Abstract
The evolution of male preferences and of female ornaments in species with traditional sex roles (i.e., polygyny) have been highlighted as areas in need of more active research by an accumulation of recent findings. The theoretical literature on these topics is relatively small and has centered on the evolution of male choice. Mathematical models have emphasized that, under polygyny, the evolution of male preferences faces much greater competition costs than does the evolution of female preferences. We discuss ways in which costly male choice can nonetheless evolve, via (1) direct selection that favors preferences, primarily through mating with highly fecund females, (2) mechanisms that rely on indirect selection, which weakly counters competitive costs of male preferences, and (3) genetic constraints, primarily in the form of pleiotropy of male and female preferences and traits. We also review a variety of mathematical models that have elucidated how costs to male preferences can be avoided. Finally, we turn our attention to the relatively scant theoretical literature on the effects of male mate choice on the evolution of female traits. We emphasize the finding that the presence of male preferences cannot be assumed to lead to the evolution of female ornaments during polygyny, and point out situations where models have elucidated ways in which female ornaments can nevertheless evolve.
Keywords: female ornaments, male mate preferences, mathematical models
Introduction
The phenomena of ornamentation in male animals and choosiness in female animals have received substantial attention from biologists since a burst of interest on the topic in the 1970s and 1980s. They are now commonly understood within the context of sexual selection; that is, female choice and male ornamentation often coexist in species where males with flashier ornaments that exploit female choice can increase their relative fitness. Although some questions remain about the evolution of male ornaments and female choice (e.g., whether good genes in males can maintain mate preferences in females; see e.g., Adkins-Regan et al. 2013), a hefty body of both empirical and theoretical literature has now accumulated (Andersson 1994; Shuster and Wade 2003; Arnqvist and Rowe 2005; Rosenthal 2017).
In contrast, a literature on the evolution of male mate choice and female ornamentation has only been accumulating in recent decades. The early research on male mate choice and female ornamentation focused primarily on species with sex role reversal, in which context the phenomena can be generally understood as the inverse of the traditional sexual selection scenario (e.g., Gwynne 1993; Jones et al. 2000). However, reports from empiricists now indicate that male mate choice and female ornamentation among species with traditional sex roles are not nearly as rare as once believed (Amundsen 2000; Bonduriansky 2001; Edward and Chapman 2011). [Note, we use the term “traditional sex roles” to describe those species that, while not always strictly polygynous, are not sex role reversed. We recognize that this term carries some unfortunate colloquial connotations (Ah-King and Ahnesjö 2013), but we opt to use it rather than introduce new and unfamiliar terminology.] Choosiness in males and sexual signals in females have been reported in many taxa (e.g., insects, Hopkins et al. 2015; fishes, Amundsen and Forsgren 2001; Massironi et al. 2005; Méndez-Janovitz and Macías Garcia 2017; lizards, Weiss 2006; Weiss et al. 2009; crustaceans, Baldwin and Johnsen 2012; primates, Huchard et al. 2009; Fitzpatrick et al. 2015).
Furthermore, many of these empirical examples are of traits that are female-specific [see Nordeide et al. (2013) for a review of mutually ornamented species]. That is, the ornaments that females of many species exhibit are not simply muted versions of traits that are found in conspecific males (although this also does occur, see below). The prevalence of this female-specific signaling in species with polygynous mating systems is important because it demonstrates that these ornaments are not explained simply by pleiotropy between male and female traits or by genetic correlations (e.g., Lande 1980; Amundsen 2000) nor are they explained by sexual selection under sex role reversal, where polygyny typically does not apply. In other words, the known natural history of male mate choice and female ornaments so far suggests that they may arise from processes that are fundamentally different in some way from the processes of sexual selection that give rise to female choice and male-specific signals.
Indeed, the recent empirical consensus—that the distribution of male mate choice and female ornamentation in polygynous species across the tree of life is not as limited as once thought—has spurred a developing body of theoretical literature as well, which we review here. The bulk of this new theoretical work dissects the processes by which male choosiness might evolve under polygyny and tries to understand the ways in which the constraints upon male mate choice might differ from its female mate choice counterpart. A small number of theoretical models have been developed that examine the evolution of female ornamentation as well. Indeed, one way to organize our thinking on the topic is as follows. Most of the male mate choice literature examines male mate choice as an outcome of selection; these models investigate the evolutionary processes that produce and constrain the evolution of such a behavior among males. A few models investigate male mate choice as a mechanism of selection; these models examine the potential for male mate choice to exert selection pressure on female traits. This distinction provides something of a scaffold for the present review. Note that although “ornaments” might be viewed as a subset of “traits” (e.g., those that are more exaggerated and/or costly), for the purposes of our review, the distinction is not necessary. Therefore, we use the terms “female ornament” and “female trait” interchangeably throughout. We turn first to a brief summary of the patterns that have been reported by the empirical and natural history literature.
Natural History of Male Mate Choice and Female Ornamentation
A survey of the empirical literature reveals different patterns between male mate choice and female choice. While females in polygynous species commonly choose males on the basis of what appear to be “arbitrary” traits (e.g., plumage characteristics or songs that bear no obvious relationship to the “quality” of the male; see Andersson 1994), this appears to be less predominant among polygnous males. Male choice, when it occurs, is most often based on traits that correlate with high fertility in females (e.g., LeBas et al. 2003; Griggio et al. 2005; Pizzolon et al. 2008), such as large body size (e.g., Olsson 1993; Verrell 1995; Ptacek and Travis 1997; Arntzen 1999; Werner and Lotem 2003; Wong and Jennions 2003; Herdman et al. 2004; Saeki et al. 2005; Byrne and Rice 2006; Méndez-Janovitz and Macías Garcia 2017; for reviews, see Andersson 1994, table 6A; Bonduriansky 2001). Male choice has also been found for other characteristics that represent a direct benefit to the male, such as readiness to mate (e.g., Rowland et al. 2002), lack of infection (e.g., Gourbal and Gabrion 2004; Wittman and Fedorka 2014), virginity (e.g., Bateman and Ferguson 2004; Carazo et al. 2004; Gaskett et al. 2004; Martel et al. 2008; MacLeod and Andrade 2014), or other indicators of reproductive potential (e.g., Craig et al. 2002; Orrell and Jenssen 2002; Ojanguren and Magurran 2004; Siefferman and Hill 2005; Simcox et al. 2005; Parga 2006; Fitzpatrick et al. 2015; Cole and Endler 2016).
