Abstract
Background and Aims
Form and function relationships in plant reproductive structures have long fascinated biologists. Although the intricate associations between specific pollinators and reproductive morphology have been widely explored among animal-pollinated plants, the evolutionary processes underlying the diverse morphologies of wind-pollinated plants remain less well understood. Here we study how this diversity may have arisen by focusing on two conifer species in the pine family that have divergent reproductive cone morphologies at pollination.
Methods
Standard histology methods, artificial wind pollination assays and phylogenetic analyses were used in this study.
Key Results
A detailed study of cone ontogeny in these species reveals that variation in the rate at which their cone scales mature means that pollination occurs at different stages in their development, and thus in association with different specific morphologies. Pollination experiments nevertheless indicate that both species effectively capture pollen.
Conclusions
In wind-pollinated plants, morphological diversity may result from simple variation in development among lineages rather than selective pressures for any major differences in function or performance. This work also illustrates the broader importance of developmental context in understanding plant form and function relationships; because plant reproductive structures perform many different functions over their lifetime, subtle differences in development may dramatically alter the specific morphologies that they use to meet these demands.
Keywords: Abies koreana, conifer, functional morphology, gymnosperm, heterochrony, pollination biology, Picea jezoensis
INTRODUCTION
Relationships among form, function and morphological evolution in plant reproductive structures have long fascinated biologists (de Candolle, 1813; Darwin, 1877; Thompson, 1942), particularly with regard to pollination. A large and diverse body of research has therefore developed that attempts to understand the mechanics of how plant reproductive structures work by investigating the intricate interactions between specialized floral organs and specific animal pollinators (Barrett, 2013; O’Meara et al., 2016; Chartier et al., 2017), as well as the evolutionary consequences of such relationships through comparative studies of diversification and trait correlation. For example, co-evolution between flowers and specialized animal pollinators is often thought to have played an important role in the diversification of many angiosperm lineages, and may perhaps underlie the unparalleled diversity of angiosperms more broadly (van der Niet and Johnson, 2012; Barrett and Harder, 2017; Pauw et al., 2017; Richman et al., 2017; Solís-Montero and Vallejo-Marín, 2017).
Insect-pollinated plants often receive the most attention in these studies, but complex form and function relationships also exist in the reproductive structures of wind-pollinated, or anemophilous, plants (Whitehead, 1969; Charlesworth, 1993; Ackerman, 2000; Richards, 2002; Weller et al., 2006; Friedman and Barrett, 2011). The ovulate structures of anemophilous plants all face similar selective pressures to maximize the capture of windborne pollen, but they exhibit a diverse range of specific morphologies, including some that have served as model systems for integrating biomechanics with pollination biology (Niklas and Paw U, 1982; Niklas, 1982, 1984; Tomlinson and Takaso, 2002; Cresswell, 2007). Understanding reproductive evolution in anemophilous plants is also important in a broad biological sense because the syndrome is pervasive; wind-pollination has evolved at least 65 times in angiosperms (Linder, 1998; Culley et al., 2002; Friedman and Barrett, 2008a, b) and includes ecologically important groups such as grasses and many temperate woody trees (Wragg and Johnson, 2011; Bogdziewicz et al., 2017). Roughly two-thirds of extant gymnosperm species are also strictly wind-pollinated (Owens et al., 1998; Nepi et al., 2017), indicating the potential importance of this syndrome in plant history prior to the evolution of angiosperms in the Early Cretaceous. Wind-pollinated species therefore account for a substantial component of plant diversity in terms of species richness, ecological importance and evolutionary history, but the processes underlying the evolution of reproductive diversity among them are not well understood.
In this study, we use conifer species in the fir (Abies) and spruce (Picea) lineages of the pine family (Pinaceae) to investigate the evolutionary mechanisms leading to reproductive diversity among wind-pollinated plants. Abies and Picea are thought to have slightly different specific pollination mechanisms, as Abies may use rainwater to move pollen into its ovules following pollination while Picea ovules exude an aqueous pollination drop to facilitate this movement (Owens et al., 1998; Chandler and Owens, 2004), but all species in both genera are strictly anemophilous. At pollination, the ovulate structures of Abies and Picea are similar in overall form but differ dramatically in the relative size and development of their constituent parts, and thus in which specific structures the plant uses to actually facilitate pollination. We integrate detailed studies of anatomy with controlled pollination experiments in order to ask why such morphological differences might arise in plants whose reproductive structures perform the same basic function. We find that the cones of these species work equally well in capturing airborne pollen, but that differences in their rate of development generate their distinctive morphologies. A comparative analysis further suggests that rate variation explains morphological patterns across the broader Pinaceae clade, demonstrating how simple differences in development may underlie the diversity of reproductive structures in wind-pollinated plants.
MATERIALS AND METHODS
Sampling
We collected seed cones of Abies koreana and Picea jezoensis from trees growing in the Arnold Arboretum of Harvard University in Boston, MA, USA (accession numbers 557-86-C and 47-95-B, respectively). We sampled cones from the autumn of 2015 (beginning in September) to the summer of 2016 (ending in July) and then again in the spring of 2017, focusing on several major developmental stages, including bud development (before and during winter), bud break, pollination and cone closure following pollination. The pollination period was defined as the interval during which ovules were actively receiving pollen, which spanned late April and early May. Sampling intensity varied by developmental stage; we collected only a few times over the winter but sampled more intensively (every 2–3 d) during the period from bud break to the end of pollination. For each sample we collected five specimens from branches at different points on the tree to ensure representative sampling.
Histological preparation and morphometric analyses
We used standard histology techniques to assess the anatomy and development of sampled cones (see Supplementary Data for details). We sectioned specimens embedded in resin blocks at 4 μm with a rotary microtome equipped with a steel knife (Microm HM360; Thermo Fisher Scientific, Waltham, MA, USA). We stained slide-mounted whole cone sections with calcofluor white for cellulose (Hughes and McCully, 1975) and with periodic acid–Schiff (PAS) reagent for insoluble polysaccharides (Feder and O’Brien, 1968). We examined and photographed fresh material using a Zeiss Discovery AxioVision stereomicroscope and stained sections with a Zeiss Axio Imager Z2 stage microscope, both equipped with Zeiss High Resolution Axiocam digital cameras (Carl Zeiss, Oberkochen, Germany). We also imaged calcofluor-stained sections using a Zeiss LSM700 confocal microscope equipped with an Axiocam HRc camera (Zeiss, Oberkochen, Germany), with excitation at 405 nm and emission detection at 465 nm wavelengths. Images of live specimens were taken with a Canon 60D DSLR camera equipped with a Tamron 90-mm macro lens.
We quantified cell size from different developmental stages using calcofluor-stained sections (which show only cell walls), using ImageJ to measure the area of cell lumens. Given the absolute differences in mature cell sizes between the two taxa, we normalized cell areas from the different cone tissues and structures (e.g. cortex or bract scales) to the maximum cell area of that tissue. We compared normalized cell size values at each developmental stage with a one-way ANOVA, and calculated least significant differences (LSDs) between sequential developmental stages. All morphometric statistical analyses in this study were performed with the SPSS 17.0 software (SPSS, Chicago, IL, USA).
