Abstract
Background
This study aimed to investigate the effect and underlying molecular mechanism of sinomenine (SIN) on ankylosing spondylitis (AS).
Material/Methods
To study the potential role of SIN in the pathogenesis of AS, an AS mouse model was established and mice were treated with different concentrations of SIN (10, 30, and 50 mg/kg, administered intraperitoneally). Markers of inflammation and oxidative stress were determined by ELISA assay. Western blot analysis and qRT-PCR were used to quantify the levels of related proteins and gene mRNA expression.
Results
The results suggest that AS mice has higher levels of TNF-α, IL-1β, and IL-6 (p<0.01 for all), and lower levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX) (p<0.01 for all). SIN treatment reduced the level of TNF-α, IL-1β, and IL-6 in a dose-dependent manner, and the levels of SOD, CAT, and GSH-PX were dose-dependently increased (p<0.05 for all). The results also revealed that NF-κBp65 expression decreased, while the level of IκB increased, in a dose-dependent manner, after SIN treatment in AS mice (p<0.05 for all). The level of p-p38 was dose-dependently reduced in AS mice by SIN treatment (p<0.05). Moreover, SIN inhibited Cox-2 expression in AS mice in a dose-dependent manner (p<0.05).
Conclusions
SIN has a beneficial role in AS through suppressing inflammatory mediators and by down-regulating oxidative stress via inhibiting the MAPKp38/NF-κB pathway and Cox-2 expression.
MeSH Keywords: Inflammation; NF-kappa B; Oxidative Stress; p38 Mitogen-Activated Protein Kinases; Sinomenium; Spondylitis, Ankylosing
Background
Ankylosing spondylitis (AS), a common and unexplained chronic inflammatory-based autoimmune disease, is characterized by ankylosis, new bone formation, and inflammation of sacroiliac joints, hip, and spine [1–5]. The pathogenesis of AS is not yet completely clear and involves a variety of factors. Many studies have shown that cytokine network abnormality is an important feature of AS pathology [6,7]. The occurrence of AS is insidious and progressive, and the major clinical manifestations of the early stage are joint pain without apparent cause, which is easily ignored by patients, leading to missed diagnosis and delay in treatment. Advanced AS may lead to spinal deformity, loss of ability to work, and disability, seriously affecting the quality of life [8–11]. The prevalence of AS in China is about 0.20% to 0.40% [12]. Almost 80% of AS patients are young adults, and 5 years after diagnosis the disability rate reaches 40% to 60% [13,14]. At present, there is no effective treatment for AS.
Sinomenine (7,8-didehydro-4-hydroxy3,7-dimethoxy-17-methyl-9α, 13α, 14α-morphinan-6-one; SIN), an extract, is extracted from the Chinese medicinal herb Sinomenium acutum. SIN is widely used in mesangial proliferative nephritis and rheumatoid arthritis treatment in China [15,16]. It has various pharmacological properties, including cytoprotection, immunosuppression, anti-cancer, and anti-inflammation effects [17–21]. However, no published study has fully investigated the effects of SIN on the development of AS and explored the potential underlying molecular mechanisms.
Therefore, the purpose of the present study was to determine whether sinomenine administration can relieve ankylosing spondylitis in an AS mouse model, and to explore the underlying molecular mechanisms.
Material and Methods
Materials
SIN was obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in saline containing 1% dimethylsulfoxide (DMSO) just before injection.
ELISA kits for the detection of TNF-α, IL-1β, IL-6, CAT, GSH-PX, and SOD were supplied by Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against NF-κBp65, IκB; MAPK p-p38; Cox-2, and β-actin, as well as the secondary antibodies, were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). All other chemicals and reagents were purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China).
Creation of the AS mouse model
The mouse models in the present experiment were treated according to the principles as set by Honghui Hospital Affiliated to Xi’an Jiaotong University. BALB/c mice were obtained from the Vital River Company (Beijing, China). For AS mouse model establishment, 3-month-old mice were injected 4 times once a week with 2 mg human proteoglycan extract dissolved in 2 mg dimethyl dioctadecyl ammonium (DDA) [22]. After the second injection, symptoms consistent with peripheral arthritis were observed, and the mice showed signs of axial skeleton ankyloses [23]. At the end of this experiment, blood was collected from the retroorbital plexus, and AS tissues were extracted for histological analysis. Suitable tissues and blood samples were immediately flash-frozen in liquid nitrogen and stored at −80°C.
