Abstract
Background
Cholera continues to be a major cause of morbidity and mortality worldwide and is now endemic in Haiti since first being introduced in 2010. Cholera and HIV have significant geographic overlap globally, but little is known about the clinical features and risk of cholera among HIV-infected people and their households.
Methods
We assessed HIV-affected households originally recruited for a randomized controlled trial of food supplements. We assessed for correlation between household and individual factors and reported history of cholera since 2010 using univariable and multivariable analyses.
Results
There were 352 HIV-infected household members, 32 with reported history of medically attended cholera, and 1968 other household members, 55 with reported history of medically attended cholera. Among HIV-infected individuals in this study, no variables correlated with reported history of cholera in univariable analyses. Among all household members, known HIV infection (adjusted odds ratio [AOR], 3.75; 95% CI, 2.43–5.79; P < .0001), source of income in the household (AOR, 1.82; 95% CI, 1.05–3.15; P = .034), time required to fetch water (AOR, 1.07 per 5-minute increase; 95% CI, 1.01–1.12; P = .015), and severe household food insecurity (AOR, 3.23; 95% CI, 1.25–8.34; P = .016) were correlated with reported history of cholera in a multivariable analysis.
Conclusions
Known HIV infection, source of household income, time required to fetch water, and severe household food insecurity were independently associated with reported history of medically attended cholera in HIV-affected households in rural Haiti. Further research is required to better understand the interactions between HIV and cholera.
Keywords: cholera, co-infection, food security, Haiti, HIV
The acute diarrheal illness caused by toxigenic Vibrio cholera remains a major cause of morbidity and mortality worldwide, with 172 454 cases and 1304 deaths reported in 42 countries in 2015 [1]. Among these, Haiti has experienced a national epidemic since the introduction of cholera in 2010 [2].
Globally, there is substantial geographic overlap between the HIV pandemic and the seventh cholera pandemic, which extends throughout Asia into Africa, Europe, and the Americas [1, 3]. However, the clinical features and risk of cholera among HIV-infected people and their households have not been well characterized. One recent study in urban Haiti found a higher than expected prevalence of HIV among people presenting to a cholera treatment center (CTC) with diarrhea [4], and 2 case–control studies also suggest a possible association between HIV and cholera, although both were at high risk of selection bias [5, 6].
This study is, to our knowledge, the first to assess risk factors for cholera within HIV-affected households—including HIV status, socioeconomic factors, water source, and food security. In it, we evaluate HIV-affected households in rural Haiti that were originally recruited to participate in a randomized controlled trial of food supplements in HIV [7].
METHODS
Participants
We analyzed data from a randomized controlled trial comparing 2 types of food supplements (ready-to-use therapeutic food [RUTF] and corn soy blend [CSB]) distributed to HIV-infected individuals receiving care at 3 health centers in the Artibonite Department of Haiti, where comprehensive medical care is provided free of charge [7]. Participants were eligible if they were 18 years of age or older, known to be HIV-infected by clinical record, and had initiated antiretroviral therapy (ART) within the prior 24 months. Individuals were ineligible for the study if another household member was eligible for food assistance (this excluded households in which 2 adults were known to have HIV infection by clinical record) or if they were pregnant at the time of enrollment. Per clinic protocol, all participants received trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis unless intolerant. The 524 participants were recruited in June 2010. We considered all other household members to be HIV status unknown. Ethical approval was granted by the Institutional Review Boards of Partners HealthCare (Boston, MA) and Zanmi Lasante (Cange, Haiti). All participants provided written informed consent.
Data Collection
The study took place during 2010–2013 and included structured survey assessments of the HIV-infected participants and their households at baseline and at 6- and 12-month follow-up time points. A household was defined as a group of persons who make common provisions for food and other essentials for living [8]. A cholera outbreak occurred in the region during the course of the original study, and a fourth follow-up time point was added at the end of the trial to assess the impact of cholera in these households [2]. All households originally enrolled in the trial were invited to participate in the survey at the fourth follow-up time point, which was completed in January 2014, 2.5 years after enrollment. This analysis is restricted to survey data from this time point. Surveys took place at the household and were directed both at the individual with known HIV infection and at other household members present at the time of survey administration.
