ABSTRACT
Background
Pigment nephropathy represents one of the most severe complications of rhabdomyolysis or hemolysis.
Methods
We performed a retrospective observational study to analyze the etiology, clinical manifestation, laboratory profile and outcome in patients with biopsy-proven pigment-induced nephropathy between January 2011 and December 2016. History, clinical examination findings, laboratory investigations and outcome were recorded.
Results
A total of 46 patients were included with mean follow-up of 14 ± 5.5 months. Mean age was 40.15 ± 12.3 years, 65% were males (male:female, 1.8:1) and ∼37 (80.4%) had oliguria. Mean serum creatinine at presentation and peak creatinine were 7.5 ± 2.2 and 12.1 ± 4.3 mg/dL, respectively. Evidence of rhabdomyolysis was noted in 26 patients (64%) and hemolysis in 20 patients (36%). Etiology of rhabdomyolysis include snake envenomation (10 patients), seizures (7), strenuous exercise (5), wasp sting (2) and rifampicin induced (2). The causes of hemolysis include rifampicin induced (7 patients), sepsis (5), malaria (3), mismatched blood transfusion/transfusion reaction (3) and paroxysmal nocturnal hemoglobinuria (2). On renal biopsy, two patients had acute interstitial nephritis and two had immunoglobulin A deposits in addition to pigment nephropathy. All except one (97.8%) required hemodialysis (HD) during hospital stay and mean number of HD sessions was 9 ± 2. A total of three patients with sepsis/disseminated intravascular coagulation died, all had associated hemolysis. On statistical analysis, there was no difference between AKI due to rhabdomyolysis and hemolysis except for high creatine phosphokinase in patients with rhabdomyolysis and Lactate dehydrogenase level in patients with hemolysis. At mean follow-up, five patients (12%) progressed to chronic kidney disease (CKD).
Conclusions
Pigment nephropathy due to rhabdomyolysis and hemolysis is an important cause of renal failure requiring HD. The prognosis was relatively good and depends on the etiology; however, long-term studies and follow-up are needed to assess the true incidence of CKD due to pigment nephropathy.
Keywords: AKI, hemolysis, pigment, renal biopsy, rhabdomyolysis
Introduction
Rhabdomyolysis-induced pigment nephropathy is common, accounting for about 7–10% of all cases of acute kidney injury (AKI) [1]. Etiology of rhabdomyolysis and pigment nephropathy differ in Western and tropical countries. There is a paucity of data in the Indian literature; hence, we intend to study the etiology, clinical manifestation, laboratory profile and outcome in patients with biopsy-proven pigment-induced nephropathy.
Materials and methods
We performed a retrospective observational study in patients admitted to the Institute of Nephrology, Madras Medical College, Chennai with various causes of AKI and renal biopsy showing pigment nephropathy during January 2011 to December 2016. Those patients with lost follow-up of at least 3 months and underlying known renal disease were excluded. Renal biopsy was done in patients who had persistent oliguria for >7 days and renal failure for >14 days despite supportive treatment. Perls staining for iron was done in all patients and immunostaining for myoglobin in selected patients. Detailed history including history of recent trauma, exertion, seizures, infections and intake of alcohol/medications, demographic data and clinical findings were noted.
Laboratory investigations included urine analysis, spot urine protein:creatinine ratio, urine myoglobin, plasma free hemoglobin, reticulocyte count blood urea, serum creatinine, sodium, potassium, calcium, prothrombin time, serum creatine phosphokinase (CPK), Lactate dehydrogenase (LDH) and liver function tests, treatment details and outcome were recorded.
All the renal biopsies were subjected to light microscopy with various stains including hemotoxylin and eosin, Periodic acid–Schiff, trichrome, Periodic Schiff-methanamine and Perls Prussian blue stain. Perls stain for iron confirms hemosiderin seen in the tubular epithelial cell cytoplasm. In the presence of intratubular pigment casts with a globular/ropy appearance, myoglobin immunohistochemistry (IHC) was done whenever possible. Hemoglobin IHC was not done due to unavailability. The etiology of rhabdomyolysis and hemolysis was ascertained by clinical history and laboratory findings.