The mice were randomly divided into 6 treatment groups (n=5 per group): (1) a control group (CG) in which healthy control mice received a basal diet [21]; (2) a control ankylosing spondylitis group (CG-AS) in which AS mice received a basal diet; (3) a sinomenine injection group (SIG) in which normal healthy mice received a basal diet and 50 mg/kg sinomenine; (4) a sinomenine injection ankylosing spondylitis group (SIG-AS10, SIG-AS30, SIG-AS50) in which AS mice received a basal diet and 10, 30, or 50 mg/kg sinomenine, respectively.
Detection of cytokines and antioxidative enzyme activities
The serum levels of tumor necrosis factor-α (TNF-α), interlukin-1β (IL-1β), and interlukin-6 (IL-6) were detected by using ELISA assay according to the manufacturer’s instructions for each kit. Levels of catalase (CAT), glutathione peroxidase (GSH-PX), and superoxide dismutase (SOD) were also determined by performing ELISA. Each experiment was independently performed 3 times.
Western blot analysis
AS tissues were extracted, and 100 μl of tissue lysis buffer (Cell Signaling Technology, Danvers, MA, USA) was used for protein collection. A BCA protein assay kit (Thermo Fisher Scientific, Inc.) was used to measure the protein concentration of samples according to the manufacturer’s instructions. The same proteins (30 μg each sample) were resolved by 12% SDS-PAGE, transferred to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA), and then blocked with 5% nonfat dried milk. The membranes were then incubated overnight at 4°C with the following primary antibodies: NF-κBp65 (1: 1000), IκB (1: 1000), p-p38 (1: 1000), Cox-2 (1: 1000), or β-actin (1: 2000). Subsequently, membranes were incubated with a HRP-conjugated secondary antibody for 1 h at 37°C. Quantity One (V4.4.0, Bio-Rad Laboratories, USA) software was used for measurement of the band intensities.
QRT-PCR
Total RNA was extracted from the AS tissues by using RNAiso Plus (Takara Bio, Dalian, China) following the manufacturer’s instructions. A reverse transcription experiment was carried out to synthesize cDNAs with the ThermoScript RT-PCR system (Invitrogen, Grand Island, NY, USA), and qPCR was performed to analyze the synthesized cDNAs. The conditions of qPCR used for amplification were as follows: 95°C for 5 min, 40 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, then 72°C for 10 min. GAPDH served as an internal control. The primer sequences for qPCR were as follows:
GAPDH-Forward: 5′GAAGGTGAAGGTCGGAGTC3′;
GAPDH-Reverse: 5′GAAGATGGTGATGGGATTTC3′;
NF-κBp65-Forward: 5′GACCTGGCATCTGTGGACAAC3′;
NF-κBp65-Reverse: 5′TCCGCAATGGAGGAGAAGTCT3′;
IκB-Forward: 5′GAGGCGTGGCAGACTATGC3′;
IκB-Reverse: 5′CTTGTACTCCGTCAGCGTGA3′;
Cox-2-Forward: 5′ACCGAGTCGTTCTGCCAATA3′;
Cox-2-Reverse: 5′CTCATGAGTGGAGGACGTCT3′.
The relative gene expression was assessed by using the 2-ΔΔCq method [24]. The experiment was repeated at least 3 times.
Statistical analysis
Data are displayed as the mean ± standard deviation (SD). Analyses were carried out using SPSS (V22.0, IBM, USA). Student’s t test or one-way ANOVA followed by Tukey’s test was used to make comparison between groups. A value of p<0.05 was considered as statistically significant.
Results
Effect of SIN on inflammatory cytokines
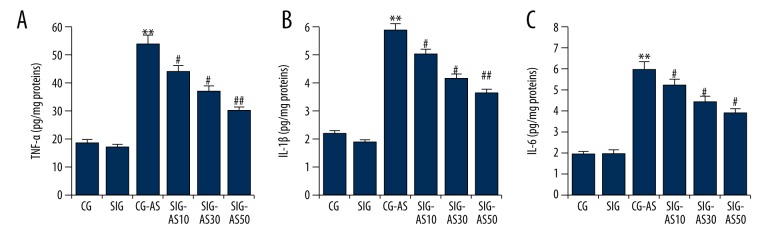
To investigate the exact beneficial role that SIN has in the animal AS models used in this experiment, the levels of TNF-α, IL-1β, and IL-6 were determined. As shown in Figure 1, TNF-α, IL-1β, and IL-6 levels were significant higher in the CG-AS group in comparison to the CG and SIG groups. Administration of SIN in SIG-AS mice decreased the levels of TNF-α, IL-1β, and IL-6 in a dose-dependent manner in comparison to those of the CG-AS mice.