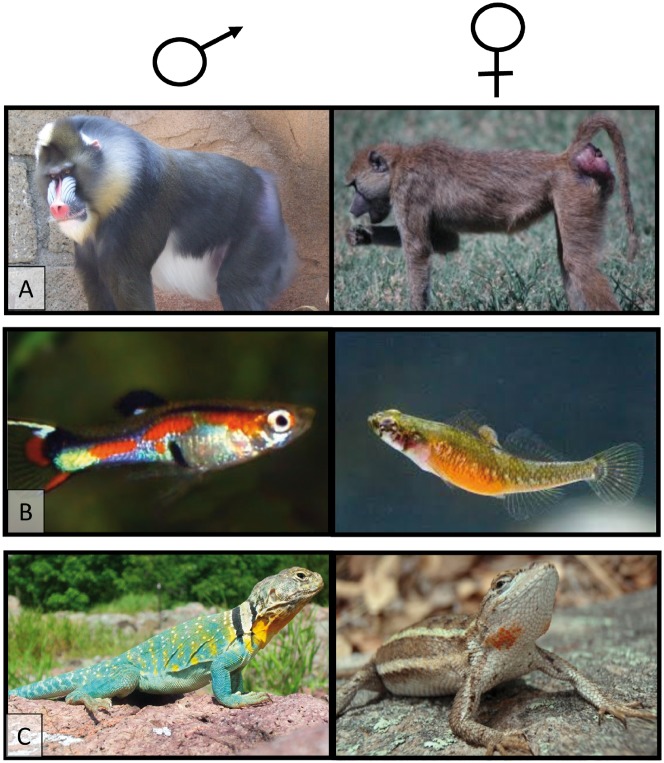
Likewise, the empirical literature suggests that common patterns of female ornamentation differ in generalizable ways from the common patterns of male ornamentation. The phrase “male ornament” usually calls to mind outlandish traits like the peacock’s train, the dances of lekking birds, or the bright color patterns of some male guppies. By comparison, the traits that have been reported as ornaments for females—while still bearing the hallmarks of secondary sexual characteristics—tend to be more subtle. For instance, no matter how conspicuous the displays of exaggerated estrous swellings by females of many species of Old World Monkeys, they pale in comparison to the flamboyant colors displayed by males mandrills (Figure 1A). Likewise, female two-spotted gobies display an orange colored belly when they are receptive (Amundsen and Forsgren 2001), but this coloration is muted relative to the flashy sexual signals commonly displayed by males of some fishes, like guppies (Brooks et al. 2001) (Figure 1B). Finally, female-spotted plateau lizards display orange throat patches during the breeding season (Weiss 2002, 2006), but are still drab overall when compared with the bright colorations in males of many species (Figure 1C). Female traits also tend to by “dynamic.” In other words, they tend to appear only around breeding time and then disappear. Although this kind of dynamic intra-individual variation in expression is observed in males of many species with visual sexual signal (e.g., birds with breeding plumage, seasonal antler growth in deer), it is also very common for males to display their extravagance year round (the many species of birds, fishes, and lizards that maintain bright coloration). In contrast, this dynamic pattern has been reported in almost all species in which females display ornaments or visual sexual signals [but see bluethroats (Amundsen et al. 1997)]. These general patterns hold for fishes (Amundsen and Forsgren 2001; Massironi et al. 2005), lizards (LeBas and Marshall 2000; Weiss 2006; Calisi et al. 2008; Weiss et al. 2009), crabs (Takahashi and Watanabe 2011; Baldwin and Johnsen 2012), and primates (Huchard et al. 2009; Fitzpatrick et al. 2014) (Figure 1).
Figure 1.
Comparison of some of the most extreme secondary sexual characters for males (left column) and females (right column) in 3 different taxa. (A) In primates, male mandrills have evolved dramatic coloration in their faces and haunches and some empirical research in a semi-natural population indicates that females prefer the more brightly colored males. In comparison, females in many species of cercopithecine primates (including the savannah baboons, pictured) have evolved exaggerated estrous swellings that appear around the time of ovulation and then disappear, coming and going each time the female experiences a sexual cycle (Nunn 1999; Fitzpatrick 2014). (B) Many species of fishes have evolved highly exaggerated coloration and courtship behavior, like the male guppy pictured here (Endler 1983). By comparison, the orange bellies that are displayed by female two-spotted gobies are more subdued and are only visible when the female is receptive (Amundsen and Forsgren 2001). (C) Finally, males in many species of lizards (e.g., collared lizards) display ornate color patterns. In those species in which females have evolved sexual signals (e.g., striped plateau lizards) the signals are muted, relative to the extreme colors seen in males of some species, and they only appear during the breeding season. Photocredits: (A) Male mandrill Mandrillus sphinx; Wikimedia commons (user: NicBar): female baboon Papio cynocephalus; CL Fitzpatrick. (B) Male guppy Poecilia wingei; Wikimedia commons (user Emilio17): female two-spotted goby Gobiusculus flavescens; Trond Amundsen. (C) Male collared lizard Crotaphytus collaris; Wikimedia commons (user: L. Dakota): female striped plateau lizard Sceloporus virgatus; Stacey Weiss.
Direct Selection against the Evolution of Male Mate Choice
The empirical patterns of male mate choice summarized above (i.e., polygynous males rarely display preferences for traits that seem to be “arbitrary”) are consistent with insights about the evolutionary processes resulting in male mate choice derived from theoretical studies. Mathematical models demonstrate that, under polygyny, direct selection will often emerge against male mate choice, such that—all else being equal—it will be unlikely to evolve. A “null model” (sensu, Prum 2010) of the evolution of male mate choice that presents this phenomenon in detail was developed by Servedio and Lande (2006). In that paper, a series of population genetic models examined the fate of an allele for male mate choice, expressed as biased courtship toward one type of female over another. The most basic of these models examined the case where the female trait was arbitrary, male preferences determined which females the male would court, and females chose from among courting males. Servedio and Lande (2006) found that in this case, the allele for male choice was always lost.
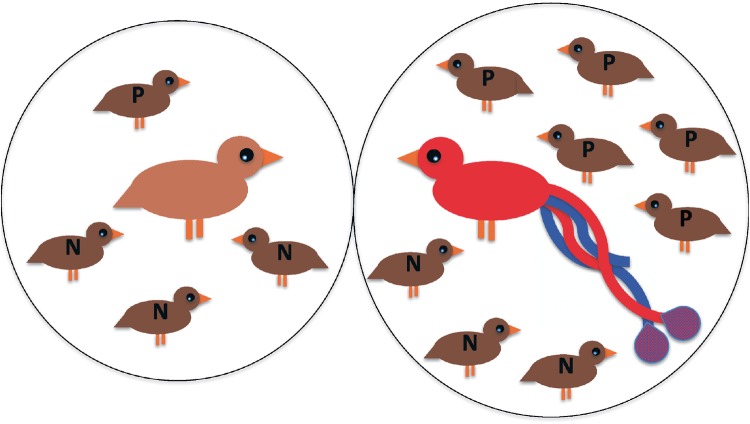
This failure to maintain the male mate choice allele under these circumstances can be explained as follows: Imagine that 2 males, one with a “preference” allele and one with an allele for “no preference,” encounter both an ornamented and an unornamented female (Figure 2). Both females represent exactly 1 mating opportunity. However, in the case of the more ornamented female, that mating opportunity also exists within a more intense competitive environment than the mating opportunity represented by the unornamented female. This difference in the intensity of competition associated with each female emerges because ornamented females will disproportionally receive courtship from other males in the population with choice alleles, while still being courted proportional to their frequency by the males that mate at random; ornamented females thus end up receiving more courtship overall. The key point is this: during strict polygyny, each female has equal mating success, regardless of her ornamentation. Thus, competition is greater for the mating opportunity that the ornamented female represents, versus the equivalent mating opportunity that the unornamented female represents. This increased competition surrounding ornamented females creates direct selection against the preference allele because males with the “preference” allele are overrepresented in the high competition situation (the ornamented female), while males with the “no-preference” allele are overrepresented in the low competition situation (the unornamented female) (Figure 2). In this null model, a male with the preference allele thus has a reduced chance of mating at all.
Figure 2.
Direct selection against a male choice allele emerges under polygyny in a “null model.” A male with a “preference” allele and a male with a “no preference” allele are faced with an ornamented and an unornamented female (center of each circle). Both males are assumed to have the same amount of energy to devote to courtship (denoted in the figure by 6 males, representing courting males, for males with both the “no preference,” “N,” and the “preference,” “P” alleles). The male with the “no preference” allele will distribute his courtship (the “N”s) randomly between the females. The male with the “preference” allele, however, will direct more courtship (the “P”s) to the ornamented female. The male with the “no preference” allele is overrepresented in courting the non-ornamented female, and is most likely to obtain a mating there (3 Ns out of 4 courting males in the figure). The male with the “preference” allele is overrepresented in courting the ornamented female (5 Ps out of 8 courting males), but not to the same extent as the “no preference” male was in courting the plain female (3/4 = 0.75 > 5/8 = 0.625). The male with the “preference” allele is thus at a competitive disadvantage due to his biased courtship, leading to direct selection against the “preference” allele. This may help to explain why highly ornamented females, such as the one in the figure, are rarely seen in nature.