All the patients received supportive treatment and forced alkaline diuresis was given when presenting without volume overload or oliguria. Indications of hemodialysis (HD) were oligoanuria, hyperkalemia (>5.5 mEq/L), metabolic acidosis and acute pulmonary edema.
AKI was defined as per Kidney Disease Improving Global Outcomes 2012 guidelines as increase in serum creatinine by ≥0.3 mg/dL within 48 h or increase in serum creatinine to 1.5 times from baseline that is known or presumed to have occurred within the prior 7 days or urine volume <0.5 mL/kg/h for 6 h. Complete recovery of kidney function was defined as a decrease in the serum creatinine level to within a normal range. Chronic kidney disease (CKD) was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2 at 3 months after the onset of AKI. The presence of rhabdomyolysis was diagnosed by clinical history and elevated serum CPK >390 IU/L (three times the normal) and hemolysis by elevated LDH (>420 IU/L), anemia (Hb<10 mg/dL) and elevated bilirubin. Data are expressed as mean (± standard deviation), median (range), numbers or percentages. The differences in variables between patients with rhabdomyolysis or hemolysis were assessed using the chi-square test. All calculations were performed using the SPSS software package (v15.0, SPSS Inc., Chicago, IL, USA). P < 0.05 was taken as statistical significance.
Results
The total number of AKI cases over 6 years was 3300, out of which 155 (4.7%) had laboratory evidence of rhabdomyolysis and 98 patients (2.9%) had evidence of hemolysis. A total of 46 patients with biopsy-proven pigment cast [26 patients (56%) with rhabdomyolysis and 20 patients (44%)] were included with mean follow-up of 14 ± 5.5 months. Those patients with clinical and laboratory evidence of rhabdomyolysis or hemolysis but no demonstrable pigment cast in renal biopsy were excluded from the study. Demographic and clinical data are given in Table 1. Mean serum CPK was 2319 ± 690 IU/L and mean serum LDH was 2128 ± 890 IU/L. Out of 26 patients with rhabdomyolysis, 8 patients had serum CPK <1500 IU/L and 18 patients had >1500 IU/L. Only five patients had severe rhabdomyolysis, as defined by serum CPK >5000 IU/L, hypoalbuminemia, hyperkalemia and hypocalcemia. Approximately 20 patients presented with hemolysis, of which the most common was rifampicin induced (7 patients), followed by sepsis (5), malaria (3), mismatched blood transfusion/transfusion reaction (3) and paroxysmal nocturnal hemoglobinuria (PNH) (2). In rifampicin-induced hemolysis, the mean duration of anti-tuberculosis treatment was 2 weeks; all the patients were on intermittent therapy. The etiology of AKI due to rhabdomyolysis and hemolysis in our study in comparison with the literature are given in Table 2. In our study, 26 out 155 rhabdomyolysis patients and 20 out of 98 hemolysis patients had pigment casts (P = 0.54). All renal biopsies revealed acute tubular injury in dilated tubules, swollen tubular epithelial cells with cytoplasmic vacuoles, sloughed off epithelial cells forming granular debris, edematous interstitium and pigment casts (Figure 1) in the tubules. None of the patients had significant glomerulosclerosis, interstitial fibrosis or tubular atrophy. Out of 26 patients with evidence of rhabdomyolysis, myoglobin immunostaining (Figure 2) was done in 18 patients and was found to be positive and all 20 biopsies with hemolysis revealed Perls stain positivity in cytoplasm. In all, two patients had acute interstitial nephritis and two had immunoglobulin A deposits in addition to pigment nephropathy. There was no significant difference noted in the morphology of pigment casts due to rhabdomyolysis or hemolyis except that Perls stain positivity in cytoplasm was seen in all 20 patients with hemolysis but only in 1 patient with rhabdomyolysis. Some minimal lymphocytic interstitial infiltrates were noted in 8 out of 26 rhabdomyolysis patients and in 6 out of 20 hemolysis patients. All except one patient (97.8%) required HD and mean number of HD sessions was 9 ± 2. A total of three patients (6.5%) died with sepsis/disseminated intravascular coagulation (DIC), all had AKI due to hemolysis etiology. Statistical analysis of variables between AKI due to rhabdomyolysis and hemolysis is given in Table 3. There was no difference between AKI due to rhabdomyolysis and hemolysis except for high CPK in patients with rhabdomyolysis and LDH level in patients with hemolysis. At mean follow-up, five patients (12%) had progressed to CKD. Out of these, two were due to severe rhabdomyolysis and three due to hemolysis.