Figure 1.
The effect of SIN on (A) TNF-α, (B) IL-1β, and (C) IL-6 in mice. ** p<0.01 vs. CG; #, ## p<0.05, 0.01 vs. CG-AS. Data are presented as mean ±SD.
Effect of SIN on antioxidative enzymes
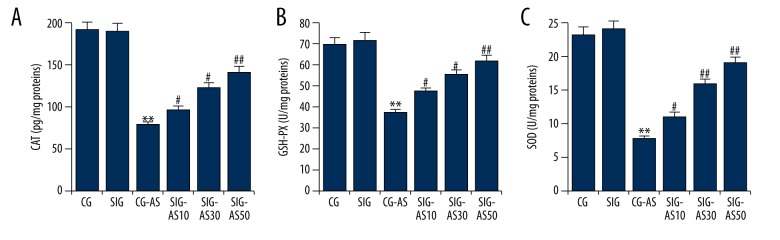
To demonstrate the beneficial role of SIN in decreasing or promoting oxidative stress in the AS mouse models, the levels of antioxidative enzymes (SOD, CAT, and GSH-PX) were measured. The results showed that the levels of SOD, CAT, and GSH-PX were significantly lower in the CG-AS group in comparison to the CG and SIG groups. SIN dose-dependently increased the levels of SOD, CAT, and GSH-PX in SIG-AS groups in comparison with the CG-AS group (Figure 2).
Figure 2.
The effect of SIN on (A) CAT, (B) GSH-PX, and (C) SOD in mice. ** p<0.01 vs. CG; #, ## p<0.05, 0.01 vs. CG-AS. Data are presented as mean ±SD.
Effect of SIN on MAPKp38/NF-κB pathway
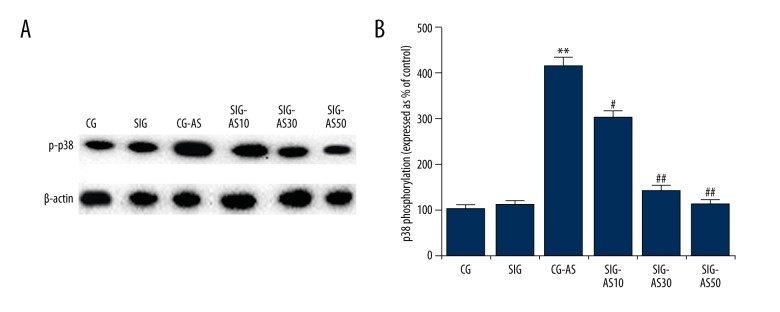
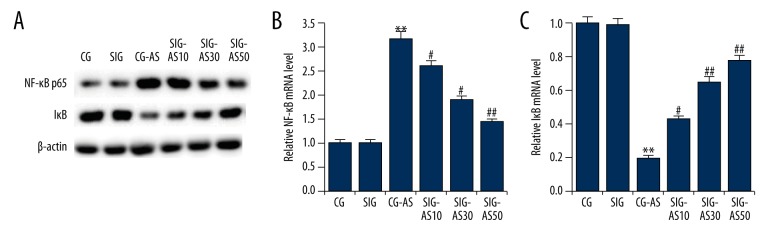
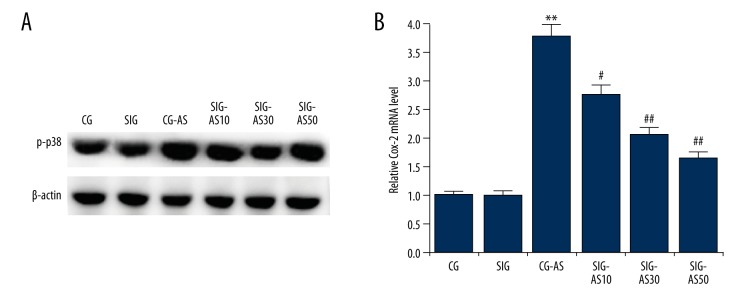
To explore the underlying molecular mechanisms of the effect of SIN on AS, we investigated the MAPKp38/NF-kB pathway and performed Western blot analysis and qRT-PCR. As shown in Figure 3, we found that the p-p38 expression level in the CG-AS group was higher than that in the CG and SIG groups. SIN dose-dependently decreased the protein level of p-p38 in the SIG-AS groups in comparison with the CG-AS group. Moreover, compared with the CG and SIG groups, in the CG-AS group NF-κBp65 was significantly increased and IκB was significantly decreased. SIN dose-dependently decreased the expression level of NF-κBp65 and increase IκB expression in SIG-AS groups in comparison with the CG-AS group (Figure 4). Taken together, the data indicate that SIN inhibits MAPKp38/NF-κB pathway activation.