Through this survey, information on the HIV-infected household members’ age, sex, marital status, and literacy was collected. Data on the household were also collected, including number of household members, source of income, electricity, access to a latrine, presence of a garden, amount of time to fetch water, water source, and use of water treatment. We assessed for self-reported history of cholera since 2010 by all household members, and whether those with a reported history sought medical attention or were hospitalized. Survey questions regarding reported history of cholera were identical to those used in the Demographic and Health Survey (DHS) performed in Haiti in 2012 [9].
We assessed food security using the Household Hunger Scale, classifying it into 1 of 3 categories: little to no hunger (score of 0–1), moderate hunger (score of 2–3), and severe hunger (score of 4–6) [10]. A previously validated poverty scorecard based on 10 indicators and specific to Haiti was used to determine the likelihood of high relative poverty, where a higher score indicates decreased probability of severe poverty [11]. CD4 count at the time of ART initiation—a measure that has been associated with 5-year outcomes including mortality—was abstracted from the medical record [12].
Statistical Analysis
For univariable comparisons, we used 2-sided Fisher exact tests for proportional data, Student t tests for normal continuous data, and Wilcoxon rank-sum tests for non-normal continuous data.
Among all households originally enrolled in the trial, we compared baseline characteristics of households that participated in the survey at the fourth follow-up time point with those that did not participate.
Among households participating in the survey at the fourth follow-up time point, we assessed for risk factors for reported history of cholera among all household members (with known HIV or HIV status unknown) using a generalized estimating equation (GEE) model with an exchangeable correlation structure to account for household clustering. In univariable models, we assessed household-level variables including number of members, source of income, access to latrine, electricity, presence of a garden, food security, poverty score, time to fetch water, use of water treatment, and water source, in addition to factors related to the household member with known HIV, including age, sex, marital status, literacy, and CD4 count at initiation of ART. We then generated a multivariable GEE model with all variables found to have P < .1 in the univariable analyses.
Within the subgroup of household members with known HIV, we assessed for associations between the above variables and reported history of cholera. If more than 1 variable was associated with reported history of cholera with P < .1 in univariable analyses, we planned to include all such variables in a multivariable logistic regression.
Statistical analysis was performed using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Of 524 HIV-affected households included in the 2010–2013 study, 352 (67%) participated in the survey at the fourth follow-up time point in 2014. At baseline, compared with those who did not participate, HIV-infected survey respondents were older (mean, 40 vs 37 years; P = .0013), more likely to be female (65% vs 55%; P = .049), and had a higher CD4 count (median, 343 vs 296 cells/mm3; P = .019). Other baseline household characteristics did not differ and have been previously described in detail [7]. Fifty-three (31%) of the HIV-infected trial participants who did not participate in the survey at the fourth follow-up time point were known to be deceased at the time.
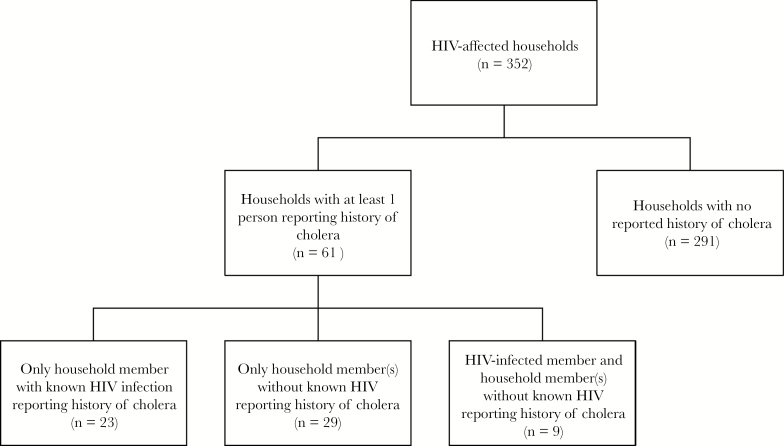
Of the 352 households that participated in the survey at the fourth follow-up time point, 61 (17%) had at least 1 household member with reported history of cholera since 2010, with a mean (SD) of 1.4 (0.8) cases per household that had at least 1 (Figure 1). All reported cases of cholera were medically attended (evaluated by a health care professional). Thirty-two (9%) of the 352 household members with known HIV infection and 55 (3%) of the 1988 household members without known HIV reported a history of cholera. Seventy-five (86%) of those who reported a history of cholera also reported being hospitalized for the episode. One person was reported to have died as a result of cholera—a 6-year-old male.