Table 1.
Demographic and clinical data in patients
| Variables | N = 46 |
|---|---|
| Males | 30 (65%) |
| Mean age (years) | 40.15 ± 12.3 |
| Oliguria at presentation | 37 (80.4%) |
| Mean creatinine at presentation (mg/dL) | 7.5 ± 2.2 |
| Mean peak serum creatinine (mg/dL) | 12.1 ± 4.3 |
| Mean serum CPK (IU/L) | 2319 ± 690 |
| Mean serum LDH (IU/L) | 2128 ± 890 |
| Mean serum potassium (mEq/L) | 4.9 ± 2.3 |
| Mean serum calcium (mg/dL) | 8.04 ± 4.5 |
| Mean serum phosphorus (mg/dL) | 4.95 ± 2.3 |
| Mean serum uric acid (mg/dL) | 5.95 ± 3.2 |
| Mean number of HD sessions | 9 ± 2 |
Table 2.
Etiology of rhabdomyolysis and hemolysis causing AKI
| Common causes | Causes in our study (n) |
|---|---|
| Rhabdomyolysis | N = 26 |
|
|
| Hemolysis | N= 20 |
|
|
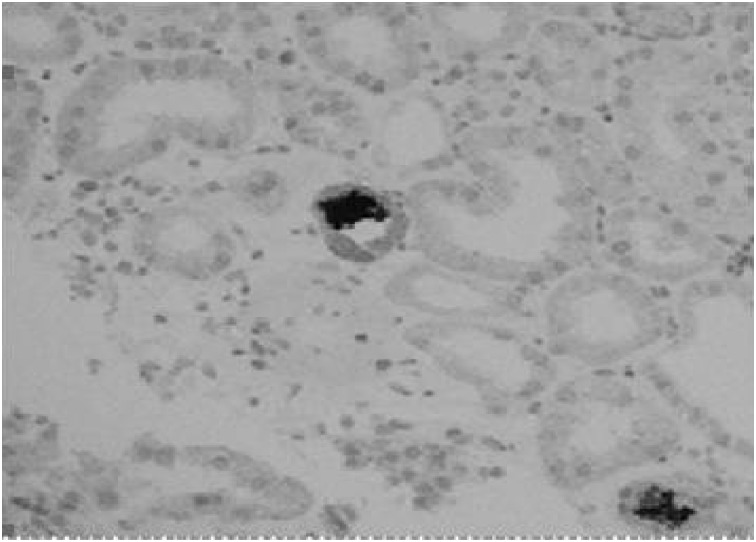
Fig. 1.
Renal biopsy showing pigment cast in the tubules.
Fig. 2.
Renal biopsy immunostaining—positive for myoglobin.
Table 3.