Figure 3.
(A, B) The effect of SIN on NF-κBp65 and IκB expression. The protein and mRNA levels of NF-κBp65 and IκB were detected by Western blotting and qRT-PCR, respectively. ** p<0.01 vs. CG; #, ## p<0.05, 0.01 vs. CG-AS. Data are presented as mean ±SD.
Figure 4.
(A–C) The effect of SIN on p-p38 expression. The protein level of p-p38 was detected by Western blotting. The expression levels of p38 phosphorylation are expressed as% of the control. ** p<0.01 vs. CG; #, ## p<0.05, 0.01 vs. CG-AS. Data are presented as mean ±SD.
Effect of SIN on Cox-2 expression
We also investigated Cox-2. As expected, compared with the CG and SIG group, the protein and mRNA levels of Cox-2 were markedly increased in the CG-AS group. Following SIN treatment, the level of Cox-2 dose-dependently decreased in the SIG-AS groups in comparison to the CG-AS group (Figure 5).
Figure 5.
(A, B) The effect of SIN on Cox-2 expression. The protein and mRNA levels of Cox-2 weres detected by Western blotting and qRT-PCR, respectively. ** p<0.01 vs. CG; #, ## p<0.05, 0.01 vs. CG-AS. Data are presented as mean ±SD.
Discussion
AS, a systemic disease of unknown etiology, is characterized by inflammation in the spine and sacroiliac joints, initially causing bone and joint erosion, and, ultimately, ankylosis and fibrosis [2–5,25]. Elevated concentrations of pro-inflammatory cytokines and reactive oxygen species (ROS) participate in this disease. Although the exact mechanism of AS remains largely unclear, pro-inflammatory mediators are strongly suspected to participate in the development of this disease. Studies have confirmed that the pro-inflammatory mediators further produce IL-6 and TNF-α by stimulating osteoclasts and other inflammatory cells [26]. ROS, known as free radicals, is produced during normal metabolic reactions in the human body. However, excessive and uncontrolled elevated levels of ROS are associated with oxidative stress [27]. CAT, GSH-PX, and SOD, known as important antioxidant enzymes, play critical roles in scavenging superoxide radicals in cells, thus reducing the toxic nature of free radicals, and thereby preventing harm [28].
As a common rattan drug, SIN has been widely used for the treatment of a variety of arthritic diseases in traditional Chinese medicine (TCM) clinics. To date, it has been demonstrated that sinomenine has a number of pharmacological activities, including immunosuppressive, anti-inflammatory, anti-rheumatic, and anti-angiogenic effects [18]. In recent years, a growing body of evidence suggests that SIN has neuroprotective effects [18,29,30]. Studies also have demonstrated that SIN has anti-tumor potential against various tumor cells, including hepatocellular carcinoma, breast cancer, and lung cancer [19,20,31].
The present study investigated the effects of SIN on AS, and an AS mouse model was created and treated with different concentrations of SIN (10, 30, and 50 mg/kg). Because pro-inflammatory cytokines and oxidative stress are involved in the development of AS, and because SIN has anti-inflammatory and antioxidant effects [18,32,33], the study determined the markers of inflammation (TNF-α, IL-1β, and IL-6) and oxidative stress (SOD, CAT and GSH-PX), showing that AS mice had higher levels of TNF-α, IL-1β, and IL-6, and lower levels of SOD, CAT, and GSH-PX. SIN treatment reduced the levels of TNF-α, IL-1β, and IL-6 in a dose-dependent manner, and the levels of SOD, CAT, and GSH-PX were dose-dependently increased. Furthermore, the present study investigated the NF-κB pathway, MAPKp38 pathway, and Cox-2, which have been reported to regulate the expression of the markers of inflammation (TNF-α, IL-1β, and IL-6) and oxidative stress (SOD, CAT and GSH-PX) [34–36]. The results suggest that SIN treatment inhibits the expression of NF-κBp65, while IκB expression was enhanced in AS mice, consistent with a previous study [37], while SIN treatment inhibited the expression of p-p38 and Cox-2.