Figure 1.
Reported history of medically attended cholera since 2010 in HIV-affected households in rural Haiti (n = 352).
Risk factors for reported history of cholera among all household members (known to have HIV or status unknown) are in Table 1. In the univariable analyses, known HIV infection, female gender of the household member with known HIV, source of income in the household, food insecurity in the household, and time required to fetch water were correlated with reported history of cholera infection, with P < .05. These variables, along with single status of the HIV-infected participant (P = .073), were included in the multivariable model. In this analysis, HIV infection (adjusted odds ratio [AOR], 3.75; 95% confidence interval [CI], 2.43–5.79; P < .0001), source of income in the household (AOR, 1.82; 95% CI, 1.05–3.15; P = .034), time to fetch water (AOR, 1.07 per 5-minute increase; 95% CI, 1.01–1.12; P = .015), and severe food insecurity in the household (AOR, 3.23; 95% CI, 1.25–8.34; P = .016) were independently associated with having a reported history of cholera.
Table 1.
Risk Factors for Reporting History of Medically Attended Cholera Since 2010 for all Household Members (Known to Have HIV or Status Unknown) in HIV-Affected Households (n = 2320a)
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | P Value | Adjusted ORb | 95% CI | P Value | |
| Known HIV infection | 3.60 | 2.35–5.50 | <.0001 | 3.75 | 2.43–5.79 | <.0001 |
| Age of household member with known HIVc | 0.98 | 0.95–1.01 | .24 | |||
| Female gender of household member with known HIV | 0.52 | 0.30–0.90 | .019 | 0.59 | 0.34–1.02 | .059 |
| Single status of household member with known HIV | 0.58 | 0.32–1.05 | .073 | 0.64 | 0.35–1.14 | .13 |
| CD4 count of household member with HIV at time of ART initiationd (n = 2135) | 1.00 | 1.00–1.00 | .91 | |||
| No. of household memberse | 1.04 | 0.95–1.13 | .41 | |||
| Source of income in household | 1.84 | 1.05–3.21 | .032 | 1.82 | 1.05–3.15 | .034 |
| Access to latrine | 0.71 | 0.41–1.24 | .23 | |||
| Poverty scoref | 0.98 | 0.95–1.01 | .11 | |||
| Household hunger scale | .021g | .018i | ||||
| Little to no hunger | Ref | |||||
| Moderate hunger in household | 2.38 | 0.92–6.11 | .072 | 2.31 | 0.89–5.98 | .087 |
| Severe hunger in household | 2.97 | 1.16–7.64 | .024 | 3.23 | 1.25–8.34 | .016 |
| Time required to fetch waterh | 1.05 | 1.01–1.10 | .013 | 1.07 | 1.01–1.12 | .015 |
| Water treatment use | 0.60 | 0.31–1.13 | .11 | |||
| Improved water sourcei | 0.63 | 0.30–1.30 | .21 | |||
| Household member with known HIV received RUTF (vs CSB) | 1.38 | 0.81–2.36 | .24 | |||
Abbreviations: CI, confidence interval; CSB, corn–soy blend; GEE, generalized estimating equations; OR, odds ratio; RUTF, ready-to-use therapeutic food.
aUnless otherwise noted.
bThe multivariable model includes HIV infection, female gender of HIV-infected household member, single status of HIV-infected household member, source of income, household hunger scale, and time to fetch water.
cPer 1-year increase.
dPer 1-cell/mm3 increase.
ePer 1–household member increase.
fPer 1-point increase in poverty score.
g P value from 2 degrees of freedom omnibus test for this covariate.
hPer 5-minute increase.
iTap, covered, protected spring, rain water, or purchased.
