Abstract
Phenotypic heterogeneity is a major barrier to understanding the genetic architecture underlying schizophrenia. Incorporating endophenotypes is one way to reduce heterogeneity and facilitate more powerful genetic analysis. Candidate endophenotypes require systematic assessment against endophenotype criteria, and a ranking of their potential utility for genetic analysis. In this study we assess 20 cognitive and personality measures in a sample of 127 families with at least 2 cases of schizophrenia per family (n = 535) plus a set of 30 control families (n = 121) against 4 endophenotype criteria: (a) be associated with the illness but not be a part of its diagnosis, (b) be heritable, (c) co-segregate with the illness in families, and (d) be found in unaffected relatives at a higher rate than in the general population. The endophenotype ranking score (endophenotype ranking variable [ERV]) was used to rank candidate endophenotypes based on their heritability and genetic correlation with schizophrenia. Finally, we used factor analysis to explore latent factors underlying the cognitive and personality measures. Evidence for personality measures as endophenotypes was at least equivalent to that of the cognitive measures. Factor analysis indicated that personality and cognitive traits contribute to independent latent dimensions. The results suggest for this first time that a number of cognitive and personality measures are independent and informative endophenotypes. Use of these endophenotypes in genetic studies will likely improve power and facilitate novel aetiological insights.
Keywords: heritability, genetic correlation, genetics, variance components analysis
Introduction
Genome wide association studies (GWAS) have accounted for a modest fraction of the heritability estimates for schizophrenia (h2~0.6–0.8)1,2; with the polygenic risk score explaining ~7% of the variation on the liability scale.3 A major barrier to elucidating schizophrenia’s genetic architecture is its phenotypic heterogeneity. Gottesman and Gould4 introduced the endophenotype concept to psychiatry as “measurable components unseen by the unaided eye along the pathway between disease and distal genotype.”
Endophenotypes represent components of liability which are narrower than a dichotomized phenotype, such as a “yes/no” clinical diagnosis. They promise improved power over affection status alone to detect risk genes by being both quantitative and closer to the level of gene action.5–7 Assuming similar levels of imprecision, many studies have demonstrated that power for gene mapping is better with a quantitatively measured phenotype than with a direct dichotomization of that phenotype.8,9 In addition, as endophenotypes should be correlated with, but not part of, the clinical diagnosis, power can be further increased by joint analyses of the dichotomous diagnostic phenotype and the quantitative correlated endophenotype.10–12 It is likely that multiple endophenotypes will be necessary to capture the complex pathophysiological processes involved in schizophrenia.13 Objectively assessing candidate endophenotypes of schizophrenia and ranking them against each other will help to prioritize the most promising endophenotypes to target in future genetic studies. This study builds on previous work in this area14,15 by assessing for the first time both cognitive and personality measures as candidate endophenotypes for schizophrenia in a sample of families multiply affected by schizophrenia.
Gottesman and Gould4 describe endophenotypes as “neurophysiological, biochemical, endocrinological, neuroanatomical, cognitive, or neuropsychological (including configured self-report data) in nature” and suggest that they should meet the following criteria: (a) be associated with the illness but not be a part of its diagnosis, (b) be heritable, (c) co-segregate with the illness in families, (d) be found in unaffected relatives at a higher rate than in the general population, and (e) be state independent. As discussed by Glahn et al,5 Gottesman and Gould’s 5 endophenotype criteria are interrelated. Both “co-segregation of endophenotypes and disease” and “unaffected relatives (who are at high genetic risk) being intermediate between cases and unaffected controls” indicate joint genetic determination (genetic correlation) between the endophenotype and disease. Endophenotype and disease must be heritable and associated with each other for genetic correlation (the proportion of trait covariance due to genetic factors) to be present. As genetic correlation indicates the endophenotype shares a biological basis with the disorder, a degree of state independence is inferred. Although the Gottesman and Gould criteria are not all independent of each other, a true endophenotype should nonetheless satisfy all the criteria assuming adequately powered samples. Therefore the candidate endophenotypes in this study were assessed against all the criteria possible from our data (a–d).
To complement this analysis, candidate endophenotypes which were associated with schizophrenia, significantly heritable and showed evidence for genetic correlation according to at least 1 of the 2 criteria (c and d), were ranked against each other using the endophenotype ranking variable (ERV10), developed by Glahn et al to prioritize endophenotypes for genetic analysis. Finally we performed a factor analysis to ascertain to what extent the cognitive and personality measures contribute to independent latent variables.