In addition to the competition cost that emerges naturally from the assumption of polygyny in the null model above, obstacles to the evolution of male preferences can arise from other causes. Several models simply assign competition costs (e.g., Fawcett and Johnstone 2003; Courtiol et al. 2016, see additional discussion of these and other models directly including costs below). Other models suggest that male mate preferences are even less likely to evolve if males are faced with multiple mating opportunities sequentially, rather than simultaneously (e.g., Barry and Kokko 2010). This result is consistent with what has been established about sequential choice using a model of female (rather than male) choice (Kokko and Ots 2006). That is, under sequential mate choice scenarios, a choosy individual rejects a mating opportunity without knowing if another mate will be encountered. As a consequence of this feature of sequential choice, the evolution of male preferences can be difficult even in situations where such preferences would otherwise be favored. In Barry and Kokko’s (2010) model, for example, males could potentially avoid the very high costs of female cannibalism through exerting mate choice, yet in their sequential choice model such a preference does not always evolve.
Whenever there is direct selection against male preference, the question arises of whether male preferences are able to evolve despite this cost. Below, we first address 3 mechanisms, opposing direct selection, opposing indirect selection, and genetic constraint, that can lead to the evolution of male preferences despite costs that arise either from the emergent competition in the null model or from other forms of direct selection against male preferences. We then review models that make assumptions which preclude competition costs of male preferences from emerging in the first place. Finally, we discuss theoretical findings regarding the potential for male preferences to place sexual selection on female traits.
How Can Male Preferences Evolve If There Is Direct Selection against Them?
Direct selection against male preferences that emerges from increased competition, or other costs, can be countered in several ways. The most effective of these possible foils for direct selection against male preferences is opposing direct selection which favors male preferences. Male preference evolution due to such direct selection is investigated by many models, as we discuss below. Less effective are processes that apply indirect selection which favors male preferences; in these scenarios, male preference alleles are statistically correlated with alleles at other loci so that changes at loci other than the preference locus increase the frequency of the preference allele as a correlated response. It is well known that indirect selection tends to be weaker than direct selection because it is mediated by the strength of imperfect genetic correlations (e.g., Kirkpatrick and Barton 1997). Nevertheless, several proposals for how male choice might evolve rely on such indirect selection. We conclude this section by a consideration of the effects of genetic constraints, mainly due to pleiotropy between the sexes at trait and preference loci.
Opposing direct selection can favor male preferences
By far the most common form of direct selection favoring male preferences in mathematical models is fecundity selection. Because fecundity selection acts upon a mated pair, instead of upon one sex or the other, a male preference for females with high fecundity will lead to fecundity selection favoring the male preference. This selection happens because the male preference will cause high fecundity females to be disproportionately paired with males with the preference allele, leading directly to increased reproductive success for males carrying such a preference. Although fecundity selection thus applies only direct selection to male preferences, the distinction between direct and indirect selection in this context can be subtle; it depends on the association between the ornamented (high-fecundity) females and males with preferences (created by non-random mating), and we are trained to think of benefits arising from associations between traits and preferences as indirect selection. In this case, the benefit to the preference allele does depend on non-random mating with the trait that is an honest indicator of high fecundity (note that the indicator must be honest), and this does form a genetic correlation between the trait and preference in the mated pair, upon which the benefit conferred to the preference allele rests. However, an individual male obtains the fecundity benefit as a consequence of the expression of his own preference, not due to the fact that he is likely to carry the preference allele because of the expression of the preference and trait in his parents.
Fecundity selection can thus be quite a strong direct force countering direct selection against male preferences. However, a male preference for fecundity-indicator traits will only evolve when the fecundity advantage is high enough to compensate for the direct selection against male preferences emerging from the null model or other forms of costs. Indeed, critical parameters representing the strength of this fecundity advantage arise in many different models of various scenarios of male choice; as such, fecundity selection is mentioned often throughout the remainder of this review. A simple demonstration of the way in which fecundity selection can counter direct selection against male preferences was illustrated by Servedio and Lande (2006), who added fecundity selection to the “null model” described above by assuming that the trait preferred by males was an honest indicator of female fecundity. They determined a threshold fecundity advantage above which male preferences would evolve, and below which the direct selection on preferences resulting from fecundity selection was still too weak to counter the direct selection against male preferences due to increased competition for preferred females. Nakahashi (2008) expanded upon these ideas using a quantitative genetic model very parallel to the null model of Servedio and Lande (2006). He showed that when fitness due to fecundity was normally distributed around an optimum trait value, the effects of fecundity selection on male preferences depend on the preference function. This preference function can take a much wider variety of forms when preferences are assumed to be continuous, as in the quantitative genetic framework, versus when they are discrete. Specifically, Nakahashi (2008) found (in his Model 2) that male preferences for more fecund females can evolve when such preferences are unimodal (“absolute” or “relative,” see Lande 1981); they will then evolve to be proportional to female fertility (e.g., will be centered around the mean of the female trait). They cannot evolve, however, if they are open-ended (or “psychophysical”), such that males prefer more extreme female traits which cannot match well with the normally distributed fecundity benefits. Nakahashi (2008) further demonstrated that unimodal preferences can instead evolve to be directional, favoring a female trait value above the current trait mean, when the female trait in question is acted upon by viability as well as fecundity selection (his Model 3; but note the trait will not necessarily be under selection due to this preference. See section on the relationship between preferences and traits below).
Other direct selection forces can arise in models that, while not explicitly fecundity selection, function in similar ways mathematically and can thus provide a direct-selection counterweight to direct selection against male preferences. For instance, Kokko and Heubel (2011) present a model that investigates the evolution of male preferences in an environment where males face the risk of being sexually parasitized. This model is based on the Amazon molly system (Poecilia formosa), in which females reproduce through gynogenesis; that is, copulation with related species is required to trigger reproduction, but offspring are all female clones of their mothers because copulating males do not contribute their genetic material to the zygotes (Hubbs and Hubbs 1932; Schlupp 2010). Such males, therefore, experience a significant fitness cost as a result of mating with an Amazon molly (but see Schlupp et al. 1994). Using individual-based simulations, the authors examine the likelihood that male preferences will evolve as a function of the density of Amazon mollies. Not surprisingly, they find that male preferences are more likely to evolve when the density of sexual parasites is high. The male preference evolution in this case can perhaps be framed as an exaggerated fecundity selection; indeed, the males who mate with Amazon mollies receive zero fecundity benefits. Another model in which direct fecundity-like benefits counterbalance the direct costs of male preferences is presented by Courtiol et al. (2016), using a game theoretical framework. In this case, the benefits are determined strictly by the environment and thus any possibility of indirect benefits is eliminated. They find that the costs of preferences—modeled as a decrease in mating rate—can be counterbalanced by direct benefits under many conditions.
An interesting set of game-theoretical models examines not only the effects of differences in female fecundity or reproductive maturity on the evolution of male mate preferences, but also the effects of variation in male quality (Fawcett and Johnstone 2003; Härdling et al. 2004; Härdling and Kokko 2005; Härdling et al., 2008; Venner et al. 2010). Male quality in these sequential-choice models is generally manifested as a male’s direct competitive ability in contests with other males to guard females [Fawcett and Johnstone 2003; Härdling et al. 2004; Härdling and Kokko 2005; Venner et al. 2010; although see Härdling et al. (2008) for a model where quality affects how quickly males can replenish sperm reserves]. These models consider a situation with limited mating opportunities; males pair, are challenged, and potentially displaced during a period of competition, gaining benefits from mating with whichever female they are paired with after the competition phase ends. They find that choice can evolve in either high-quality males, low-quality males, or both, depending on exact conditions. All of these models find some parameter ranges where both low- and high-quality males prefer more fecund females, as is expected because of the direct benefit that such a preference can incur. Some models produce the similarly intuitive outcome in which only high-quality males express a preference (or express a stronger preference; e.g., Fawcett and Johnstone 2003; Härdling and Kokko 2005). However, some surprising results also emerge, including a case in which only low-quality males have a preference (e.g., Härdling et al. 2004; Härdling and Kokko 2005), for example, when encounters are scarce and low-quality males easily lose guarded mates at a high cost to themselves (Härdling and Kokko 2005).