Conclusions
The present study shows that SIN has a beneficial role in AS through preventing inflammatory mediators, and by down-regulating oxidative stress via inhibiting NF-κB, MAPKp38 pathway, and Cox-2 expression. These results provide a theoretical basis for the treatment of AS. SIN plays a protective role in the progression of AS, and thus may serve as a novel therapeutic target for AS.
Footnotes
Source of support: Departmental sources
Conflict of interests.
None.
References
- 1.Yu H, Liu Y, Zhang L, et al. FOXO1 gene confers genetic predisposition to acute anterior uveitis with ankylosing spondylitis. Invest Ophthalmol Vis Sci. 2014;55:7970–74. doi: 10.1167/iovs.14-15460. [DOI] [PubMed] [Google Scholar]
- 2.Liu YF, Dong H, Tu SH, et al. Etanercept in the treatment of ankylosing spondylitis: A systematic review and meta-analysis. Exp Ther Med. 2014;8:1585–92. doi: 10.3892/etm.2014.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Lin S, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev. 2014;11:CD004800. doi: 10.1002/14651858.CD004800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danve A, Reddy A, Vakil-Gilani K, et al. Routine Assessment of Patient Index Data 3 score (RAPID3) correlates well with Bath Ankylosing Spondylitis Disease Activity index (BASDAI) in the assessment of disease activity and monitoring progression of axial spondyloarthritis. Clin Rheumatol. 2015;34:117–24. doi: 10.1007/s10067-014-2827-4. [DOI] [PubMed] [Google Scholar]
- 5.Svealv BG, Tang MS, Klingberg E, et al. Prevalence of diastolic dysfunction in patients with ankylosing spondylitis: A cross-sectional study. Scand J Rheumatol. 2015;44:111–17. doi: 10.3109/03009742.2014.953201. [DOI] [PubMed] [Google Scholar]
- 6.Ranganathan V, Gracey E, Brown MA, et al. Pathogenesis of ankylosing spondylitis – recent advances and future directions. Nat Rev Rheumatol. 2017;13:359–67. doi: 10.1038/nrrheum.2017.56. [DOI] [PubMed] [Google Scholar]
- 7.Gracey E, Qaiyum Z, Almaghlouth I, et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis. 2016;75:2124–32. doi: 10.1136/annrheumdis-2015-208902. [DOI] [PubMed] [Google Scholar]
- 8.Lubrano E, Spadaro A, Amato G, et al. Tumour necrosis factor alpha inhibitor therapy and rehabilitation for the treatment of ankylosing spondylitis: A systematic review. Semin Arthritis Rheum. 2015;44:542–50. doi: 10.1016/j.semarthrit.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Fan D, Liu L, Ding N, et al. Male sexual dysfunction and ankylosing spondylitis: a systematic review and metaanalysis. J Rheumatol. 2015;42:252–57. doi: 10.3899/jrheum.140416. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Yu K, Guo R, et al. Ankylosing spondylitis: Correlations between clinical and MRI indices of sacroiliitis activity. Clin Radiol. 2015;70:62–66. doi: 10.1016/j.crad.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Gao H, Li X, Wang C. Ankylosing spondylitis with idiopathic spinal cord herniation. Spine J. 2015;15:552–53. doi: 10.1016/j.spinee.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Dean LE, Jones GT, MacDonald AG, et al. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650–57. doi: 10.1093/rheumatology/ket387. [DOI] [PubMed] [Google Scholar]
- 13.Haywood KL, Packham JC, Jordan KP. Assessing fatigue in ankylosing spondylitis: The importance of frequency and severity. Rheumatology (Oxford) 2014;53:552–56. doi: 10.1093/rheumatology/ket397. [DOI] [PubMed] [Google Scholar]
- 14.Gan FY, Fei YY, Li MT, et al. The characteristics of patients having ankylosing spondylitis associated with Takayasu’s arteritis. Clin Rheumatol. 2014;33:355–58. doi: 10.1007/s10067-013-2444-7. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Li F, Wang D, et al. Sinomenine inhibits the expression of PDL1 in the peripheral blood mononuclear cells of mesangial proliferative nephritis patients. Mol Med Rep. 2013;7:1223–28. doi: 10.3892/mmr.2013.1302. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Liu L, Qi C, et al. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Med. 2008;74:1423–29. doi: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Zhang J, Hou W, et al. Immunoregulatory effects of sinomenine on the T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of mesangial proliferative nephritis. Int Immunopharmacol. 2009;9:894–99. doi: 10.1016/j.intimp.