection and drug administration.
In conclusion, this study was one of the first to examine the effects of mood stabilizers, lithium and VPA, on the class of synapsin phosphoproteins in vivo. There was a significant effect of lithium and VPA on synapsins I, IIa, and IIb. There was a region-specific increase by lithium of synapsin I in the striatum, synapsin IIa in the hippocampus and PFC, and synapsin Iib in the hippocampus and striatum. These findings may provide further insight on the specific roles of synapsins in the therapeutic effects of these mood stabilizers. Overall, these findings help to elucidate the mechanisms through which lithium and VPA contrast in downstream targets from antipsychotics, and provide deeper insight on the involvement of synapsins in psychiatric illnesses such as bipolar disorder.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Funding
This work was supported by the Canadian Institute of Health Research (grant no. 126004).
Statement of Interest
None.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Benicio Frey, Ashley Bernardo, Dima Malkawi, Khaled Nawar, William Brett McIntyre, and all the members of the Mishra laboratory for their help and support throughout the project.
References
- Almutawaa W, Kang NH, Pan Y, Niles LP(2014)Induction of neurotrophic and differentiation factors in neural stem cells by valproic acid. Basic Clin Pharmacol Toxicol 115:216–221. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013)Diagnostic and statistical manual of mental disorders, DSM-IV-TR. Washington, DC: American Psychiatric Association. [Google Scholar]
- Baldelli P, Fassio A, Valtorta F, Benfenati F(2007)Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci 27:13520–13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo A, Prashar S, Molinaro L, Mishra RK(2017)Synapsin II. In: Encycopedia of signaling molecules (Choi S, ed), pp1–11. New York: Springer. [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F(2010)The synapsins: key actors of synapse function and plasticity. Prog Neurobiol 91:313–348. [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Chuang DM(1999)Lithium activates the serine/threonine kinase akt-1 and suppresses glutamate-induced inhibition of akt-1 activity in neurons. Proc Natl Acad Sci USA 96:8745–8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CT, Wang Z, Hunsberger JG, Chuang DM(2013)Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65:105–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong VZ, Young LT, Mishra RK(2002)Cdna array reveals differential gene expression following chronic neuroleptic administration: implications of synapsin II in haloperidol treatment. J Neurochem 82:1533–1539. [DOI] [PubMed] [Google Scholar]
- Chong VZ, Skoblenick K, Morin F, Xu Y, Mishra RK(2006)Dopamine-D1 and -D2 receptors differentially regulate synapsin II expression in the rat brain. Neuroscience 138:587–599. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Hawton K, Stockton S, Geddes JR(2013)Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. Bmj 346:f3646. [DOI] [PubMed] [Google Scholar]
- Cruceanu C, Alda M, Grof P, Rouleau GA, Turecki G(2012)Synapsin II is involved in the molecular pathway of lithium treatment in bipolar disorder. PLoS One 7:e32680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruceanu C, Kutsarova E, Chen ES, Checknita DR, Nagy C, Lopez JP, Alda M, Rouleau GA, Turecki G(2016)DNA hypomethylation of synapsin II cpg islands associates with increased gene expression in bipolar disorder and major depression. BMC Psychiatry 16:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Daniel E, Mudge AW, Maycox PR(2005)Comparative analysis of the effects of four mood stabilizers in SH-SY5Y cells and in primary neurons. Bipolar Disord 7:33–41. [DOI] [PubMed] [Google Scholar]
- Fajutrao L, Locklear J, Priaulx J, Heyes A(2009)A systematic review of the evidence of the burden of bipolar disorder in europe. Clin Pract Epidemiol Ment Health 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Chi P, Blanpied TA, Xu Y, Magarinos AM, Ferreira A, Takahashi RH, Kao HT, McEwen BS, Ryan TA, Augustine GJ, Greengard P(2002)Regulation of neurotransmitter release by synapsin III. J Neurosci 22:4372–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ(2004)Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci 24:11368–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Cheng Q, Greengard P, Augustine GJ(2008)Synapsin iia controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci 28:10835–10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK(2004)Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry 9:734–755. [DOI] [PubMed] [Google Scholar]