Variables between AKI due to rhabdomyolysis and hemolysis
| Variables | Rhabdomyolysis | Hemolysis | P-value |
|---|---|---|---|
| Number | 26 | 20 | |
| Mean age (years) | 41.1 | 39.2 | 0.91 |
| Mean CPK (IU/L) | 3581 | 1057 | 0.002 |
| Mean LDH (IU/L) | 1294 | 2962 | 0.0001 |
| Mean potassium (mEq/L) | 5.2 | 4.61 | 0.88 |
| Mean calcium (mg/dL) | 8.06 | 8.03 | 0.97 |
| Mean phosphorus (mg/dL) | 4.6 | 5.3 | 0.45 |
| Mean uric acid (mg/dL) | 4.7 | 7.2 | 0.20 |
| Mean number of HD sessions | 9.3 | 8.6 | 0.71 |
| Death | 0 | 3 | 0.05 |
Discussion
The causes of pigment-induced nephropathy are rhabdomyolysis, intravascular hemolysis and bile pigment nephropathy due to cholestasis. Rhabdomyolysis refers to disintegration of striated muscle, resulting in the release of muscular cell constituents into the circulation [2]. The mechanisms of renal toxicity by myoglobin, a 17.8-kDa protein, are renal vasoconstriction, formation of intratubular casts and the direct toxicity of myoglobin to kidney tubular cells [3, 4]. Myoglobin is filtered by the glomeruli, gets concentrated along the renal tubules and precipitates with the Tamm–Horsfall protein, a process favored by acidic urine. It appears in the urine only when the renal threshold of 0.5–1.5 mg/dL of myoglobin is exceeded. Tubule obstruction occurs usually at the distal tubules, and direct tubule toxicity occurs in the proximal tubules. Serum CPK is the most sensitive enzyme marker of muscle injury. Though serum myoglobin levels peak before serum CPK, it has a rapid and unpredictable metabolism. The measurement of serum myoglobin has a low sensitivity for the diagnosis of rhabdomyolysis [5]. There is no defined threshold value of serum CPK above which the risk of AKI is markedly increased, although values >5000 U/L have been reported to increase the risk of AKI, but only five patients in our study had such high values [6, 7]. Rhabdomyolysis-induced AKI was first described by Meyer-Betz in 1911 [8]. Approximately 10–50% of patients with rhabdomyolysis develop AKI, and it contributes to 5–25% of all cases of AKI [9, 10]. In our study, rhabdomyolysis contributed to ∼4.7% of total AKI.
In a retrospective study involving 126 patients with severe rhabdomyolysis with a 9-year follow-up, the incidence of AKI was 58%. The causes of rhabdomyolysis [11] include immobilization due to illicit drugs abuse (27.8%), infectious disease (19.8%), trauma (7.1%), stroke (4.8%), surgery (3.2%) and other (30.2%), whereas it was due to snake envenomation (38%), seizures (30%), strenuous exercise (19%), wasp sting (6.5%) and rifampicin induced (6.5%) in our study. Death was significantly higher among patients with AKI, compared with patients without AKI (19.2% versus 3.6%). The risk factors for AKI include peak CPK, hypoalbuminemia, metabolic acidosis and decreased prothrombin time. The mortality rate varied from 3.5% to 22% [3, 12, 13]. None of our patients with rhabdomyolysis-induced AKI died. Hyperkalemia, hyperuricemia and hypocalcemia are other common complications of rhabdomyolysis [14]. The main step in management remains the early, aggressive repletion of fluids. Administration of sodium bicarbonate, which results in an alkaline urine, was first proposed by Bywaters and Beall, though studies did not show encouraging results [15, 16]. Long-term survival among patients with rhabdomyolysis and AKI is reported to be close to 80% [17].