Characteristics of the individuals with known HIV, grouped by whether they reported a personal history of cholera, are presented in Table 2. There were no statistically significant differences between the groups. Nearly two-thirds of the HIV-infected household members were female, half were single, and their mean household size (SD) was 5.6 (2.3) people. Most participants were poor and food insecure. Less than half had electricity or a source of income. The median CD4 count at the time of ART initiation (interquartile range [IQR]) was 237 (103–321) cells/mm3, and mean time on ART at the time of the survey at the fourth follow-up time point (SD) was 42 (7.8) months. The great majority of households in both groups reported access to a safe water source and water treatment, and more than half used a latrine. Households in which the HIV-infected household member reported a history of cholera required a median (IQR) of 20 (10–30) minutes to fetch water, compared with 15 (5–30) minutes for those in which the HIV-infected household member had no reported history of cholera (P = .062).
Table 2.
Characteristics of Participants with Known HIV Grouped by Whether they Reported a Personal History of Medically Attended Cholera Since 2010 (n = 352a)
| No Reported History of Cholera | Reported History of Cholera | ||
|---|---|---|---|
| n = 320a | n = 32a | P Value | |
| Age, mean (SD), y | 43 (11) | 41 (13) | .51 |
| Female | 207 (65) | 17 (53) | .20 |
| Single | 168 (53) | 13 (41) | .20 |
| Literate | 177 (55) | 16 (50) | .57 |
| Months on ARTb, mean (SD) | 42 (7.6) | 43 (9.5) | .62 |
| CD4 count at time of ART initiation, median (IQR) (n = 327) | 237 (102–321) | 240 (121–325) | .66 |
| No. of members in household, mean (SD) | 5.6 (2.3) | 5.6 (1.9) | .93 |
| Source of income in household | 143 (45) | 19 (59) | .12 |
| Access to latrine | 203 (63) | 19 (59) | .65 |
| Electricity in household | 139 (43) | 12 (38) | .52 |
| Garden | 104 (33) | 9 (28) | .61 |
| Poverty score, mean (SD)c | 27 (10) | 24 (9) | .12 |
| Household hunger scale | .67 | ||
| Little or no hunger | 51 (16) | 3 (9) | |
| Moderate hunger | 172 (54) | 17 (53) | |
| Severe hunger | 96 (30) | 12 (38) | |
| Time to fetch water, median (IQR), min | 15 (5–30) | 20 (10–30) | .062 |
| Water treatment use | 272 (85) | 25 (78) | .29 |
| Access to improved water sourced | 281 (88) | 26 (81) | .29 |
| Received RUTF (vs CSB) | 176 (55) | 19 (59) | .59 |
Data are presented as No. (%) unless otherwise specified. For continuous variables, median (IQR) is shown for non–normally distributed variables, and mean (SD) is shown for normally distributed variables.
Abbreviations: ART, antiretroviral therapy; CSB, corn–soy blend; IQR, interquartile range; RUTF, ready-to-use therapeutic food.
aUnless otherwise noted.
bAs of January 2014.
cRange of possible scores: 0 (worst) to 85 (best).
dTap, covered, protected spring, rain water, or purchased.
Missing data were less than 1% for all variables except CD4 count at initiation of ART, which was missing from 25 (7%) of the participants with known HIV infection. There was a similar percentage of missing CD4 counts among those who reported a history of cholera and those who did not.