Functional morphology
We tested whether cone morphology influences pollination function through natural and artificial pollination experiments using Abies and Picea cones. For natural pollination, we sampled cones every other day during the pollination period, dissected them, and recorded the position of pollen grains on a normalized grid system imposed over the cone scales (see following paragraph). For artificial pollination experiments, we first collected cones that had opened but had not yet received pollen and then artificially pollinated them in a wind tunnel (housed in the Brown Design Space at Brown University) in batches of four at low and medium wind speeds (2.5 and 5.0 m s−1, respectively). For each batch of cones, ~250 mg of conspecific pollen was introduced into the wind tunnel 0.5 m upstream of the cones. Because A. koreana and P. jezoensis pollen grains are similar in size (81.2 ± 3.6 [s.e.] and 73.0 ± 1.4 µm, respectively), roughly similar amounts of pollen were used in each experiment (Supplementary Data Videos S1 and S2).
To compare the distribution of pollen grains in the taxa, we carefully dissected and removed individual cone scales, which were then photographed on their adaxial and abaxial surfaces. We discarded cone scales where dissection had disturbed the distribution of pollen grains. To quantify the position of pollen grains on the scales, we adjusted and fitted each image to a grid composed of 250 × 250 µm squares, which were used as coordinates to quantify position and number of pollen grains (Supplementary Data Fig. S2). The number of pollen grains in each grid cell was recorded and this distribution was statistically analysed using a non-parametric multivariate ANOVA-type test in the R package npmv (Orme, 2013; Ellis et al., 2017), where the grid coordinates of each counted pollen grain were used as the raw multivariate data. We also compared the number of pollen grains directly captured by the ovules by counting the number of pollen grains found adhering to the integuments surrounding the micropyle of each imaged ovule. We then divided this number by the average projected integumentary area for Abies and Picea in order to calculate a normalized value for pollen grains per given ovule (Supplementary Data).
RESULTS
Cone development
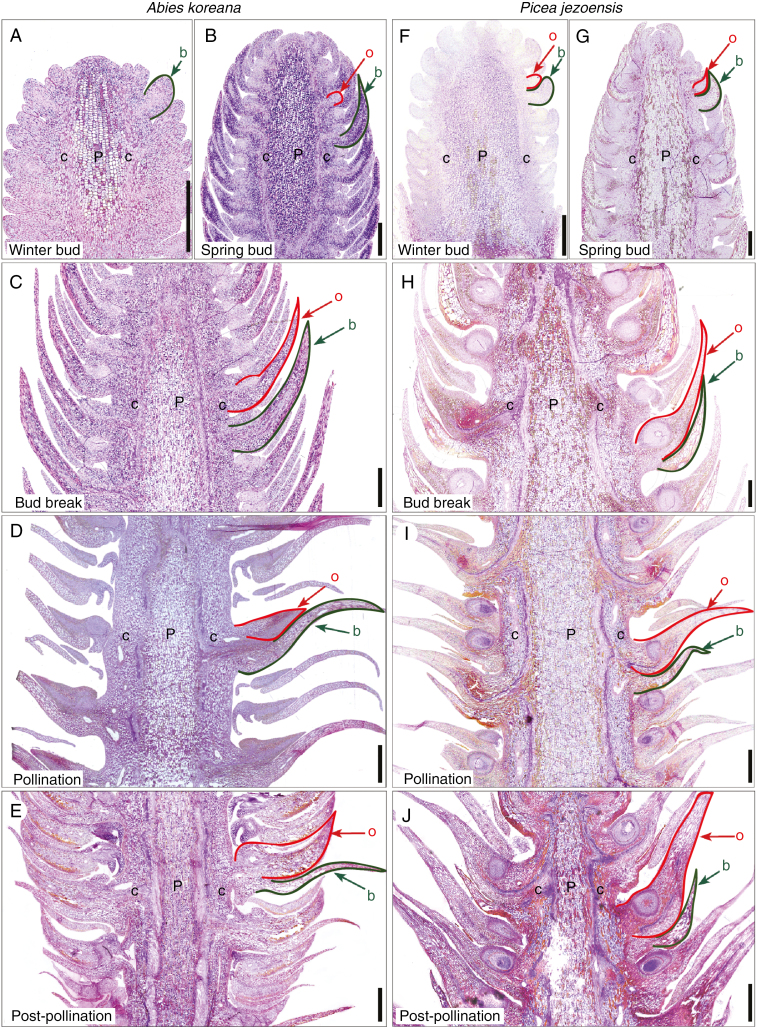
The seed cones of conifers are compact branching systems composed of reiterated units that typically consist of two separate structures: a bract, or modified leaf, that subtends an ovuliferous scale, which is a modified seed-bearing shoot (Florin, 1951; Owens et al., 1998; Taylor et al., 2009). The terminology associated with this basic structure can be complex, but for simplicity we here use the term ‘cone scale’ when referring to this entire complex and the terms ‘bract scale’ and ‘ovuliferous scale’ when referring to its component parts. Abies koreana and P. jezoensis both share this basic cone arrangement, which begins to develop in late summer when cones are still in bud. From then until next spring (approximately early April), young seed cones consist of closely packed cone scales (Fig. 1A, B). Between mid and late April (15 April for Abies; 22 April for Picea), seed cones of both species break bud and elongate; this increases the distance between adjacent cone scales and creates openings through which windborne pollen can reach ovules positioned in the interior of the cone (Fig. 1C, D; see also Niklas, 1982; Tomlinson and Takaso, 2002). The cone scales of these two species differ in structure during pollination, however; bract scales are larger than ovuliferous scales in Abies while the opposite is the case in Picea (Fig. 1C, D). Following pollination, rapid growth of the ovuliferous scales in both taxa closes gaps in the cone and seals it off (Fig. 1E, F).
Fig. 1.
Morphological development in Abies koreana and Picea jezoensis seed cones. (A) A. koreana cones around bud break but before pollination; bract scales are the only visible structures. (B) P. jezoensis cones following bud break, with both bract scales and ovuliferous scales visible. (C) A. koreana cones at pollination, showing gaps between bract scales where pollen can enter the cone. (D) P. jezoensis cone at pollination; red structures are ovuliferous scales and the much smaller bract scales are no longer visible. (E) A. koreana after pollination; ovuliferous scales have expanded to fill spaces between bracts, which are now visible only as thin pointed structures. (F) P. jezoensis cone following pollination; growth of the ovuliferous scale bases has closed the gaps between them, while the entire cone has also shifted position.