Hemolysis is the second most common cause of pigment nephropathy. Deposits of iron and hemosiderosis in the kidney have been observed in diseases with intravascular hemolysis, including PNH, valvular heart diseases and prosthetic heart valve implants, genetic hemoglobinopathies, malaria and transfusion of stored red blood cells [18–21]. Mechanical trauma to erythrocytes liberates hemoglobin into plasma, which is bound by haptoglobin. This complex, taken up by the reticuloendothelial cells, is degraded. When plasma haptoglobin is fully saturated, free plasma hemoglobin dissociates to dimeric hemoglobin, which in turn dissociates into heme and globin [22]. Heme proteins can cause AKI through decreased renal perfusion, direct cytotoxicity and intratubular casts formed from the interaction of heme proteins with Tamm–Horsfall protein. Episodes of AKI, especially those that fail to completely resolve, predispose to CKD [23, 24]. At mean follow-up, five (12%) patients had progressed to CKD. Out of these, two were due to severe rhabdomyolysis and three due to hemolysis.
AKI is a common but serious complication following cardiopulmonary bypass (CPB) and cardiac surgeries, and carries a poor prognosis. Hemodynamic/inflammatory factors and the release of labile iron, resulting in reactive oxygen species (ROS), are the major determinants of cardiac surgery-associated AKI [25]. Biomarkers like neutrophil gelatinase-associated lipocalin (NGAL), liver-type fatty acid-binding protein and alpha-1 microglobulin predict the development of CPB-associated AKI, while hepcidin isoforms predict protection from AKI. NGAL participates in local iron transport while liver-type fatty acid-binding protein and alpha-1 microglobulin function as high-affinity heme-binding proteins and hepcidin in iron sequestration. Novel biomarkers point toward free iron-mediated (hemoglobin-induced) renal injury to be an important mechanism of AKI and to result in pigment nephropathy in these patients. Alkalinization of urine with sodium bicarbonate might protect from tubular cast formation from met-hemoglobin, proximal tubular cell necrosis by reduced endocytotic hemoglobin uptake and free iron-mediated ROS production and related injury [26].
In a study of 14 patients of PNH by Ram et al., AKI was noted in six (42.8%) patients, five of whom had HD [27]. Renal biopsy was done in four patients and all showed prominent hemosiderin pigments and acute tubular necrosis. At the end of 3 months after discharge, all patients had normal renal function.
Envenomation/poisonings, malaria, infections and sepsis often cause both rhabdomyolysis and hemolysis [28]. There was no difference in the morphology of pigment casts or extent of tubular injury due to rhabdomyolysis or hemolysis in our study. We noted that Perls staining of cytoplasm with pigment cast points toward hemolysis as the etiology of AKI. In addition to clinical and laboratory evidence, immunohistochemistry of pigment casts will be helpful in clinching the etiology, though is not essential.
Snake envenomation is the most common cause of rhabdomyolysis and rifampicin is the most common cause of hemolysis causing pigment nephropathy in our study. It has a relatively good prognosis depending on the underlying etiology, though long-term follow-up is needed to ascertain the burden of pigment-induced nephropathy to CKD incidence in the future.
Conflict of interest statement
None declared.