DISCUSSION
In this study of 352 households affected by HIV in rural Haiti, comprised of 2320 total household members, we found that known HIV infection, source of income in the household, time required to fetch water, and severe food insecurity in the household were independently associated with a reported history of medically attended cholera.
V. cholerae was inadvertently introduced into Haiti in October 2010, leading to a national epidemic that has caused more than 800 000 suspected cases and nearly 10 000 deaths [13, 14]. The Artibonite Department had 116 069 suspected cases and 1126 deaths over the first 2 years of the epidemic, with an aggregate case fatality rate of 1%. Cholera transmission is by the fecal–oral route and is thus closely linked to inadequate sanitation and an unsafe water supply. For these reasons, the burden of disease is highest among the poor, who also tend to have more limited access to life-saving medical treatment.
Although cholera is not typically regarded as an opportunistic infection, little is known about the clinical features or risk of cholera in the setting of HIV infection, a disease with substantial geographic overlap with cholera [1, 3]. In 1 study, 13.6% of people presenting over the course of a year to a CTC in urban Haiti were found to be HIV-infected, as compared with an estimated 1.9% adult HIV prevalence in that region [4]. Half as many HIV-infected individuals as those without HIV infection ultimately had a positive stool culture for cholera, indicating that other causes of diarrhea may have contributed to some presentations. Regardless, there was a high prevalence of HIV even among people who eventually had a positive cholera stool culture, suggesting the possibility of an association between HIV and cholera infection. This is supported by 2 case–control studies of culture-confirmed cholera compared with noncholera diarrhea in Mozambique, although both were at high risk of selection bias [5, 6]. Mathematical modeling suggests that an association between HIV and cholera may also exist in the endemic setting [15]. Beyond this, evidence is limited to several small case series [16, 17].
In this context, we identified known HIV infection as strongly associated with a reported history of cholera, with a nearly 4-fold increase in the odds compared with other household members after adjusting for potential confounders. The biological plausibility of this association lies in the deleterious impact of HIV infection on gastrointestinal mucosal immunity and epithelial function, which can persist even after prolonged ART [18–20].
There are other potential factors that may contribute to an association between HIV and a reported history of cholera. In some cases, other causes of diarrhea related to HIV infection may have been misattributed to cholera, as suggested by the previously mentioned study, which found a discrepancy in the rate of positive stool culture for V. cholerae between HIV-infected and uninfected individuals presenting with acute watery diarrhea [4]. The HIV-infected participants in that study, however, had a median CD4 count of 222 cells/mm3, and nearly two-thirds were newly diagnosed with HIV at the time of presentation. HIV-infected individuals in our study, on the other hand, were all on ART and had a median CD4 count of 343 cells/mm3 at the start of the study. All tolerating participants were also receiving TMP-SMX prophylaxis. Higher CD4 counts and TMP-SMX prophylaxis are both associated with lower risk of acute diarrhea in HIV-infected people [21, 22], in particular, acute diarrhea requiring hospitalization outside of the context of an ongoing cholera outbreak [22].
It is also possible that household members with HIV may have been more embedded within the health care system and thus more likely to present to care and be diagnosed with cholera when ill. On the other hand, the majority of individuals reporting a history of cholera in the study also reported being hospitalized with the illness. As hospitalization is reserved for moderately and severely dehydrated patients, it seems likely that, at the height of the cholera epidemic, when public health messaging was focused on seeking care for diarrhea, subjects would have presented for care with these illnesses regardless of their prior connection to the HIV clinic.