- Gray JD, McEwen BS(2013)Lithium’s role in neural plasticity and its implications for mood disorders. Acta Psychiatr Scand 128:347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Benfenati F, Doussau F, Nairn AC, Czernik AJ, Augustine GJ, Greengard P(2005)Structural domains involved in the regulation of transmitter release by synapsins. J Neurosci 25:2658–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, Hammer RE, Südhof TC(1999)A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 24:377–387. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ(1996)Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA 93:3679–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile BM, Guillot TS, Venton BJ, Wetsel WC, Augustine GJ, Wightman RM(2010)Synapsins differentially control dopamine and serotonin release. J Neurosci 30:9762–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y(1995)Differential modulation of brain immediate early genes by intraperitoneal licl. Neuroreport 7:289–293. [PubMed] [Google Scholar]
- Lazzara CA, Kim YH(2015)Potential application of lithium in Parkinson’s and other neurodegenerative diseases. Front Neurosci 9:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Pirooznia M, Guintivano J, Ly M, Ewald ER, Tamashiro KL, Gould TD, Moran TH, Potash JB(2015)Search for common targets of lithium and valproic acid identifies novel epigenetic effects of lithium on the rat leptin receptor gene. Transl Psychiatry 5:e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W.(2007)The pharmacokinetics of antiepileptic drugs in rats: consequences for maintaining effective drug levels during prolonged drug administration in rat models of epilepsy. Epilepsia 48:1245–1258. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Tanious M, Das P, Coulston CM, Berk M(2013)Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs 27:135–153. [DOI] [PubMed] [Google Scholar]
- Medrihan L, Cesca F, Raimondi A, Lignani G, Baldelli P, Benfenati F(2013)Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels. Nat Commun 4:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti B, Polazzi E, Contestabile A(2009)Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr Mol Pharmacol 2:95–109. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Brown RE(2006)Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookubo M, Kanai H, Aoki H, Yamada N(2013)Antidepressants and mood stabilizers effects on histone deacetylase expression in C57BL/6 mice: brain region specific changes. J Psychiatr Res 47:1204–1214. [DOI] [PubMed] [Google Scholar]
- Patel JP, Frey BN(2015)Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder?Neural Plast 2015:708306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P(2009)Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ(2008)Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- Skoblenick KJ, Argintaru N, Xu Y, Dyck BA, Basu D, Tan ML, Mazurek MF, Mishra RK(2010)Role of AP-2alpha transcription factor in the regulation of synapsin II gene expression by dopamine D1 and D2 receptors. J Mol Neurosci 41:267–277. [DOI] [PubMed] [Google Scholar]
- Song J, Sjölander A, Joas E, Bergen SE, Runeson B, Larsson H, Landén M, Lichtenstein P(2017)Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry 174:795–802. [DOI] [PubMed] [Google Scholar]
- Song SH, Augustine GJ(2015)Synapsin isoforms and synaptic vesicle trafficking. Mol Cells 38:936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproule B.(2002)Lithium in bipolar disorder: can drug concentrations predict therapeutic effect?Clin Pharmacokinet 41:639–660. [DOI] [PubMed] [Google Scholar]
- Tan ML, Dyck BA, Gabriele J, Daya RP, Thomas N, Sookram C, Basu D, Ferro MA, Chong VZ, Mishra RK(2014)Synapsin II gene expression in the dorsolateral prefrontal cortex of brain specimens from patients with schizophrenia and bipolar disorder: effect of lifetime intake of antipsychotic drugs. Pharmacogenomics J 14:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Thatcher L, Usen N, Hyde TM, Kleinman JE, Freed WJ(2002)Reduction of synapsin in the hippocampus of patients with bipolar disorder and schizophrenia. Mol Psychiatry 7:571–578. [DOI] [PubMed] [Google Scholar]
- Villanueva M, Thornley K, Augustine GJ, Wightman RM(2006)Synapsin II negatively regulates catecholamine release. Brain Cell Biol 35:125–136. [DOI] [PubMed] [Google Scholar]
- Vos T, et al. (2012)Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AJ, Goodwin GM, De Souza R, Green AR(1986)The pharmacokinetic profile of lithium in rat and mouse; an important factor in psychopharmacological investigation of the drug. Neuropharmacology 25:1285–1288. [DOI] [PubMed] [Google Scholar]
- Zanni G, Michno W, Di Martino E, Tjärnlund-Wolf A, Pettersson J, Mason CE, Hellspong G, Blomgren K, Hanrieder J(2017)Lithium accumulates in neurogenic brain regions as revealed by high resolution ion imaging. Sci Rep 7:40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.