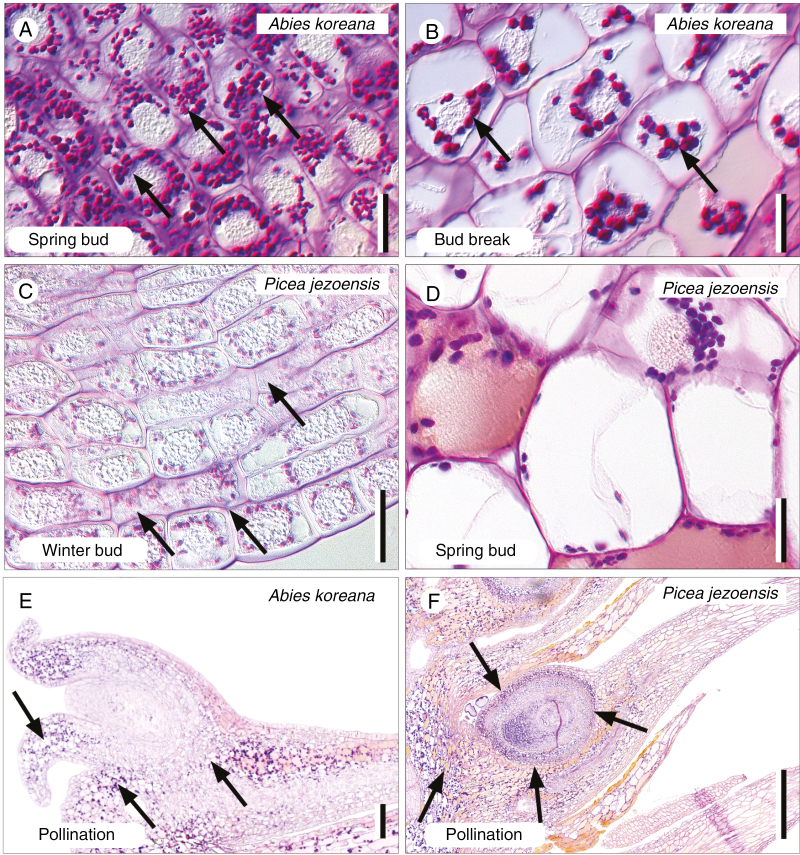
Anatomical sections reveal the developmental patterns underlying these morphological changes. Longitudinal sections of the youngest Abies buds (summer to autumn) show three different tissues: a central pith of small closely stacked cells, a cortex, and numerous cone-scale primordia (at this stage consisting only of a bract scale; Fig. 2A). Ovuliferous scale primordia became visible just prior to bud break (Fig. 2B). Large amounts of starch were also deposited in cone tissues around this time, signalling the beginning of pronounced cell proliferation and expansion (Fig. 3A). During bud break, pith cells elongated and lengthened the cones while initial cell proliferation in the cone scales and cortex widened them (Fig. 2C, 3B; Supplementary Data Fig. S1). Just prior to pollination, cells of the bract scale proliferated and elongated, dramatically increasing bract size compared with underdeveloped ovuliferous scales (Fig. 2D). The funnel-shaped integument of Abies ovules, where pollen is first captured, developed as the cones opened but Abies ovuliferous scales grew only slightly during cone opening and the pollination period (Figs 2D and 3E). Extensive cell proliferation and elongation in the ovuliferous scales did not occur until the end of the pollination period (Fig. 2E). Growth throughout the ovuliferous scale then caused them to swell uniformly, resulting in the closure of gaps between adjacent cone scales.
Fig. 2.
Seed cone anatomy and developmental in Abies koreana (A–E) and Picea jezoensis (F–J). (A) Abies cone before winter dormancy. Note that ovuliferous scale primordia have not formed. (B) Cone in early spring prior to bud break, showing initial differentiation of ovuliferous scale. (C) Cone in spring around bud break, when bract scales and ovuliferous scales are roughly equal in length. (D) Cone at pollination, showing spaces between bract scale/ovuliferous scale complexes and the large bract scale with small ovuliferous scale. (E) Cone following pollination, after ovuliferous scales have expanded to fill gaps between adjacent bract scale/ovuliferous scale complexes. (F) Picea cone before winter dormancy. Note that ovuliferous scale primordia are present. (G) Cone in early spring with more developed bract scale/ovuliferous scale complexes. (H) Cone during bud break. Note that ovuliferous scales have already elongated beyond the bract scales, whose cells are fully mature. (I) Cone at pollination showing enlarged ovuliferous scales and much smaller bract scales. (J) Cone after pollination. Note the expanded ovuliferous scale bases that have reoriented the bract scale/ovuliferous scale complexes into an imbricated arrangement. Abbreviations: b, bract scales (highlighted in green); c, cortical tissue; P, pith tissue; o, ovuliferous scales (highlighted in red). Scale bars (A–C, F–H) = 500 µm; (D, E, I, J) = 1000 µm.
Fig. 3.
Polysaccharides in Abies koreana and Picea jezoensis cone tissues through development. (A) Massive starch accumulation (arrows) within the cells of bract scales in the spring buds of A. koreana. (B) Starch (arrows) depletes concomitantly with cell elongation in the bract scales of A. koreana during bud break. (C) Starch (arrows) accumulates in the bract scale primordia of the pre-winter buds of P. jezoensis. (D) In contrast, starch is depleted from cells of the bract scales within the spring buds of P. jezoensis. (E) Starch (arrows) accumulates around the ovules of A. koreana at the pollination stage, when the seed cones open. (F) Similarly, starch accumulates around the ovules of P. jezoensis at the pollination stage. Scale bars (A–D) = 20 µm; (E, F) = 100 µm.
Anatomical development in young Picea cones was similar to that of Abies (Fig. 2F), but was accelerated in the cone scales. Picea ovuliferous scale primordia were present by winter, long before those of Abies. Starch also accumulated in the pith and bracts of Picea prior to winter (Fig. 3C); it was used up and depleted by the time it was just beginning to accumulate in Abies. In contrast to Abies, Picea bract-scale cells stopped growing and sclerified prior to pollination. The distinctive integumentary arms of Picea, which capture windborne pollen, developed around the time of cone opening, and pollination itself (Fig. 2I) occurred after initial cell proliferation and elongation in the ovuliferous scales (Figs 2G, H and 3D). Following pollination, cells in the basal and abaxial parts of the ovuliferous scales proliferated and elongated, which swelled their bases and repositioned them from a horizontal to a more highly angled orientation relative to the cone axis (Figs 2I, J and 3F). This combination of volume increase through cell proliferation and repositioning through imbrication sealed the cone.