Food insecurity is defined as a persistent lack of access to food in adequate quantity or quality [23]. The impact of food insecurity on health outcomes has been most well studied in the setting of HIV infection, where it has been associated with lower adherence to ART, high-risk sexual behavior, poor virologic control, and increased risk of mortality [24–30]. The relationship between food insecurity and cholera, on the other hand, is not well understood. One study in a Haitian urban center found that a diverse diet (1 element of food security) was associated with decreased risk of cholera in a multivariable model [31]. In our study, severe food insecurity in the household was independently associated with a household member reporting a history of cholera. Food insecurity could plausibly increase risk of cholera through 2 distinct mechanisms: directly, through increased risk of symptomatic cholera in a malnourished state; and indirectly, by increasing the likelihood of riskier behavior, including drinking from less safe water sources or consuming unsafe food. On the other hand, because the survey was not performed concurrently with the reported episode of cholera, a given household’s level of food insecurity may not have been the same at the time of the infection. This raises the possibility of reverse causality—that an episode of cholera in the household could influence subsequent risk of household food insecurity, potentially through direct or indirect costs of illness and health care. The directionality of this association, in addition to its generalizability to HIV-unaffected households, needs further investigation.
We also found that the amount of time required to fetch water was independently associated with a reported history of cholera. This finding is consistent with a prior study of a cholera outbreak in Tanzania, which found that a requirement of more than 10 minutes to get to a water source was associated with increased risk for cholera in multivariable analysis [32]. The length of time required to reach a water source may be a proxy measure of unsafe water use or lower socioeconomic status. Households that are further from a water source may also store water for a longer time at home, allowing the potential inoculum of V. cholerae in contaminated water to grow.
The independent risk factors for reported history of cholera identified in this study were not found to be associated with cholera in the subgroup of individuals with known HIV infection. However, differences in these covariates (time to fetch water, severe food insecurity, source of household income) trended in the same direction as in the group of all household members, suggesting that with a larger sample size these relationships might become apparent.
This study has some limitations. We relied on self-report to ascertain history of cholera, which is subject to recall bias. However, all people who reported a history of cholera sought medical attention, and the vast majority were hospitalized, events that are likely to be recalled within a household, particularly within the relatively short time since the epidemic began. We did not have microbiologic confirmation of cholera cases, and thus some cases may have been related to other causes of acute watery diarrhea. Regardless, clinical diagnosis of cholera was the norm at this point in the epidemic in Haiti and is consistent with the World Health Organization (WHO) case definition of cholera—a patient 5 years of age or older with acute watery diarrhea (3 or more unformed stools within 24 hours), with or without vomiting, in an area with a known cholera epidemic [33]. Laboratory surveillance in Haiti during this time period found that around two-thirds of stool samples collected from hospitalized patients with acute watery diarrhea grew V. cholerae in culture [13, 34].
The HIV-affected households that participated in the survey at the fourth follow-up time point contained HIV-infected individuals who were on ART and had relatively higher CD4 counts at baseline. Furthermore, more females participated in the survey at the fourth follow-up time point. Our study did not provide insight into why this might be the case, but those with a higher CD4 count at baseline had a higher likelihood of survival 2.5 years after enrollment in the survey. Men travel frequently for work in Haiti, and it is possible that this accounts for differential follow-up over time. We cannot rule out differential loss to follow-up among people with and without cholera, although the cholera attack rate in our population was similar to that reported in Haiti at the time [13]. We caution against generalizing these results to different HIV-affected populations (ie, households of newly diagnosed people or those with advanced disease), although it seems unlikely that untreated HIV would have less association with cholera. Furthermore, because of survivorship bias, our findings may not apply to households in which the person with known HIV died (from cholera or other causes) before the survey at the fourth follow-up time point, although it is unlikely that people in these households had a lower risk of cholera overall.
Although households with more than 1 adult with known HIV infection would have been ineligible for the original study, we did not definitively know the HIV serostatus of the nonindex household members. However, incomplete ascertainment of HIV infection among other household members would bias an association between HIV and reported history of cholera toward the null. Because the precise timing of self-reported cholera was unknown, we were unable to incorporate CD4 count at the time of reported cholera into our analysis.
CONCLUSIONS
This observational study of 352 HIV-affected households in rural Haiti, comprised of 2320 total members, is the first to characterize reported history of medically attended cholera in households affected by HIV. We found that known HIV infection, time required to fetch water, source of household income, and severe food insecurity were independently associated with a reported history of cholera. Further research is required to better understand the interactions between HIV and cholera and to explore the relationship and directionality of the association between food insecurity and cholera.