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Qian L, Xu Z, Zhang W, et al. Sinomenine, a natural dextrorotatory morphinan analog, is antiinflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu XL, Zeng J, Chen YL, et al. Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: Involvement of cell cycle arrest and apoptosis induction. Int J Oncol. 2013;42:229–38. doi: 10.3892/ijo.2012.1704. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Li PP, Liu C, et al. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial–mesenchymal transition and cancer stemness. Oncotarget. 2017;8:13560–74. doi: 10.18632/oncotarget.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol. 2011;11:373–76. doi: 10.1016/j.intimp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Haynes KR, Pettit AR, Duan R, et al. Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors. Arthritis Res Ther. 2012;14(6):R253. doi: 10.1186/ar4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren W, Duan J, Yin J, et al. Dietary l-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. 2014;46:2403–13. doi: 10.1007/s00726-014-1793-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Lu X, Cao Y. MicroRNA and signal transduction pathways in tumor radiation response. Cell Signal. 2013;25:1625–34. doi: 10.1016/j.cellsig.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montala N, Juanola X, Collantes E, et al. Prevalence of vertebral fractures by semiautomated morphometry in patients with ankylosing spondylitis. J Rheumatol. 2011;38:893–97. doi: 10.3899/jrheum.100851. [DOI] [PubMed] [Google Scholar]
- 26.Robinson PC, Brown MA. Genetics of ankylosing spondylitis. Mol Immunol. 2014;57:2–11. doi: 10.1016/j.molimm.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Okano K, Kimura K, Tanaka Y, et al. Direct measurement of reactive oxygen species in leukocytes during hemodialysis therapy. Int J Clin Exp Med. 2015;8:20959–64. [PMC free article] [PubMed] [Google Scholar]
- 28.Kozaci LD, Sari I, Alacacioglu A, et al. Evaluation of inflammation and oxidative stress in ankylosing spondylitis: A role for macrophage migration inhibitory factor. Mod Rheumatol. 2010;20:34–39. doi: 10.1007/s10165-009-0230-9. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Wang H, Li L, et al. Sinomenine provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE pathway. Front Neurosci. 2016;10:580. doi: 10.3389/fnins.2016.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu J, Wang M, Zhang J, et al. The neuroprotection of Sinomenine against ischemic stroke in mice by suppressing NLRP3 inflammasome via AMPK signaling. Int Immunopharmacol. 2016;40:492–500. doi: 10.1016/j.intimp.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Jiang SL, Gao YB, Hou W, et al. Sinomenine inhibits A549 human lung cancer cell invasion by mediating the STAT3 signaling pathway. Oncol Lett. 2016;12:1380–86. doi: 10.3892/ol.2016.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan H, Shu Q, Guan X, et al. Sinomenine protects PC12 neuronal cells against H2O2-induced cytotoxicity and oxidative stress via a ROS-dependent Up-regulation of endogenous antioxidant system. Cell Mol Neurobiol. 2017;37:1387–98. doi: 10.1007/s10571-017-0469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin T, Yin S, Yang J, et al. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFβ signaling. Toxicol Appl Pharmacol. 2016;304:1–8. doi: 10.1016/j.taap.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Xing Y, Ji Q, Li X, et al. Asiaticoside protects cochlear hair cells from high glucose-induced oxidative stress via suppressing AGEs/RAGE/NF-κB pathway. Biomed Pharmacother. 2017;86:531–36. doi: 10.1016/j.biopha.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Dai JP, Wang QW, et al. Emodin inhibition of influenza a virus replication and influenza viral pneumonia via the Nrf2, TLR4, p38/JNK and NF-kappaB pathways. Molecules. 2017;22(10) doi: 10.3390/molecules22101754. pii: E1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozturk H, Gezici A, Ozturk H. The effect of celecoxib, a selective COX-2 inhibitor, on liver ischemia/reperfusion-induced oxidative stress in rats. Hepatol Res. 2006;34:76–83. doi: 10.1016/j.hepres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Fang Y, Huang W, et al. Effect of sinomenine on cytokine expression of macrophages and synoviocytes in adjuvant arthritis rats. J Ethnopharmacol. 2005;98:37–43. doi: 10.1016/j.jep.2004.12.022. [DOI] [PubMed] [Google Scholar]