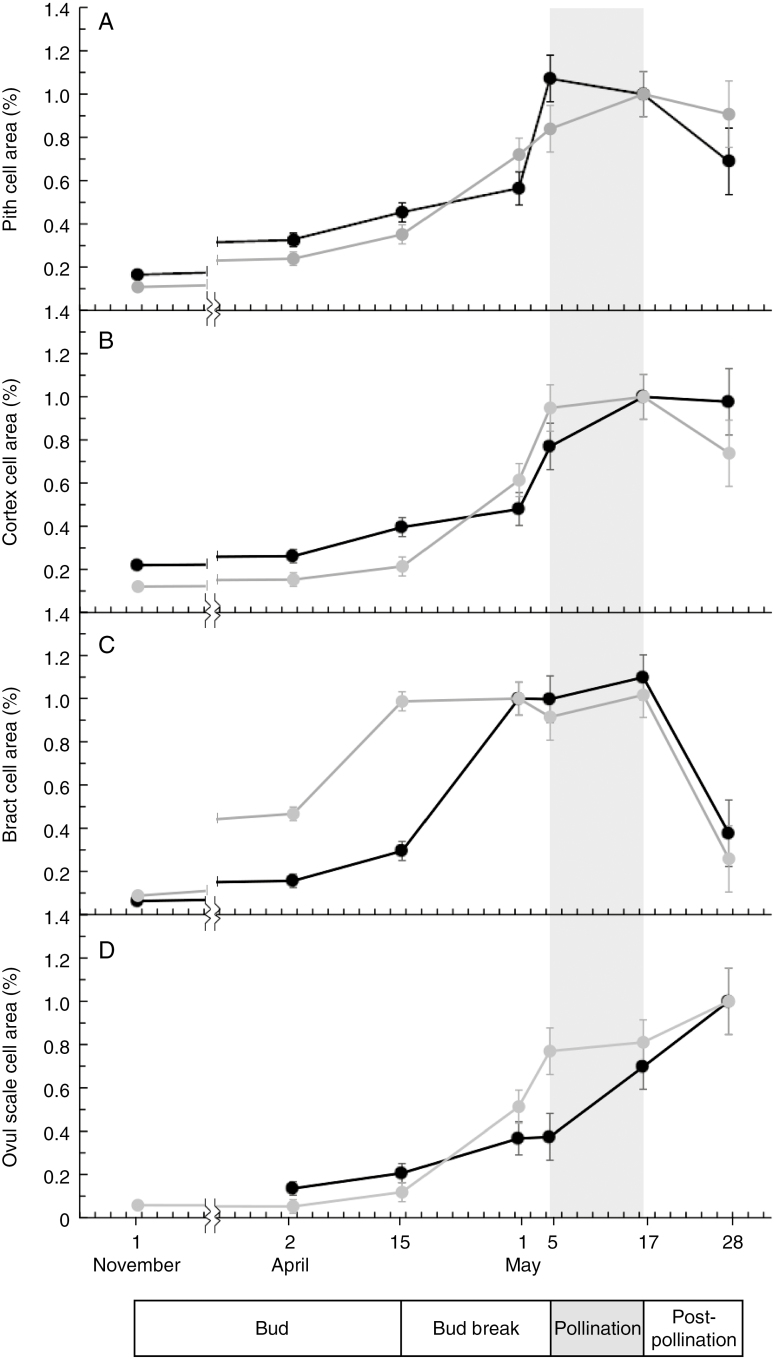
Cell area measurements further support the patterns discussed above (Fig. 4). Cell proliferation in both Abies and Picea occurred early in the development of the pith, cortex and bract scales, but expansion occurred closer to pollination (Fig. 4A, B; their subsequent size decrease was due to wall thickening and consequently smaller lumens). Bract-scale cells in both Abies and Picea matured and increased in relative area before those of the ovuliferous scales (Fig. 4C, D), but this process occurred later Abies than in Picea (Fig. 4C, D).
Fig. 4.
Relative cell size changes in tissues of Abies koreana (black) and Picea jezoensis (grey) cones during early development. Relative cell area was calculated by comparing the size of a sample of cells at different developmental stages in specific tissue with a representative mature cell; in some cases cells could be greater in size (>100%) than the representative cell. Error bars show least significant distance (LSD) between species at each developmental stage at P < 0.05, and the broken line indicates the winter break in sampling. (A) Changes in cone pith cell area, showing the sharp increase in size around bud break with a decrease following pollination due to cell wall thickening and shrinking of cell lumens. (B) Changes in cortex cell area, with a pattern similar to pith cells. (C) Changes in bract-scale cell area, showing an increase in Picea cell size well before those of Abies, which reach mature size just prior to pollination. The dramatic decrease in bract size following pollination is due to crushing from expanding ovuliferous scales. (D) Ovuliferous scale cell area, showing that Picea cell area begins to increase just prior to pollination whereas Abies cell area increases during and after pollination.
Cone function
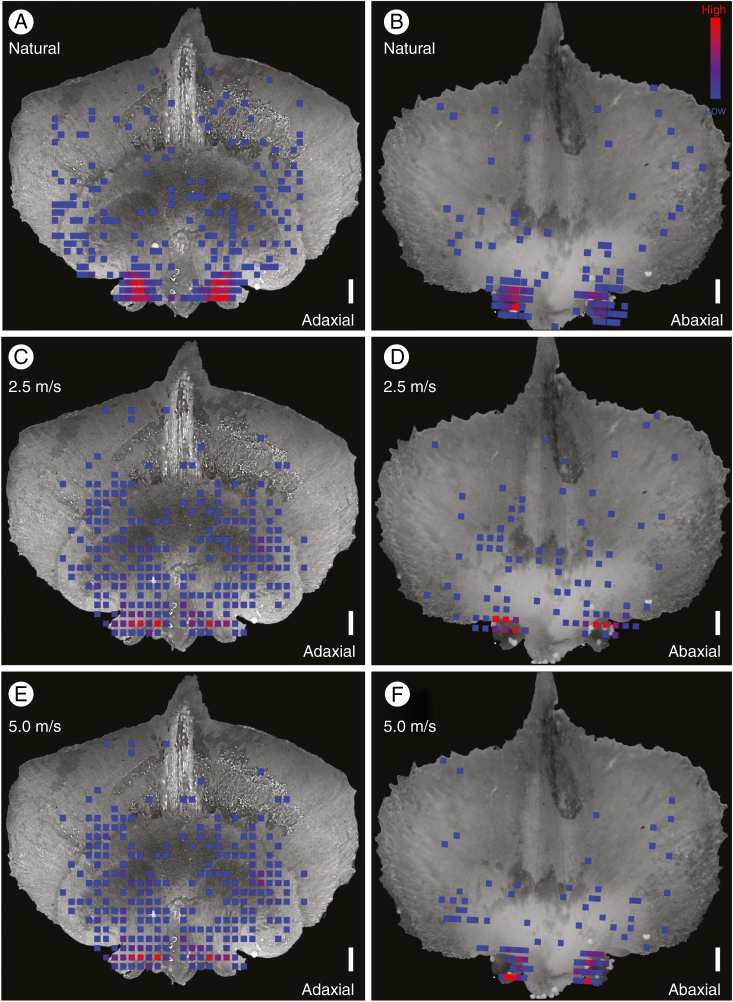
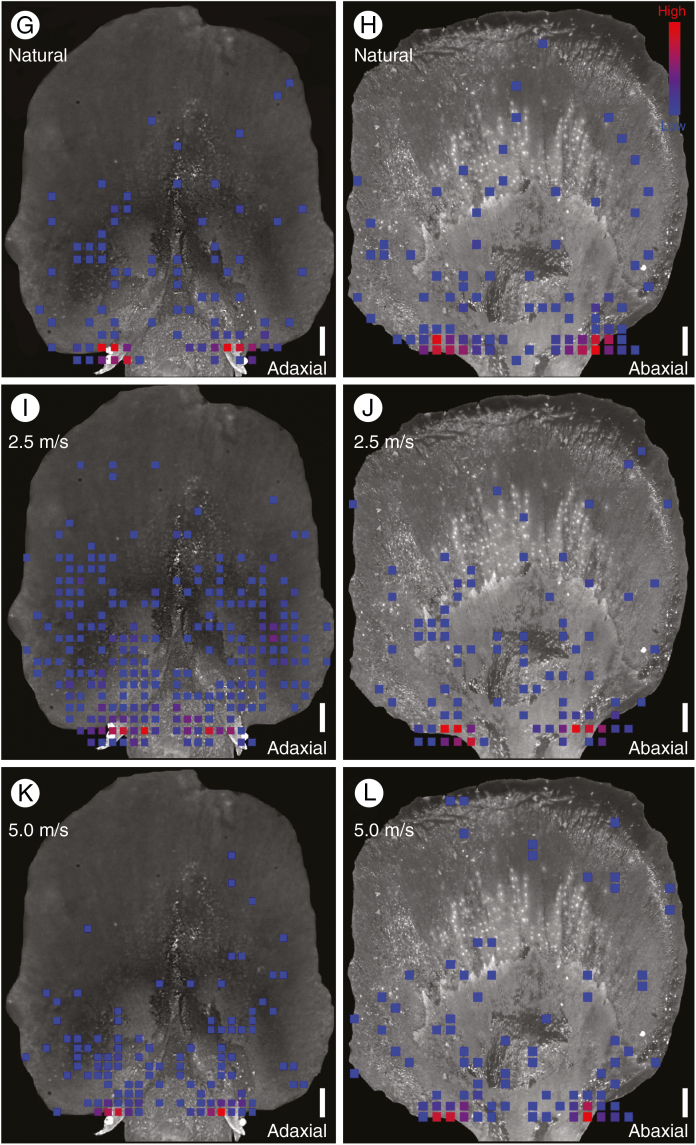
Despite morphological and structural differences in the cone scales of Abies and Picea, the overall ability of their cones to facilitate pollination was not obviously different. Pollen grains in both Abies and Picea were similarly concentrated on the integument and around the micropyle in both natural and artificial pollination experiments (Fig. 5; Supplementary Data Videos S1 and S2). Statistical tests did suggest that the distribution of pollen grains across the cone scales was different in Abies and Picea (Supplementary Data Table S1), but this was primarily due to differences in integumentary morphology. The broad funnel-shaped integument of Abies caused a slight shift in the location of peak pollen accumulation relative to Picea, which has a smaller, narrower integument and thus a more localized area of peak pollen accumulation (Fig. 5). Different wind regimes also changed the distribution of pollen grains across the surface of the cone scales (Fig. 5; note the greater abundance of pollen grains on the ovuliferous scales of artificially pollinated specimens, particularly at lower wind speeds), but in all cases the greatest concentration of pollen was found around the micropyle.
Fig. 5.