Acknowledgments
We thank the study participants. We are grateful to the Zanmi Lasante staff in Haiti.
Financial support. This work was supported by the National Institute of Child Health and Human Development and the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant numbers R01HD057627, R01AI099243 to L.C.I.), with help from the Harvard University Center for AIDS Research (CFAR), an National Institutes of Health–funded program (P30 AI060354).
Potential conflicts of interest. The authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. WHO. Cholera, 2015. Wkly Epidemiol Rec 2016; 38:433–40. [PubMed] [Google Scholar]
- 2. CDC. Update: cholera outbreak - Haiti, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1473–9. [PubMed] [Google Scholar]
- 3. UNAIDS. Global AIDS Update. Geneva: World Health Organization; 2016. [Google Scholar]
- 4. Sévère K, Anglade SB, Bertil C et al. Clinical features of human immunodeficiency virus-infected patients presenting with cholera in Port-au-Prince, Haiti. Am J Trop Med Hyg 2016; 95:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Seidlein L, Wang XY, Macuamule A et al. Is HIV infection associated with an increased risk for cholera? Findings from a case-control study in Mozambique. Trop Med Int Health 2008; 13:683–8. [DOI] [PubMed] [Google Scholar]
- 6. Sema Baltazar C, Langa JP, Dengo Baloi L et al. Multi-site cholera surveillance within the African Cholera Surveillance Network shows endemicity in Mozambique, 2011–2015. PLoS Negl Trop Dis 2017; 11: e0005941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ivers LC, Teng JE, Jerome JG et al. A randomized trial of ready-to-use supplementary food versus corn-soy blend plus as food rations for HIV-infected adults on antiretroviral therapy in rural Haiti. Clin Infect Dis 2014; 58: 1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. United Nations Department of Economic and Social Affairs Statistics Division. Principles and Recommendations for Populations and Housing Censuses: Revision 3. New York: United Nations; 2017. [Google Scholar]
- 9. Cayemittes M, Busangu MF, Bizimana JdD et al. Haïti Enquête Mortalité, Morbidité et Utilisation des Services 2012. Calverton, MD: Ministère de la Santé Publique et de la Population - MSPP/Haïti, Institut Haïtien de l’Enfance - IHE and ICF International; 2013. [Google Scholar]
- 10. Deitchler M, Ballard T, Swindale A, Coates J.. Introducing a Simple Measure of Household Hunger for Cross-Cultural Use. Washington, DC: Food and Nutrition Technical Assistance II project, AED; 2011. [Google Scholar]
- 11. Schreiner M. A simple poverty score care for Haiti. http://www.microfinance.com/English/Papers/Scoring_Poverty_Haiti.pdf. Accessed October 22. [Google Scholar]
- 12. May MT, Vehreschild JJ, Trickey A et al. ; Antiretroviral Therapy Cohort Collaboration (ART-CC) Mortality according to CD4 count at start of combination antiretroviral therapy among HIV-infected patients followed for up to 15 years after start of treatment: collaborative cohort study. Clin Infect Dis 2016; 62:1571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barzilay EJ, Schaad N, Magloire R et al. Cholera surveillance during the Haiti epidemic–the first 2 years. N Engl J Med 2013; 368:599–609. [DOI] [PubMed] [Google Scholar]
- 14. Ministere Sante Publique et de la Population (MSPP) – Direction d’Epidémiologie de Laboratoire et de Recherches (DELR). Rapport du Réseau National de Surveillance - sites choléra 40ème semaine épidémiologique 2017. http://mspp.gouv.ht/site/downloads/Profil%20statistique%20Cholera%2040eme%20SE2017%20partiel.pdf. Accessed 24 October 2017. [Google Scholar]
- 15. Mushayabasa S, Bhunu CP. Is HIV infection associated with an increased risk for cholera? Insights from a mathematical model. Biosystems 2012; 109:203–13. [DOI] [PubMed] [Google Scholar]