One particularly interesting recurrent result from the family of game theoretical models described above is the emergence of assortative mating, in which high-quality males prefer high-quality females, while low-quality males do not just opportunistically pair with the remaining females [as in Rowell and Servedio (2009), described below], but instead actively express a preference for low-quality females (Fawcett and Johnstone 2003; Härdling and Kokko 2005; Härdling et al. 2008; Venner et al. 2010). This outcome, which has been termed “prudent choice” (Härdling and Kokko 2005) or “reversed male mate choice” (Venner et al. 2010), occurs because it may be too costly for a low-quality male to attempt to pair with a high-quality female if he is likely to lose her to a high-quality male in a costly encounter (or, similarly, simply end up losing her late in a season and remaining unmated).
Finally, another possible direct benefit to male preferences arises if females have a preference for males that court them more, provided that preference is disproportionately large compared with the relative amount with which they are courted by each type of male (South et al. 2012). Returning to Figure 2, such a preference would mean, for example, that un-ornamented females would be more than 3/4 times as likely to mate with males without a preference allele and, similarly, ornamented females would be more than 5/8 times as likely to mate with males with a preference. South et al. (2012) found that male preferences are likely to evolve more easily if females have a fixed strength of preference for a male that courts more and if the preferred female trait were more common. This result suggests that the biology of the female response to male courtship may be an important factor in the evolution of male preferences.
Opposing indirect selection can sometimes favor male preferences
As stated above, indirect selection is expected to be less efficient than direct selection at opposing direct competition costs that males face if they bias their courtship toward certain females. This can be seen in the contrast between the version of the “null model” of Servedio and Lande (2006) that includes fecundity selection, which is a form of direct selection favoring males preferences (see above), and an otherwise identical version that includes instead a direct viability advantage to females with the trait (Servedio 2007). Viability selection on the trait acts only on females, not on the mated pair, and so any advantage to the male preference allele is due to indirect rather than direct selection, as a result of the fact that females with the trait allele tend to also carry the preference allele. Servedio (2007) found that such a viability advantage to the trait is very inefficient at driving male preference evolution; it was found to move the preference only slightly upward in frequency even when viability selection was of a value to maximize preference evolution (paradoxically, stronger viability selection favoring the trait does not always increase the equilibrium frequency of a preference allele, see Servedio 2007). In a parallel quantitative genetic model where males prefer a high-viability female trait, Nakahashi (2008) found that unimodal male preferences could not evolve, and while very strong open-ended male preferences could evolve the dynamics were quite unusual and not biologically likely (they were oscillatory with ever-increasing absolute values; his Model 1).
One situation in which indirect selection in favor of male preferences can be extremely strong is when female traits indicate species identity. This was assessed in a model of the process of reinforcement—the evolution of premating isolation due to selection against hybrids—by Servedio (2007). In this model, the female trait is locally adapted and 2 separate loci determine whether individuals are “purebreds” or hybrids, and hence selected against. Two types of male preferences were considered, one in which there are separate male preference loci and female trait loci (a “preference/trait” model), and the other which examines the evolution of an allele that causes a male to prefer a female that shares whatever trait he himself carries (a “matching” model; for a review of the difference between these types of mating models, see Kopp et al. 2018). In both of these scenarios, the male preference allele that leads to reproductive isolation in the focal population becomes correlated with both the trait locus and the loci that are under selection against hybrids, causing indirect selection to favor the preference through multiple channels. Male preferences were generally able to evolve under such selection, although in some cases not as quickly as female preference would in a parallel situation [Servedio 2007, see also Servedio and Dukas (2013) for a description of how within-generational learning would affect this process].
Genetic constraint
Finally, it is possible that male preferences may evolve because of genetic constraints that affect loci involved in preferences or traits. Early observations of female traits, for example, noted that they often seemed to reflect male traits expressed in that same species, but in a more subdued manner (Darwin 1871; Amundsen 2000); such an effect could potentially occur if traits were at least partially pleiotropic (e.g., Lande 1980). Similarly, female preferences may have pleiotropic expressions in males. Using population genetic models, Servedio and Lande (2006) analyzed both of these cases. They first examined the pleiotropy of both trait and preference expression across the sexes, such that male traits were weakly expressed in females and female preferences were weakly expressed in males. They found that male preferences could indeed evolve as a pleiotropic expression of female preference, although the presence of this pleiotropic effect reduced the parameter space in which female preference could occur, indicating that the expression in males was generally having an inhibitory effect. In the case in which only trait expression was pleiotropic and there were separate loci for male and female preferences, only slight increases in the frequency of a male preference were found, and this occurred under restricted conditions. While other types of genetic constraints on preferences and traits may be envisaged, to date this seems to be a restricted avenue for the evolution of male preference.
When Direct Selection against Male Preferences Is Absent
Perhaps the simplest way to prevent the occurrence of the direct selection against male preference that arises from the increased competition emergent in the null model is to violate the assumption of strict polygyny, whereby in the null model females have equal mating success, regardless of their traits. There are several ways in which such an assumption can be violated, and not all of these will remove direct selection against a male preference allele.
Females may, for example, have higher mating success if they are courted more. If the greater mating success of preferred females is a function of the extra courtship that they receive, this can counter, or in extreme cases remove, the competitive mating disadvantage that males with a preference are subjected to in the null model shown in Figure 2. Nakahashi (2008), for example, directly considers the possibility that females that are not courted by enough males can be childless (his Model 4, note that preferred females also, independently, have higher fecundity in this model). This probability ranges from all females mating to the chance of going unmated being proportional to the degree to which that type of female is preferred (his R = 1, at which point male preferences have no cost). He finds that the evolution of strong male preferences can occur, but it requires a large chance of a female not mating. In some cases, when a female’s chances of going unmated are relatively high, a runaway process can even occur, especially if males are of high quality (non-genetic quality is included in Nakahashi’s Model 5). Only when there is such a runaway can the female trait evolve to be exaggerated beyond its fecundity optimum. If the mating success of a female is not proportional to the courtship that she receives, but is instead a fixed benefit of having the preferred trait, this is simply a biological reinterpretation of the same mathematical relationship that underlies preferences for more fecund females. In this case the male competition cost is still present, but is simply countered by the direct selective benefit of mating with a female that has a trait that indicates higher mating success (Servedio and Lande, 2006; the threshold fecundity benefit described above can be interpreted as a threshold of higher mating success in this scenario).
Another extreme departure from strict polygyny is the case of monogamy, where both males and females have equal mating success. Logically, if monogamy occurs with an equal sex ratio at the time of mating, and all mates are of equal quality in terms of direct fecundity benefits, there will be neither sexual selection nor fecundity selection on either males or females. Models of this scenario that have explicitly considered male choice have, however, generally included fecundity differences in either the male or female, as well as other forms of selection. These models have found that male preferences can evolve relatively easily in portions of the parameter space (e.g., Ihara and Aoki 1999, models 1 and 2; Stern and Servedio 2017; see also the model of post-pairing male choice by Lyu et al. 2017).