Heat maps showing the total distribution and frequency of pollen grains on the bract scale/ovuliferous scale complexes of Abies koreana (A–F) and Picea jezoensis (G–L) under natural (A, B, G, H) and artificial pollination regimes at two different wind speeds (C–F, I–L). The colour spectrum indicates frequency of observed pollen grains (blue, 1 grain; red, ≥5 grains). All panels show aggregated data from multiple complexes superimposed on a representative image of a cone scale for visual comparison. In Abies, the reduced ovuliferous scale can be seen only in the adaxial (top) surface view (A, C, E), along with the funnel-shaped integuments that protrude outwards from the scale. Ovule micropyles face downwards, however, and are visible in the abaxial (bottom) view (B, D, F). Pollen grains were found both around the integument and inside the micropyle. In Picea, micropylar arms are more clearly exposed in the adaxial view (G, I, K). The reduced bract scale can be seen only in the abaxial view (H, J, L). In all cases, note the high concentration of pollen grains on the integuments surrounding the micropyles. Scale bars = 500 µm.
Although Abies ovules did collect a larger absolute number of pollen grains than Picea ovules, the number of pollen grains per ovule was not significantly different after controlling for differences in integument (means of 9.83 and 12.40 grains µm−2, respectively, among pooled experimentally pollinated ovules; not significant using a Tukey test for ANOVA among treatments; Supplementary Data Table S2). Our results then suggest that the different cone and cone scale morphologies of Abies and Picea are similarly effective in funnelling windborne pollen inside the cone and into the ovules, regardless of any later mechanisms that may operate to move pollen into the ovules, such as pollination drops or rainwater.
DISCUSSION
Abies and Picea (as well as other Pinaceae: Owens and Smith, 1964, 1965; Powell, 1970; Owens and Molder, 1974; Tompsett, 1978) share a similar overall sequence of cone development that ultimately shifts cell proliferation and maturation in a centrifugal direction, away from the cone axis and towards the ovuliferous scales. Just prior to pollination in both taxa, the elongation of axial pith cells and the proliferation of cortex cells both lengthens and widens the cones; this has the effect of pulling and pushing the scales apart and thus creating open spaces through which windborne pollen can flow. In parallel to the cone axis, cone scales exhibit their own developmental trajectory where cell proliferation and maturation begin in the bract scales but then shift to the ovuliferous scales. Following pollination, cone growth becomes concentrated almost entirely in the ovuliferous scales; their continued development seals off the spaces initially created by axis elongation and isolates pollinated ovules from the outside world.
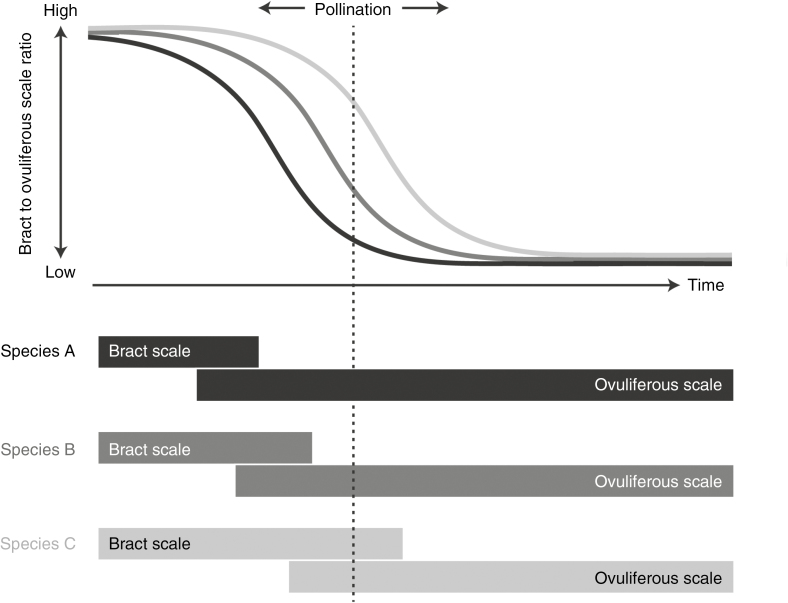
Given that pollination in Abies and Picea occurs at the exact same time, the relative dominance of ovuliferous scales in Picea must reflect an accelerated rate of development (Fig. 6). Such differences are especially likely to translate into morphological disparity in Pinaceae because pollination occurs early in cone ontogeny (Owens et al., 1981; Takaso and Owens, 1995; Owens and Morris, 1998; Dörken and Nimsch, 2014), precisely when they are undergoing a major shift from bract-scale-dominated to ovuliferous-scale-dominated growth (Fig. 6). The specific morphologies expressed by various lineages will then be sensitive to cone scale development rate, and Pinaceae do indeed show wide variability in the relative size and development of bract scales and ovuliferous scales (Fig. 7). Although pollination early in ontogeny most likely reflects selective pressures that are unrelated to specific cone morphologies, such as minimizing up-front tissue investment and pollinating early in the growing season in order to minimize interference from mature leaves (Whitehead, 1969; Niklas, 1985, 1992), they may ultimately be responsible for linking morphological diversity to variation in developmental patterns.