- 16. Utsalo SJ, Eko FO, Umoh F, Asindi AA. Faecal excretion of Vibrio cholerae during convalescence of cholera patients in Calabar, Nigeria. Eur J Epidemiol 1999; 15:379–81. [DOI] [PubMed] [Google Scholar]
- 17. Rey JL, Milleliri JL, Soares JP et al. HIV seropositivity and cholera in refugee children from Rwanda. AIDS (London, England) 2005; 9: 1203–4. [DOI] [PubMed] [Google Scholar]
- 18. Shacklett BL, Anton PA. HIV infection and gut mucosal immune function: updates on pathogenesis with implications for management and intervention. Curr Infect Dis Rep 2010; 12:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Estes J, Baker JV, Brenchley JM et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis 2008; 198:456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuetz A, Deleage C, Sereti I et al. ; RV254/SEARCH 010 and RV304/SEARCH 013 Study Groups Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 2014; 10:e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brink AK, Mahé C, Watera C et al. Diarrhea, CD4 counts and enteric infections in a community-based cohort of HIV-infected adults in Uganda. J Infect 2002; 45:99–106. [DOI] [PubMed] [Google Scholar]
- 22. Campbell JD, Moore D, Degerman R et al. HIV-infected Ugandan adults taking antiretroviral therapy with CD4 counts >200 cells/μL who discontinue cotrimoxazole prophylaxis have increased risk of malaria and diarrhea. Clin Infect Dis 2012; 54:1204–11. [DOI] [PubMed] [Google Scholar]
- 23. Ivers L. Food Insecurity and Public Health. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2015. [Google Scholar]
- 24. Aibibula W, Cox J, Hamelin AM et al. Food insecurity and low CD4 count among HIV-infected people: a systematic review and meta-analysis. AIDS Care 2016; 28:1577–85. [DOI] [PubMed] [Google Scholar]
- 25. Chop E, Duggaraju A, Malley A et al. Food insecurity, sexual risk behavior, and adherence to antiretroviral therapy among women living with HIV: a systematic review. Health Care Women Int 2017; 38:927–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ivers LC, Cullen KA, Freedberg KA et al. HIV/AIDS, undernutrition, and food insecurity. Clin Infect Dis 2009; 49:1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feldman MB, Alexy ER, Thomas JA, Gambone GF, Irvine MK. The association between food insufficiency and HIV treatment outcomes in a longitudinal analysis of HIV-infected individuals in New York City. J Acquir Immune Defic Syndr 2015; 69:329–37. [DOI] [PubMed] [Google Scholar]
- 28. Singer AW, Weiser SD, McCoy SI. Does food insecurity undermine adherence to antiretroviral therapy? A systematic review. AIDS Behav 2015; 19:1510–26. [DOI] [PubMed] [Google Scholar]
- 29. Weiser SD, Fernandes KA, Brandson EK et al. The association between food insecurity and mortality among HIV-infected individuals on HAART. J Acquir Immune Defic Syndr 2009; 52:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weiser SD, Tsai AC, Gupta R et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in a resource-poor setting. AIDS 2012; 26:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dunkle SE, Mba-Jonas A, Loharikar A et al. Epidemic cholera in a crowded urban environment, Port-au-Prince, Haiti. Emerg Infect Dis 2011; 17:2143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Acosta CJ, Galindo CM, Kimario J et al. Cholera outbreak in Southern Tanzania: risk factors and patterns of transmission. Emerg Infect Dis 2001; 7:583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. WHO Global Task Force on Cholera Control. Cholera Outbreak: Assessing the Outbreak Response and Improving Preparedness, 2004. Geneva: World Health Organization; 2004. [Google Scholar]
- 34. Steenland MW, Joseph GA, Lucien MA et al. Laboratory-confirmed cholera and rotavirus among patients with acute diarrhea in four hospitals in Haiti, 2012-2013. Am J Trop Med Hyg 2013; 89:641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]