However, it should be noted that in contrast to the case of monogamy with equal sex ratios, if there are more males than females some males will go without a mate and therefore direct selection against male preferences will still emerge during monogamous mating. A similar effect can also occur when encounter rates between the sexes are low, and choosy males risk not mating at all. Male choice can still evolve in such models, even with added competition costs, but fecundity selection or another direct benefit of such a preference is again generally required (e.g., see mate guarding models of “serial monogamy,” Fawcett and Johnstone 2003; Härdling et al. 2004, 2008; Härdling and Kokko 2005). Presumably, such benefits of choice would have to be stronger than in the case of equal sex ratios above, although we are not aware of a model that has reported on an explicit comparison of these cases.
Under certain specific assumptions, limited access to females can have a diametrically opposite effect to that described in the monogamy models directly above; it can lead to selection for, instead of against, male preferences. In moths, for example, male preferences for species-specific pheromone blends can determine the ability of males to find females at all (Linn et al. 1997; Gould et al. 2010). In such cases, males without a preference will be directly selected against because they do not approach females, and thus are not visible to females at the short range at which females make mating decisions. Several researchers recognized that male preferences in such a case can be accurately represented mathematically as the equivalent of the male trait in a female-choice sexual selection model (because females are more likely to mate with males with a preference than one without), while female pheromones can likewise be represented by the female preference in a female-choice model (Phelan 1992; Butlin and Trickett 1997; Bengtsson and Lofstedt 2007; Bergen et al. 2012). Thus, one can conclude that male preferences can evolve in this scenario.
The direct selection due to competition will be lessened, or even completely countered, if males that have a preference have more energy available to put into courtship than males that have no preference. This extra overall courtship of males with a preference could, for example, allow them to compete equally for matings from unornamented females while still being over-represented in their courtship toward ornamented females (e.g., Servedio and Lande 2006); they are thus not at a competitive disadvantage with either type of female. To our knowledge, there are no empirical explorations of whether courtship would be expected to be equivalent in males with versus without a preference, although one could imagine that a relationship between preferences and courtship ability or resources for courtship might be realistic. Indeed, preferences have been shown to evolve in males with more resources or higher quality in several models (Ihara and Aoki 1999; Fawcett and Johnstone 2003; Härdling et al. 2004, 2008; Härdling and Kokko 2005; Venner et al. 2010). Ihara and Aoki (1999), for example, consider male choice under polygyny when the ability of males to mate with the maximum number of females depends on whether the males themselves are “resource rich” or “resource poor,” and resources affect the fecundity of the mated pair. They find that if resources are paternally inherited (their Model 4), resources become positively correlated with male preferences for an attractive but costly female trait. This correlation provides indirect selection favoring a male preference allele, as resource poor males who sometimes obtain no mate are likely to have no preference.
Finally, males themselves may be behaviorally capable of eliminating the costs of high competition on preference alleles that emerge in the null model above if they are aware of the competitive landscape and direct their courtship in a way to avoid high competition situations. Rowell and Servedio (2009) considered this situation by assuming that males can distribute their courtship following an ideal-free distribution where females are the limiting resource. They found that when males are capable of moving out of a high competition situation some degree of assortment generally results. Males that do not have preferences will tend to be the first to move out of situations of high competition, leaving ornamented females to males with the preference and thus tending themselves to court unornamented females. This movement and assortment has the consequence of removing the competitive disadvantage of males with the preference allele, and preferences can then be maintained polymorphically in the population. Preferences generally evolve only to low frequencies, however, under this mechanism.
The Relationship between Male Mate Preferences and Female Traits
Above, we have discussed the various models that provide explanations for the evolution of male preferences in contexts in which they might not otherwise have been expected to evolve. We now turn our attention to the handful of models that examine the potential for male mate choice to drive the evolution of ornamentation in females in polygynous mating systems. Although there are not very many theoretical models that investigate this potential evolutionary process, the few that have been developed are important because, as we detail below, they challenge the conventional wisdom that empirical evidence of male mate choice can be interpreted as evidence for selection on female traits. In fact, theoretical models suggest that although male preferences may evolve in species with traditional sex roles, the potential for that behavior to function as a mechanism of selection, thereby driving the evolution of female traits, is highly constrained.
This point is demonstrated most explicitly by a recent model published by the authors of this review (Fitzpatrick and Servedio 2017), which demonstrates that—in polygynous mating systems—the potential for male mate choice to drive the evolution of a female trait is not only limited, but is only possible under very circumscribed conditions. We develop a population genetic model that, in addition to trait and preference loci (expressed in females and males, respectively), includes a “quality” locus expressed only in males. Quality is modeled as a fecundity benefit to the mated pair such that a female mated to a high-quality male produces more offspring. This male quality locus allows the model to formalize the hypothesis that, even under polygyny, females might experience sexual selection as a result of competition for mates of superior quality [see Fitzpatrick (2015) for a review of the literature that invokes this common hypothesis]. The central parameter of interest in the model is ρ, which allows for an interaction between the male quality and preference loci so that when ρ > 1, males who have both high-quality and the preference allele have higher mating success than they would if the effects of each allele functioned independently. We demonstrate that simply the presence of mate preferences among males in combination with variation in quality is insufficient to cause the evolution of an allele for a costly female trait. The only conditions under which the presence of male mate preferences have a measurable impact on the evolution of the female trait are when: (1) ρ > 1, which biases the mate pairings so that ornamented females mate non-randomly with choosy, high-quality males; (2) ornamented females have a level of intrinsic fecundity that is large enough to allow male preference evolution, but not so great that it swamps the fecundity benefit that females might experience by mating with a high-quality male. These conditions are not only quite circumscribed, but might or might not be biologically realistic. Furthermore, even when male preferences in combination with male quality can influence female trait evolution in this model, the effect is weak. Thus, one conclusion from this model is that direct fecundity benefits delivered to females via male preferences are not a likely explanation for female trait evolution.
One scenario in which the concept that ρ > 1, as in Fitzpatrick and Servedio (2017), might be realistic is laid out by Ihara and Aoki (1999). In order to draw this comparison, let us highlight the consequence of ρ > 1 that is important for female trait evolution; the necessary feature for male preferences in combination with male quality to drive the evolution of female traits is that females with the trait must be more likely to mate with the high-quality males. In the models of sexual selection by male mate choice in human populations presented by Ihara and Aoki (1999), preferred female traits can evolve when resource-rich males are able to mate according to their preferences because they pair before resource-poor males do. That is, the model does not assume an association among males between preferences and resources per se, but males with more resources are more able to mate according to their preferences. The females with the preferred trait thus end up mating non-randomly with resource-rich males. These models are presented within the context of both monogamous mating systems and polygynous ones (with no discounting of resources per female for multiply-mated resource-rich males in the latter case). Male preference-mediated non-random mating (between resource-rich males and ornamented females) functions in both contexts to drive trait evolution in females. While mating priority is given to resource-rich males in many human cultures [which Ihara and Aoki (1999) explicitly model], the extent to which biological analogs are found in non-human animals is an open empirical question.
Two models have addressed the potential for male mate choice to drive the evolution of exaggerated female traits specifically among non-human primates (Pagel 1994; Nakahashi 2016)—perhaps because the exaggerated estrous swellings (“sexual swellings”) displayed by many species of primates are so commonly cited as an example of sexual selection on female traits (e.g., see p. 201 in Davies et al. 2012). Both models use a game theoretical framework. Consistent with the conditions necessary for the evolution of male preferences outlined above, both models assume that the female trait (exaggerated swellings) indicates something that represents a fitness benefit to males. It is well known that sexual swellings correspond with ovulation, to varying degrees across species (Nunn 1999; Alberts and Fitzpatrick 2012). Although Pagel (1994) is presented as a model that explains the evolution of exaggerated swellings in primates, the math of the model is primarily concerned with the evolution of preferences among males (see above) and female traits are implicitly assumed to evolve due to the presence of male preferences. Nakahashi (2016) developed another, more detailed model, also using game theoretical techniques. However, the feature of female ornamentation represented by this model is a function of time rather than morphology; that is, the model asks about the conditions that favor a strategy in which females signal receptivity for longer than they are actually fertile (“exaggerated signal”) versus a strategy in which females signal receptivity for fewer days than they are actually fertile (“concealed ovulation”). The fitness benefit that females can receive under some conditions is modeled as a direct benefit: the mitigation of infanticide risk by the confusion of paternity that occurs when females are not monopolized by the alpha male and can therefore mate with multiple males. Not surprisingly, he finds that an exaggerated signal in females (which can take 2 different forms) can evolve when the costs of mounting the strategy are smaller than the benefits obtained. It is interesting to note, however, that the exaggerated signal only evolves when the benefits only outweigh the costs by a small amount.