Fig. 6.
Conceptual figure illustrating how different developmental trajectories in the bract scale/ovuliferous scale complexes of conifers may generate morphological diversity at pollination. Bars in the lower panel indicate the initiation and cessation of growth in the bract scales and ovuliferous scales in three hypothetical species of conifers. The plot in the upper panel depicts hypothetical curves comparing the size of bract scales to the ovuliferous scales at different points in their development (the exact shape of the curve would depend on growth rates in each structure). The specific morphology each species exhibits at pollination depends on when exactly pollination intersects these curves. Note that the greatest potential diversity in morphologies occurs relatively early in cone development, during the time period when species transition from bract-dominated to ovuliferous scale-dominated forms.
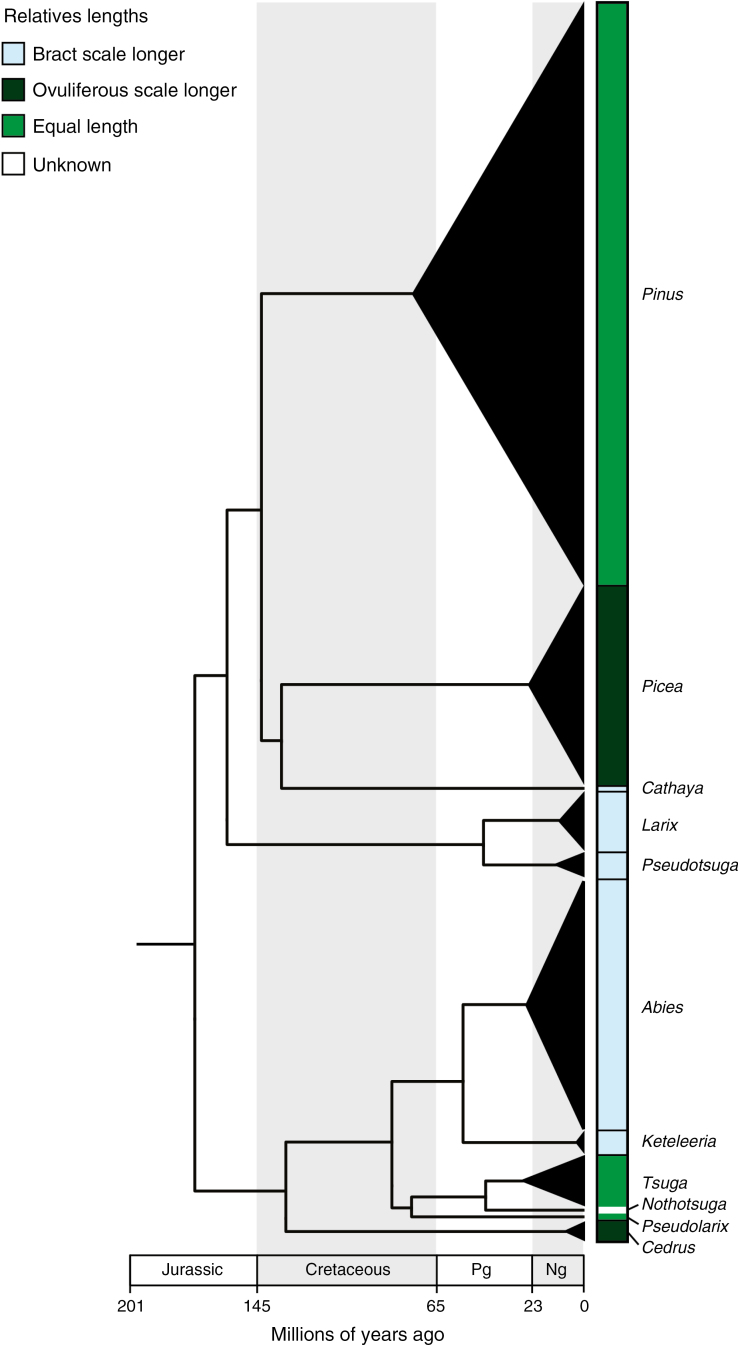
Fig. 7.
Time-calibrated molecular phylogeny from Leslie et al. (2012) showing the distribution of pollination-stage seed cone morphologies across Pinaceae. These morphologies include Abies-like (bract scales longer), Picea-like (ovuliferous scales longer) and intermediate (bract and ovuliferous scales roughly equal in length). The width of the black triangles is proportional to the relative species richness among extant genera, and the apex of the triangle corresponds to the estimated crown divergence ages within that genus. The relative sizes of bract scales and ovuliferous scales are roughly constant within extant genera. Pg, paleogene; Ng, neogene.
Despite significant developmental and structural differences between Abies and Picea cone scales, the cones themselves present similar forms to ambient wind patterns and we find no obvious differences in the efficiency with which they collect pollen. These results are also consistent with previous studies showing that Pinaceae cones from a wide range of taxa can effectively gather pollen (Niklas and Paw U, 1982; Niklas, 1982, 1984). Although it is difficult to conclusively prove that cone morphologies across lineages are exactly equivalent in function, we see no evidence that the diversity of Pinaceae pollination-stage morphologies is due to selection for differences in performance. Mapping cone traits on a time-calibrated molecular phylogeny (Fig. 7; phylogeny from Leslie et al., 2012) also indicates that the current suite of Pinaceae pollination-stage morphologies arose during the Cretaceous radiation of the group (Gernandt et al., 2016; Smith et al., 2017) but have since remained static, complicating any straightforward correlation between pollination-stage cone morphology and present-day climate or ecology.
In the absence of clear functional differences among cones, neutral variation in cone scale development appears to be the primary reason for diverse pollination-stage morphologies exhibited by Pinaceae. Such differences in developmental rate, or heterochrony in a broad sense (Gould, 1977; McKinney and McNamara, 1991; Klingenberg, 1998; Beldade et al., 2002; Webster and Zelditch, 2005), have been widely explored as a mechanism underlying the evolution of morphological diversity in animals and in plants (Jernvall, 2000; Li and Johnston, 2000; Herrel and Gibb, 2005; Kavanagh et al., 2007; Cartolano et al., 2015) and are especially likely to influence relationships among form, function and morphological diversity in plant reproductive structures because of the nature of their biology. All seed plant reproductive structures, from angiosperm flowers to conifer cones, undergo significant functional changes over their lifetimes; they initially facilitate pollination but then shift to protecting and ultimately dispersing seeds. These vastly disparate functional roles demand different morphologies to perform them, and therefore require an extended period of development to accommodate these changes (Sattler, 1990, 1992). Reproductive structures as a whole could then be thought of as having a ‘four-dimensional’ morphology, where the performance of multiple functions is nested within a larger context of ontogenetic shape change. Differences in development may alter this trajectory and therefore change the specific structures that plants use to meet their functional demands, as in our studied species of Abies and Picea.