References
- 1. El-Abdellati E, Eyselbergs M, Sirimsi H. et al. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: acute kidney injury. Ann Intensive Care 2013; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vanholder R, Sever MS, Erek E. et al. Rhabdomyolysis. J Am Soc Nephrol 2000; 11: 1553–1561 [DOI] [PubMed] [Google Scholar]
- 3. Bosch X, Poch E, Grau JM.. Rhabdomyolysis and acute kidney injury. N Engl J Med 2009; 361: 62–72 [DOI] [PubMed] [Google Scholar]
- 4. Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 1996; 49: 314–326 [DOI] [PubMed] [Google Scholar]
- 5. Lappalainen H, Tiula E, Uotila L. et al. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med 2002; 30: 2212–2215 [DOI] [PubMed] [Google Scholar]
- 6. Fernandez WG, Hung O, Bruno GR. et al. Factors predictive of acute renal failure and need for hemodialysis among ED patients with rhabdomyolysis. Am J Emerg Med 2005; 23: 1–7 [DOI] [PubMed] [Google Scholar]
- 7. Bagley WH, Yang H, Shah KH.. Rhabdomyolysis. Intern Emerg Med 2007; 2: 210–218 [DOI] [PubMed] [Google Scholar]
- 8. Woodrow G, Brownjohn AM, Turney JH.. The clinical and biochemical features of acute renal failure due to rhabdomyolysis. Ren Fail 1995; 17: 467–474 [DOI] [PubMed] [Google Scholar]
- 9. Huerta-Alardin AL, Varon J, Marik PE.. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care 2005; 9: 158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Visweswaran P, Guntupalli J.. Rhabdomyolysis. Crit Care Clin 1999; 15: 415–428 [DOI] [PubMed] [Google Scholar]
- 11. Rodríguez E, Soler MJ, Rap O. et al. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS One 2013; 8: e82992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petejova N, Martinek A.. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care 2014; 18: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zimmerman JL, Shen MC.. Rhabdomyolysis. Chest 2013; 144: 1058–1065 [DOI] [PubMed] [Google Scholar]
- 14. Cervellin G, Comelli I, Lippi G.. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med 2010; 48: 749–756 [DOI] [PubMed] [Google Scholar]
- 15. Schiffl H. Prevention of acute kidney injury by intravenous sodium bicarbonate: the end of a saga. Crit Care 2014; 18: 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown CV, Rhee P, Chan L. et al. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma 2004; 56: 1191–1196 [DOI] [PubMed] [Google Scholar]
- 17. Knochel JP. Rhabdomyolysis and myoglobinuria. Annu Rev Med 1982; 33: 435–443 [DOI] [PubMed] [Google Scholar]
- 18. Ballarin J, Arce Y, Torra Balcells R. et al. Acute renal failure associated to paroxysmal nocturnal haemoglobinuria leads to intratubular haemosiderin accumulation and CD163 expression. Nephrol Dial Transplant 2011; 26: 3408–3411 [DOI] [PubMed] [Google Scholar]
- 19. Balwani MR, Kute VB, Shah PR. et al. Manifestation of paroxysmal nocturnal hemoglobinuria as repeated acute kidney injury. J Nephropharmacol 2015; 5: 116–118 [PMC free article] [PubMed] [Google Scholar]
- 20. Ackermann D, Vogt B, Gugger M. et al. Renal haemosiderosis: an unusual presentation of acute renal failure in a patient following heart valve prosthesis. Nephrol Dial Transplant 2004; 19: 2682–2683 [DOI] [PubMed] [Google Scholar]
- 21. Tombe M. Images in clinical medicine. Hemoglobinuria with malaria. N Engl J Med 2008; 358: 1837. [DOI] [PubMed] [Google Scholar]
- 22. Schaer DJ, Buehler PW, Alayash AI. et al. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 2013; 121: 1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tracz MJ, Alam J, Nath KA.. Physiology and patho-physiology of heme: implications for kidney disease. J Am Soc Nephrol 2007; 18: 414–420 [DOI] [PubMed] [Google Scholar]
- 24. Qian Q, Nath KA, Wu Y. et al. Hemolysis and acute kidney failure. Am J Kidney Dis 2010; 56: 780–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haase M, Haase-Fielitz A, Bagshaw SM. et al. Cardiopulmonary bypass-associated acute kidney injury: a pigment nephropathy? Contrib Nephrol 2007; 156: 340–353 [DOI] [PubMed] [Google Scholar]
- 26. Haase M, Bellomo R, Haase-Fielitz A.. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol 2010; 55: 2024–2033 [DOI] [PubMed] [Google Scholar]
- 27. Ram R, Adiraju KP, Gudithi S. et al. Renal manifestations in paroxysmal nocturnal hemoglobinuria. Indian J Nephrol 2017; 27: 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duvic C, Rabar D, Didelot F. et al. Acute renal failure during severe malaria: physiopathology and therapeutic management. A propos of 2 cases. Med Trop 2000; 60: 267–270 [PubMed] [Google Scholar]