Female traits that exist in the context of male mate choice may, as we have stressed above, often indicate high fecundity. One way in which this association may occur is if males are attracted to females that have a trait that indicates that they are better resource competitors (e.g., the “armament-ornament” hypothesis or a “dual-utility” trait, see Berglund et al. 1996; Hunt et al. 2009). This process has been invoked with respect to female ornamentation in a number of empirical studies (e.g., Griggio et al. 2010; Crowhurst et al. 2012) and its feasibility was demonstrated for male preferences and female traits in a population genetic model of monogamous populations by Stern and Servedio (2017). It is possible to interpret the assumption of high fecundity in females in polygyny models, such as those discussed elsewhere in this review, as due to the ability of the females to gather more resources, and the preferred trait being an honest indicator of this ability. However, none of the models above explicitly model resource competition for a limited resource by females both with and without a trait.
Finally, Lyu et al. (2017) present a model that examines the potential for post-mating male mate choice—expressed in the form of allocation of paternal care to the brood versus engaging in extra-pair copulations—to shape female traits, envisaged in this model as a post-mating signal (e.g., egg color; Moreno and Osorno 2003; Soler et al. 2008). This 2 locus population genetic model indicates that, provided the female trait elicits enough paternal care, it can evolve despite a fecundity cost; direct fecundity costs of producing the preferred trait in these cases are offset by the fecundity benefit provided by the males through parental care. Due to tradeoffs that arise in this model, it can also yield cases of sexual conflict where male preferences and female traits cycle evolutionarily.
A central take home message from this review of the literature of male mate choice and female sexual signals is that, although there is a dearth of mathematical models that explicitly examine the potential for male mate choice to promote the evolution of sexual signals in females; this relationship is often assumed. Indeed, most models that include female traits simply assume that the trait is associated with high fecundity or is an indicator of some aspect of fertility, such that male mate preferences can evolve, but do not otherwise focus specifically on the effects that these male preferences might place on female traits.
Summary and Conclusions
Although the number of mathematical models that investigate the evolution of male mate choice has grown in recent decades, the literature on this topic remains relatively small. The subset of these papers investigating how this behavioral trait can drive trait evolution in females is smaller still. Nonetheless, empirical examples of both male preferences and female-specific traits are now well documented. Thus, one of our conclusions is that many more interesting questions remain and there is more work to be done.
As we have discussed, many models have either assumed that male choice includes explicit costly contests between males, or have concluded that competitive costs emerge from the very nature of male preferences under polygyny. The evolution of male choice is thus expected to be more difficult than the evolution of female choice, and as such the benefits that males acquire by their choices must be great if male preferences are to be explained. We have discussed many different types of benefits that have been considered in mathematical models, some of which apply direct selection, and hence are quite effective at allowing male preference evolution, and other, less effective benefits, that operate through indirect selection (or via genetic constraints). There are doubtless, however, other specific mechanisms of benefits that have yet to be discovered in natural systems; we suggest that an awareness of the costs inherent in male choice should prompt researchers to assess whether costs are indeed present in their systems (as we point out, there are also many mechanisms that remove costs), and if so, prompt investigation into benefits that can explain the persistence of male preferences.
We have also stressed that the mere presence of male preferences does not mean that there is sexual selection favoring female traits. The presence of female traits, especially during polygyny, thus also warrants further investigation, regardless of whether male preferences are also found in the system. The mathematical models surrounding both of these phenomena have therefore elucidated and motivated several fruitful areas for further investigation.
Future Directions
This review points to areas of theoretical research that are ripe for further investigation and we specify a few of them here. With respect to questions about the evolution of male mate choice, we note an absence of models that examine the potential for heritable genetic quality in females to drive the evolution of male mate preferences. Furthermore, our review emphasizes that there are many questions about the evolution of ornamentation in females that remain unexplored. For instance, there are virtually no models that explore the potential for visual sexual signals to evolve in females as a consequence of fitness benefits that are not strictly reproductive (e.g., protection from predation or access to resources that is mediated in some way by the male mate). These types of models would, specifically, be investigating the potential for “social selection” to be a common explanation for the patterns of female ornaments within and across taxa. Indeed, we anticipate that these questions about social selection and female ornaments will produce an increasingly fruitful and vibrant area of future research.
Acknowledgments
We thank Marcella Willett for the artwork in Figure 2. We thank the guest editor, Ingo Schlupp, as well as 3 anonymous reviewers, whose comments on previous versions improved the quality of this manuscript.
Funding
This work was supported by the National Institutes of Health award 5T32HD049336-12 [to C.L.F.].
References
- Adkins-Regan E, Akçay E, Alonzo S, Bailey N, Crawford J. et al. , 2013. Sexual selection studies: progress, challenges, and future directions. Final Report from a NESCent Catalyst Meeting Durham NC, 15–17 July 2013. Revision 1.1, 11 December 2013. Available at the PeerJ website.