Such developmental variation is likely to be especially important in the evolution of wind-pollinated reproductive structures because their functional constraints appear to be relatively lax, at least in the sense that a wide diversity of specific forms are capable of effectively facilitating pollination. Without strong selective pressures constraining evolution, simple variation in either the rate or specific patterns of development can readily generate morphological diversity over evolutionary time. Such a process need not be limited to wind-pollinated plants, however; many animal-pollinated lineages are also generalists and their morphologies function well across a range of pollinators (Rech et al., 2016; Wozniak and Sicard, 2017). Variation in their rate of development, either in the flower as a whole or in specific floral organs, may then also translate into a wide diversity of viable morphologies (Diggle, 2014). In a broader sense, the potential role of developmental variation in structuring both the evolution of reproductive disparity and specific patterns of trait variation needs further investigation, and this work illustrates how even relatively straightforward form and function relationships in plants can be strongly influenced by the developmental context in which they occur.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Video S1: high-speed time lapse showing the artificial pollination of Abies koreana seed cones. Video S2: high-speed time lapse showing the artificial pollination of Picea jezoensis seed cones. Fig. S1: relationship between total seed cone length and cell area of the pith in Abies koreana and Picea jezoensis. Fig. S2: representative image of P. jezoensis for pollen grain distribution analysis. Table S1: differences in the distributions of pollen grains on the bract scale/ovuliferous scale complexes of Abies and Picea. Table S2: pollen grains in or on the micropyles of Abies koreana and Picea jezoensis.
ACKNOWLEDGEMENTS
We are grateful to the Arnold Arboretum of Harvard University for providing access to plant collections, sampling equipment and imaging facilities, as well as William E. (Ned) Friedman for laboratory space, supplies and support. We would like to thank Callin Switzer for help with high-speed video and Kenneth Breuer for providing lighting equipment and helpful guidance on using the wind tunnel. We would also like to thank Benjamin Lyons and the Brown Design Space for additional help with wind tunnel experiments, and the two anonymous reviewers for their thoughtful suggestions.
LITERATURE CITED
- Ackerman JDD. 2000. Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives. Plant Systematics and Evolution 222: 167–185. [Google Scholar]
- Barrett SCH. 2013. The evolution of plant reproductive systems: how often are transitions irreversible?Proceedings of the Royal Society B: Biological Sciences 280: 20130913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD. 2017. The ecology of mating and its evolutionary consequences in seed plants. Annual Review of Ecology, Evolution, and Systematics 48: 135–157. [Google Scholar]
- Beldade P, Koops K, Brakefield PM. 2002. Developmental constraints versus flexibility in morphological evolution. Nature 416: 844–847. [DOI] [PubMed] [Google Scholar]
- Bogdziewicz M, Szymkowiak J, Kasprzyk I, et al. 2017. Masting in wind‐pollinated trees: system‐specific roles of weather and pollination dynamics in driving seed production. Ecology 98: 2615–2625. [DOI] [PubMed] [Google Scholar]
- de Candolle AP. 1813. Théorie élémentaire de la botanique. Paris: Deterville Press. [Google Scholar]
- Cartolano M, Pieper B, Lempe J, et al. 2015. Heterochrony underpins natural variation in Cardamine hirsuta leaf form. Proceedings of the National Academy of Sciences of the USA 112: 10539–10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LM, Owens JN. 2004. The pollination mechanism of Abies amabilis. Canadian Journal of Forest Research 34: 1071–80. [Google Scholar]
- Charlesworth D. 1993. Why are unisexual flowers associated with wind pollination and unspecialized pollinators?American Naturalist 141: 481–490. [Google Scholar]
- Chartier M, Löfstrand S, von Balthazar M, et al. 2017. How (much) do flowers vary? Unbalanced disparity among flower functional modules and a mosaic pattern of morphospace occupation in the order Ericales. Proceedings of the Royal Society B: Biological Sciences 284: 20170066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell JE, Henning K, Pennel C, et al. 2007. Conifer ovulate cones accumulate pollen principally by simple impaction. Proceedings of the National Academy of Sciences of the USA 104: 18141–18144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley TM, Weller SG, Sakai AK. 2002. The evolution of wind pollination in angiosperms. Trends in Ecology and Evolution 17: 361–369. [Google Scholar]
- Darwin C. 1877. The different forms of flowers on plants of the same species. London: John Murray. [Google Scholar]
- Diggle PK. 2014. Modularity and intra-floral integration in metameric organisms: plants are more than the sum of their parts. Philosophical Transactions of the Royal Society Series B: Biological Sciences 369: 20130253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörken VM, Nimsch H. 2014. Morpho‐anatomical investigations of cones and pollen in Cathaya argyrophylla Chung and Kuang (Pinaceae, Coniferales) under systematical and evolutional aspects. Feddes Repertorium 125: 25–38. [Google Scholar]
- Ellis AR, Burchett WW, Harrar SW, Bathke AC. 2017. Nonparametric inference for multivariate data: the R package npmv. Journal of Statistical Software 76: 1–18. [Google Scholar]
- Feder N, O’Brien TP. 1968. Plant microtechnique: some principles and new methods. American Journal of Botany 55: 123–142. [Google Scholar]
- Florin R. 1951. Evolution in cordaites and conifers. Acta Horti Bergiani 15: 285–388. [Google Scholar]
- Friedman J, Barrett SCH. 2008a A phylogenetic analysis of the evolution of wind pollination in the angiosperms. International Journal of Plant Sciences 169: 49–58. [Google Scholar]
- Friedman J, Barrett SCH. 2008b Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Annals of Botany 103: 1515–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Barrett SCH. 2011. The evolution of ovule number and flower size in wind-pollinated plants. American Naturalist 177: 246–257. [DOI] [PubMed] [Google Scholar]
- Gernandt DS, Holman G, Campbell C, et al. 2016. Phylogenetics of extant and fossil Pinaceae: methods for increasing topological stability. Botany 94: 863–884. [Google Scholar]
- Gould SJ. 1977. Ontogeny and phylogeny. Cambridge, MA: Harvard University Press. [Google Scholar]
- Herrel A, Gibb AC. 2005. Ontogeny of performance in vertebrates. Physiological and Biochemical Zoology 79: 1–6. [DOI] [PubMed] [Google Scholar]
- Hughes J, McCully ME. 1975. The use of an optical brightener in the study of plant structure. Stain Technology 50: 319–329. [DOI] [PubMed] [Google Scholar]
- Jernvall J. 2000. Linking development with generation of novelty in mammalian teeth. Proceedings of the National Academy of Sciences of the USA 97: 2641–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh KD, Evans AR, Jernvall J. 2007. Predicting evolutionary patterns of mammalian teeth from development. Nature 449: 427–432. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. 1998. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biological Reviews 73: 79–123. [DOI] [PubMed] [Google Scholar]