- Ah-King M, Ahnesjö I, 2013. The “sex role” concept: an overview and evaluation. Evol Biol 40:461–470. [Google Scholar]
- Alberts S, Fitzpatrick C, 2012. Paternal care and the evolution of exaggerated sexual swellings in primates. Behav Ecol 23:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen T, 2000. Why are female birds ornamented? Trends Ecol Evol 15:149–155. [DOI] [PubMed] [Google Scholar]
- Amundsen T, Forsgren E, 2001. Male mate choice selects for female coloration in a fish. Proc Natl Acad Sci USA 98:13155–13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen T, Forsgren E, Hansen LTT, 1997. On the function of female ornaments: male bluethroats prefer colourful females. Proc R Soc B 264:1579–1586. [Google Scholar]
- Andersson M, 1994. Sexual Selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- Arnqvist G, Rowe L, 2005. Sexual Conflict. Princeton: Princeton University Press. [Google Scholar]
- Arntzen J, 1999. Sexual selection and male mate choice in the common toad Bufo bufo. Ethol Ecol Evol 11:407–414. [Google Scholar]
- Baldwin J, Johnsen S, 2012. The male blue crab Callinectes sapidus uses both chromatic and achromatic cues during mate choice. J Exp Biol 215:1184–1191. [DOI] [PubMed] [Google Scholar]
- Barry KL, Kokko H, 2010. Male mate choice: why sequential choice can make its evolution difficult. Anim Behav 80:163–169. [Google Scholar]
- Bateman PW, Ferguson JWH, 2004. Male mate choice in the Botswana armoured ground cricket Acanthoplus discoidalis (Orthoptera: tettigoniidae; Hetrodinae). Can, and how, do males judge female mating history? J Zool 262:305–309. [Google Scholar]
- Bengtsson BO, Lofstedt C, 2007. Direct and indirect selection in moth pheromone evolution: population genetical simulations of asymmetric sexual interactions. Biol J Linn Soc 90:117–123. [Google Scholar]
- Bergen EL, Rowell JT, Gould F, Servedio MR, 2012. Stochasticity in sexual selection enables divergence: implications for moth phermone evolution. Evol Biol 39:271–281. [Google Scholar]
- Berglund A, Bisazza A, Pilastro A, 1996. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biological Journal of the Linnean Society 58:385–399. [Google Scholar]
- Bonduriansky R, 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev Camb Phil Soc 76:305–339. [DOI] [PubMed] [Google Scholar]
- Brooks R, Endler JA, Url S, 2001. Direct and indirect sexual selection and quantitative genetics of male traits in guppies Poecilia reticulata. Evolution 55:1002–1015. [DOI] [PubMed] [Google Scholar]
- Butlin RK, Trickett AJ, 1997. Can population genetic simulations help to interpret pheromone evolution? In: Cardé RT, Minks AK, editors. Insect Pheromone Research. Boston, MA: Springer; 548–562. [Google Scholar]
- Byrne PG, Rice WR, 2006. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc R Soc B 273:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisi RM, Malone JH, Hews DK, 2008. Female secondary coloration in the Mexican boulder spiny lizard is associated with nematode load. J Zool 276:358–367. [Google Scholar]
- Carazo P, Sanchez E, Font E, Desfilis E, 2004. Chemosensory cues allow male Tenebrio molitor beetles to assess the reproductive status of potential mates. Anim Behav 68:123–129. [Google Scholar]
- Cole GL, Endler JA, 2016. Male courtship decisions are influenced by light environment and female receptivity. Proc R Soc B 283:20160861.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtiol A, Etienne L, Feron R, Godelle B, Rousset F, 2016. The evolution of mutual mate choice under direct benefits. Am Nat 188:521–538. [DOI] [PubMed] [Google Scholar]
- Craig AS, Herman LM, Pack AA, 2002. Male mate choice and male–male competition coexist in the humpback whale Megaptera novaeangliae. Can J Zool 80:745–755. [Google Scholar]
- Crowhurst CJ, Zanollo V, Griggio M, Robertson J, Kleindorfer S, 2012. White flank spots signal feeding dominance in female diamond firetails Stagonopleura guttata. Ethology 118:63–75. [Google Scholar]
- Darwin C, 1871. Descent of Man, and Selection in Relation to Sex. London: Penguin Books. [Google Scholar]
- Davies NB, Krebs JR, West SA, 2012. An Introduction to Behavioural Ecology. 4th edn.Sunderland: Sinauer Associates. [Google Scholar]
- Edward DA, Chapman T, 2011. The evolution and significance of male mate choice. Trends Ecol Evol 26:647–654. [DOI] [PubMed] [Google Scholar]
- Endler JA, 1983. Natural and sexual selection on color patterns in poeciliid fishes. Environ Biol Fish 9:173–190. [Google Scholar]
- Fawcett TW, Johnstone RA, 2003. Mate choice in the face of costly competition. Behav Ecol 14:771–779. [Google Scholar]
- Fitzpatrick C, Altmann J, Alberts S, 2014. Sources of variance in a female fertility signal: exaggerated estrous swellings in a natural population of baboons. Behav Ecol Sociobiol 68:1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CL, 2015. Expanding sexual selection gradients; a synthetic refinement of sexual selection theory. Ethology 121:207–217. [Google Scholar]
- Fitzpatrick CL, Altmann J, Alberts SC, 2015. Exaggerated sexual swellings and male mate choice in primates: testing the reliable indicator hypothesis in the Amboseli baboons. Anim Behav 104:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CL, Servedio MR, 2017. Male mate choice, male quality, and the potential for sexual selection on female traits under polygyny. Evolution 71:174–183. [DOI] [PubMed] [Google Scholar]
- Gaskett A, Herberstein M, Downes B, Elgar M, 2004. Changes in male mate choice in a sexually cannibalistic orb-web spider (Araneae: araneidae). Behaviour 141:1197–1210. [Google Scholar]
- Gould F, Estock M, Hillier NK, Powell B, Groot AT. et al. , 2010. Sexual isolation of male moths explained by a single pheromone response QTL containing four receptor genes. Proc Natl Acad Sci USA 107:8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourbal BEF, Gabrion C, 2004. A study of mate choice in mice with experimental Taenia crassiceps cysticercosis: can males choose? Can J Zool 82:635–643. [Google Scholar]
- Griggio M, Valera F, Casas A, Pilastro A, 2005. Males prefer ornamented females: a field experiment of male choice in the rock sparrow. Anim Behav 69:1243–1250. [Google Scholar]
- Griggio M, Zanollo V, Hoi H, 2010. Female ornamentation, parental quality, and competitive ability in the rock sparrow. J Ethol 28:455–462. [Google Scholar]
- Gwynne DT, 1993. Food quality controls sexual selection in Mormon crickets by altering male mating investment. Ecology 74:1406–1413. [Google Scholar]
- Härdling R, Gosden T, Aguilée R, 2008. Male mating constraints affect mutual mate choice: prudent male courting and sperm-limited females. Am Nat 172:259–271. [DOI] [PubMed] [Google Scholar]
- Härdling R, Kokko H, 2005. The evolution of prudent choice. Evol Ecol Res 7:697–715. [Google Scholar]
- Härdling R, Kokko H, Elwood RW, 2004. Priority versus brute force: when should males begin guarding resources? Am Nat 163:240–252. [DOI] [PubMed] [Google Scholar]
- Herdman EJE, Kelly CD, Godin JGJ, 2004. Male mate choice in the guppy Poecilia reticulata: do males prefer larger females as mates? Ethology 110:97–111. [Google Scholar]
- Hopkins J, Baudry G, Candolin U, Kaitala A, 2015. I’m sexy and I glow it: female ornamentation in a nocturnal capital breeder. Biol Lett 11:20150599.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs C, Hubbs L, 1932. Apparent parthenogenesis in nature, in a form of fish of hybrid origin. Science 76:628–630. [DOI] [PubMed] [Google Scholar]
- Huchard E, Benavides JA, Setchell JM, Charpentier MJE, Alvergne A. et al. , 2009. Studying shape in sexual signals: the case of primate sexual swellings. Behav Ecol Sociobiol 63:1231–1242. [Google Scholar]
- Hunt J, Breuker CJ, Sadowski JA, Moore AJ, 2009. Male-male competition, female mate choice and their interaction: Determining total sexual selection. J Evol Biology 22:13–26. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Aoki K, 1999. Sexual selection by male choice in monogamous and polygynous human populations. Theor Popul Biol 55:77–93. [DOI] [PubMed] [Google Scholar]
- Jones AG, Rosenqvist G, Berglund A, Avise JC, 2000. Mate quality influences multiple maternity in the sex-role-reversed pipefish Syngnathus typhle. Oikos 90:321–326. [Google Scholar]
- Kirkpatrick M, Barton NH, 1997. The strength of indirect selection on female mating preferences. Proc Natl Acad Sci USA 94:1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Heubel KU, 2011. Prudent males, group adaptation, and the tragedy of the commons. Oikos 120:641–656. [Google Scholar]
- Kokko H, Ots I, 2006. When not to avoid inbreeding. Evolution 60:467–475. [PubMed] [Google Scholar]
- Kopp M, Servedio MR, Mendelson TC, Safran RJ, Rodríguez RL. et al. , 2018. Mechanisms of assortative mating in speciation with gene flow: connecting theory and empirical research. The American Naturalist 191:1–20. [DOI] [PubMed] [Google Scholar]
- Lande R, 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34:292–305. [DOI] [PubMed] [Google Scholar]
- Lande R, 1981. Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci USA 78:3721–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBas NR, Hockham LR, Ritchie MG, 2003. Nonlinear and correlational sexual selection on “honest” female ornamentation. Proc R Soc B 270:2159–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBas NR, Marshall NJ, 2000. The role of colour in signalling and male choice in the agamid lizard Ctenophorus ornatus. Proc R Soc B 267:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn CE, Young MS, Gendle M, Glover TJ, Roelofs WL, 1997. Sex phermone blend discrimination in two races and hybrids of the European corn borer moth Ostrinia nubilalis. Physiol Entomol 22:212–223. [Google Scholar]
- Lyu N, Servedio MR, Lloyd H, Sun Y-H, 2017. The evolution of postpairing male mate choice. Evolution 71:1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod EC, Andrade MCB, 2014. Strong, convergent male mate choice along two preference axes in field populations of black widow spiders. Anim Behav 89:163–169. [Google Scholar]
- Martel V, Damiens D, Boivin G, 2008. Male mate choice in Trichogramma turkestanica. J Insect Behav 21:63–71. [Google Scholar]
- Massironi M, Rasotto MB, Mazzoldi C, 2005. A reliable indicator of female fecundity: the case of the yellow belly in Knipowitschia panizzae (Teleostei: Gobiidae). Mar Biol 147:71–76. [Google Scholar]
- Méndez-Janovitz M, Macías Garcia C, 2017. Do male fish prefer them big and colourful? Non-random male courtship effort in a viviparous fish with negligible paternal investment. Behav Ecol Sociobiol 71:160. [Google Scholar]
- Moreno J, Osorno JL, 2003. Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806. [Google Scholar]
- Nakahashi W, 2008. Quantitative genetic models of sexual selection by male choice. Theoretical population biology 74:167–181. [DOI] [PubMed] [Google Scholar]
- Nakahashi W, 2016. Coevolution of female ovulatory signals and male–male competition in primates. J Theor Biol 392:12–22. [DOI] [PubMed] [Google Scholar]
- Nordeide JT, Kekäläinen J, Janhunen M, Kortet R, 2013. Female ornaments revisited: are they correlated with offspring quality? J Anim Ecol 82:26–38. [DOI] [PubMed] [Google Scholar]
- Nunn C, 1999. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav 58:229–246. [DOI] [PubMed] [Google Scholar]
- Ojanguren AF, Magurran AE, 2004. Uncoupling the links between male mating tactics and female attractiveness. Proc R Soc B 271:S427–S429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, 1993. Male preference for large females and assortative mating for body size in the sand lizard Lacerta agilis. Behav Ecol Sociobiol 32:337–341. [Google Scholar]
- Orrell KS, Jenssen TA, 2002. Male mate choice by the lizard Anolis carolinensis: a preference for novel females. Anim Behav 63:1091–1102. [Google Scholar]
- Pagel M, 1994. The evolution of conspicuous oestrous advertisement in Old World monkeys. Anim Behav 47:1333–1341. [Google Scholar]
- Parga JA, 2006. Male mate choice in Lemur catta. Int J Primatol 27:107–131. [Google Scholar]
- Phelan P, 1992. Evolution of sex phermones and the role of asymmetric tracking In: Roitberg B, Isman M, editors. Insect Chemical Ecology: An Evolutionary Approach. New York: Chapman and Hall, 265–314. [Google Scholar]
- Pizzolon M, Rasotto MB, Mazzoldi C, 2008. Male lagoon gobies, Knipowitschia panizzae, prefer more ornamented to larger females. Behav Ecol Sociobiol 62:521–528. [Google Scholar]
- Prum RO, 2010. The Lande–Kirkpatrick mechanism is the null model of evolution by intersexual selection: implications for meaning, honesty, and design in intersexual signals. Evolution 64:3085–3100. [DOI] [PubMed] [Google Scholar]
- Ptacek MB, Travis J, 1997. Mate choice in the Sailfin molly Poecilia latipinna. Evolution 51:1217–1231. [DOI] [PubMed] [Google Scholar]
- Rosenthal GG, 2017. Mate Choice; the Evolution of Sexual Decision Making from Microbes to Humans. Princeton: Princeton University Press. [Google Scholar]
- Rowell JT, Servedio MR, 2009. Gentlemen prefer blondes: the evolution of mate preference among strategically allocated males. Am Nat 173:12–25. [DOI] [PubMed] [Google Scholar]
- Rowland WJ, Grindle N, Maclaren RD, Granquist R, 2002. Male preference for a subtle posture cue that signals spawning readiness in female sticklebacks. Anim Behav 63:743–748. [Google Scholar]
- Saeki Y, Kruse K, Switzer P, 2005. Male preference for large females and female reproductive condition in the Japanese beetle Popillia japonica Newman (Coleoptera: scarabaeidae). J Kans Entomol Soc 78:13–19. [Google Scholar]
- Schlupp I, 2010. Mate choice and the Amazon molly: how sexuality and unisexuality can coexist. J Hered 101:S55–S61. [DOI] [PubMed] [Google Scholar]
- Schlupp I, Marler C, Ryan MJ, 1994. Benefit to male Sailfin mollies of mating with heterospecific females. Science 263:373–374. [DOI] [PubMed] [Google Scholar]
- Servedio MR, 2007. Male versus female mate choice: sexual selection and the evolution of species recognition via reinforcement. Evolution 61:2772–2789. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Dukas R, 2013. Effects on population divergence of within-generational learning about prospective mates. Evolution 67:2363–2375. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Lande R, 2006. Population genetic models of male and mutual mate choice. Evolution 60:674–685. [PubMed] [Google Scholar]
- Shuster SM, Wade MJ, 2003. Mating Systems and Strategies. Princeton: Princeton University Press. [Google Scholar]
- Siefferman L, Hill GE, 2005. Male eastern bluebirds trade future ornamentation for current reproductive investment. Biol Lett 1:208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox H, Colegrave N, Heenan A, Howard C, Braithwaite VA, 2005. Context-dependent male mating preferences for unfamiliar females. Anim Behav 70:1429–1437. [Google Scholar]
- Soler JJ, Navarro C, Contreras TP, Avilés JM, Cuervo JJ, 2008. Sexually selected egg coloration in spotless starlings. Am Nat 171:183–194. [DOI] [PubMed] [Google Scholar]
- South SH, Arnqvist G, Servedio MR, 2012. Female preference for male courtship effort can drive the evolution of male mate choice. Evolution 66:3722–3735. [DOI] [PubMed] [Google Scholar]
- Stern CA, Servedio MR, 2017. Evolution of a mating preference for a dual-utility trait used in intrasexual competition in genetically monogamous populations. Ecol Evol 7:8008–8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Watanabe M, 2011. Male mate choice based on ontogenetic colour changes of females in the damselfly Ischnura senegalensis. J Ethol 29:293–299. [Google Scholar]
- Venner S, Bernstein C, Dray S, Bel-Venner M, 2010. Make love not war: when should less competitive males choose low-quality but defendable females? Am Nat 175:650–661. [DOI] [PubMed] [Google Scholar]
- Verrell PA, 1995. Males choose larger females as mates in the salamander Desmognathus santeetlah. Ethology 99:162–171. [Google Scholar]
- Weiss SL, 2002. Reproductive signals of female lizards: pattern of trait expression and male response. Ethology 813:793–813. [Google Scholar]
- Weiss SL, 2006. Female-specific color is a signal of quality in the striped plateau lizard Sceloporus virgatus. Behav Ecol 17:726–732. [Google Scholar]
- Weiss SL, Kennedy EA, Bernhard JA, 2009. Female-specific ornamentation predicts offspring quality in the striped plateau lizard Sceloporus virgatus. Behav Ecol 20:1063–1071. [Google Scholar]
- Werner NY, Lotem A, 2003. Choosy males in a haplochromine cichlid: first experimental evidence for male mate choice in a lekking species. Anim Behav 66:293–298. [Google Scholar]
- Wittman T, Fedorka KM, 2014. Male mate choice for unparasitized females in Drosophila melanogaster. J Insect Behav 28:37–43. [Google Scholar]
- Wong BBM, Jennions MD, 2003. Costs influence male mate choice in a freshwater fish. Proc R Soc B 270:S36–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]