- Leslie AB, Beaulieu J, Hardeep R, Crane P, Donoghue M, Mathews S. 2012. Hemisphere-scale differences in conifer evolutionary dynamics. Proceedings of the National Academy of Sciences of the USA 109: 16217–16221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Johnston MO. 2000. Heterochrony in plant evolutionary studies through the twentieth century. Botanical Review 66: 57–88. [Google Scholar]
- Linder HP. 1998. Morphology and the evolution of wind pollination. In: Owens SJ, Rudall PJ, eds. Reproductive biology in systematics, conservation and economic botany. London: Royal Botanic Gardens Kew. [Google Scholar]
- McKinney ML, McNamara KJ. 1991. Heterochrony: the evolution of ontogeny. New York: Plenum Press. [Google Scholar]
- Nepi M, Little S, Guarieri M, et al. 2017. Phylogenetic and functional signals in gymnosperm ovular secretions. Annals of Botany 120: 923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Niet T, Johnson SD. 2012. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends in Ecology and Evolution 27: 353–361. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. 1982. Simulated and empiric wind pollination patterns of conifer ovulate cones. Proceedings of the National Academy of Sciences of the USA 79: 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. 1984. The motion of windborne pollen grains around conifer ovulate cones: implications on wind pollination. American Journal of Botany 71: 356–374. [Google Scholar]
- Niklas KJ. 1985. The aerodynamics of wind pollination. Botanical Review 51: 328–386. [Google Scholar]
- Niklas KJ. 1992. Plant biomechanics: an engineering approach to plant form and function. Chicago: University of Chicago Press. [Google Scholar]
- Niklas KJ, Paw UKT. 1982. Pollination and airflow patterns around conifer ovulate cones. Science 30: 442–444. [DOI] [PubMed] [Google Scholar]
- O’Meara BC, Smith SD, Armbruster WS, et al. 2016. Non-equilibrium dynamics and floral trait interactions shape extant angiosperm diversity. Proceedings of the Royal Society B: Biological Sciences 283: 20152304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme D. 2013. The caper package: comparative analysis of phylogenetics and evolution in R. R package version 0.5.2.https://cran.r-project.org/web/packages/caper/vignettes/caper.pdf [Google Scholar]
- Owens JN, Molder M. 1974. Bud development in western hemlock. II. Initiation and early development of pollen cones and seed cones. Canadian Journal of Botany 52: 283–294. [Google Scholar]
- Owens JN, Morris SJ. 1998. Factors affecting seed and cone development in Pacific silver fir (Abies amabilis). Canadian Journal of Forest Research 28: 1146–1163. [Google Scholar]
- Owens JN, Smith FH. 1964. The initiation and early development of the seed cone of Douglas fir. Canadian Journal of Botany 42: 1031–1047. [Google Scholar]
- Owens JN, Smith FH. 1965. Development of the seed cone of Douglas-fir following dormancy. Canadian Journal of Botany 43: 317–332. [Google Scholar]
- Owens JN, Simpson SJ, Molder M. 1981. Sexual reproduction of Pinus contorta. I. Pollen development, the pollination mechanism, and early ovule development. Canadian Journal of Botany 59: 1828–1843. [Google Scholar]
- Owens JN, Takaso T, Runions CJ. 1998. Pollination in conifers. Trends in Plant Science 3: 479–485. [Google Scholar]
- Pauw A, Kahnt B, Kuhlmann M, et al. 2017. Long-legged bees make adaptive leaps: linking adaptation to coevolution in a plant–pollinator network. Proceedings of the Royal Society B: Biological Sciences 284: 20171707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell GR. 1970. Postdormancy development and growth of microsporangiate and megasporangiate strobili of Abies balsamea. Canadian Journal of Botany 48: 419–428. [Google Scholar]
- Rech AR, Dalsgaard B, Sandel B, et al. 2016. The macroecology of animal versus wind pollination: ecological factors are more important than historical climate stability. Plant Ecology and Diversity 9: 253–262. [Google Scholar]
- Richards JH. 2002. Flower and spikelet morphology in sawgrass, Cladium jamaicense Crantz (Cyperaceae). Annals of Botany 90: 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman SK, Irwin RE, Nelson CJ, Bronstein JL. 2017. Facilitated exploitation of pollination mutualisms: fitness consequences for plants. Journal of Ecology 105: 188–196. [Google Scholar]
- Sattler R. 1990. Towards a more dynamic plant morphology. Acta Biotheoretica 38: 303–315. [Google Scholar]
- Sattler R. 1992. Process morphology: structural dynamics in development and evolution. Canadian Journal of Botany 70: 708–714. [Google Scholar]
- Smith SY, Stockey RA, Rothwell GW, Little SA. 2017. A new species of Pityostrobus (Pinaceae) from the Cretaceous of California: moving towards understanding the Cretaceous radiation of Pinaceae. Journal of Systematic Palaeontology 15: 69–81. [Google Scholar]
- Solís‐Montero L, Vallejo‐Marín M. 2017. Does the morphological fit between flowers and pollinators affect pollen deposition? An experimental test in a buzz‐pollinated species with anther dimorphism. Ecology and Evolution 7: 2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaso T, Owens JN. 1995. Ovulate cone morphology and pollination in Pseudotsuga and Cedrus. International Journal of Plant Sciences 156: 630–639. [Google Scholar]
- Taylor EL, Taylor TN, Krings M. 2009. Paleobotany: the biology and evolution of fossil plants, Boston, MA: Academic Press. [Google Scholar]
- Thompson DW. 1942. On growth and form. Cambridge: Cambridge University Press. [Google Scholar]
- Tomlinson PB, Takaso T. 2002. Seed cone structure in conifers in relation to development and pollination: a biological approach. Canadian Journal of Botany 80: 1250–1273. [Google Scholar]
- Tompsett PB. 1978. Studies of growth and flowering in Picea sitchensis (Bong.) Carr. 2. Initiation and development of male, female and vegetative buds. Annals of Botany 42: 889–900. [Google Scholar]
- Webster M, Zelditch ML. 2005. Evolutionary modifications of ontogeny: heterochrony and beyond. Paleobiology 31: 354–372. [Google Scholar]
- Weller SG, Sakai AK, Culley TM, Campbell DR, Dunbar-Wallis AK. 2006. Predicting the pathway to wind pollination: heritabilities and genetic correlations of inflorescence traits associated with wind pollination in Schiedea salicaria (Caryophyllaceae). Journal of Evolutionary Biology 19: 331–342. [DOI] [PubMed] [Google Scholar]
- Whitehead DR. 1969. Wind pollination in the angiosperms: evolutionary and environmental considerations. Evolution 23: 28–35. [DOI] [PubMed] [Google Scholar]
- Wozniak NJ, Sicard A. 2017. Evolvability of flower geometry: convergence in pollinator-driven morphological evolution of flowers. Seminars in Cell and Developmental Biology. doi: 10.1016/j.semcdb.2017.09.028. [DOI] [PubMed] [Google Scholar]
- Wragg PD, Johnson SD. 2011. Transition from wind pollination to insect pollination in sedges: experimental evidence and functional traits. New Phytologist 191: 1128–1140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.