Cognitive variables are among the most often-cited candidate endophenotypes in the psychiatric literature.5 Cognitive deficits across multiple domains are a core feature of schizophrenia,16–18 present in up to 80% of cases,19 predate illness onset, and do not result from the positive or negative symptoms of the disorder, or antipsychotic treatment.20–23 Studies of healthy individuals24–27 and families with schizophrenia15,28,29 have shown cognitive deficits to be significantly heritable (h2~0.4–0.8 across different tests). The genetic correlation between cognitive traits and schizophrenia has been estimated by comparing unaffected relatives with healthy controls. A large meta-analysis30 reported modest but reliable relative-control differences in attention/working memory, verbal memory, visual memory, executive function, spatial ability, motor function, language and general intelligence. There is evidence that a substantial proportion of the correlation observed among cognitive variables is due to a shared genetic basis.25–28,31
Abnormal premorbid personality traits have long been described in schizophrenia patients, as well as in their biological relatives.32,33 These personality differences are consistent and pervasive,34–40 predate psychotic illness and are stable over the course of psychotic illness.41 Although they meet all the criteria for potential endophenotypes,42 personality measures have not been well studied as such, possibly be due to the fact that they have a history of being designed based on clinical observations and may have been considered too close to the clinical diagnosis of schizophrenia to be useful endophenotypes. However, accumulating evidence demonstrates that personality traits represent important potential endophenotypes which are independent of, but causally related to, the disease process of schizophrenia itself,43 discussed in more detail below. In this study we assessed personality using 2 instruments—the Schizotypal Personality Questionnaire (SPQ44), which measures schizotypy, and the Temperament and Character Inventory (TCI), a self-report questionnaire designed to quantify individual differences on each of the temperament and character dimensions outlined in Cloninger’s psychobiological model.45
The term “schizotype”46 was introduced to describe a continuum of schizophrenic-like differences in perceptual, cognitive, and affective experiences. Schizotypy was originally interpreted under a quasi-dimensional model, whereby a small group of individuals, labeled “schizotypes,” are differentiated from the rest of the population in a categorical fashion.46,47 However recent neurobiological, neuropsychological, social and environmental evidence (reviewed in Nelson et al48) supports a fully dimensional model of schizotypy.49 This model posits a continuum between schizotypy in healthy populations and disorders on the schizophrenia spectrum, consistent with the majority of current theories pertaining to schizophrenia, which describe continuity between clinical and nonclinical psychosis populations.50 Under the fully dimensional model, high schizotypy is strongly associated with an increased risk of schizophrenia-spectrum psychopathology,51,52 although it is not part of its diagnosis, and schizotypy may also relate to a range of psychotic disorders other than schizophrenia.51 In addition, it has been repeatedly noted that many healthy individuals with high schizotypy not only function well but may benefit from their anomalous perceptual and other experiences and exhibit adaptive strengths such as creativity.48 Schizotypy is a stable trait with high re-test reliability53,54 which does not solely manifest during acute phases of illness (ie, is state-independent). Heritability estimates for total schizotypy in community samples are variable, likely reflecting the heterogeneity of schizotypal traits (h2~0.15–0.70).55–59 Few studies have looked for evidence of genetic correlation between schizotypy and schizophrenia; however, there is some evidence that unaffected relatives of probands with schizophrenia display higher schizotypy than healthy controls.60,61 Impaired cognition is not necessarily associated with high levels of schizotypy in general populations62,63 or in samples of schizophrenia patients,64 and schizotypy and cognitive measures are likely to represent distinct endophenotypes of schizophrenia.
In addition to schizotypy and schizophrenia being phenotypically independent, a recent overview51 of genetic studies suggests that they are influenced by at least 2 different groups of genetic variants. The first group is postulated to explain mainly schizotypy variance and increased proneness for psychosis, regardless of clinical diagnosis. The second group conveys unspecific neuronal fragility and susceptibility to environmental insults, associated with the risk of transition between being well (but possibly with healthy high schizotypy) and clinical schizophrenia, which is likely to be common to many disorders. The relationship between schizophrenia-associated genetic variants and schizotypy supports this hypothesis. Although several genes implicated in the aetiology of schizophrenia have also been associated with schizotypy, few of these genes have achieved genome-wide significance in large case-control GWAS of schizophrenia.51 In these GWAS, both cases and controls are treated as homogeneous groups and therefore GWAS may predominantly pick up the second group, the more general “disease resilience”-associated variants. This is supported by a cross-disorder GWAS which found substantial overlap between genetic risk variants for 5 psychiatric disorders65 and by a study showing that genetic risk for schizophrenia from case-control GWAS (summarized using polygenic risk scores) is associated with negative symptoms but not with schizotypy in nonclinical populations.66,67 Individuals with high but healthy schizotypy would not be expected to have a high polygenic risk score for schizophrenia, as those with high polygenic risk scores and high schizotypic traits would be more likely to develop spectrum conditions than to be in a healthy sample. Unlike schizotypy, polygenic risk score for schizophrenia is associated with lower cognitive ability in nonclinical populations,68–71 indicating that poor cognition may be more correlated with the “susceptibility to disease” variants picked up by GWAS than schizotypy is. Previous research from the Western Australian Family Study of Schizophrenia (WAFSS) has shown that in this sample, patients with schizophrenia can be separated into distinct subtypes characterized by either cognitive deficit, or personality factors (heavily weighted on schizotypal symptoms), using grade of membership analysis.17 The group has previously demonstrated genetic linkage between the cognitively deficit group and the MHC region later implicated in the large schizophrenia GWAS.
There is evidence for distinct and separable constructs within schizotypy,72 with the most consistent48,73 being a 3-factor model comprising Cognitive-Perceptual Dysfunction (Ideas of Reference, Magical Thinking, Unusual Perceptual Experiences, Suspiciousness), Interpersonal-Affective Deficits (Social Anxiety, No Close Friends, Suspiciousness and Constricted Affect), and Disorganization (Odd Speech and Odd Behavior) which broadly correspond to the positive, negative and disorganized dimensions of schizophrenia respectively.74 High “positive” schizotypy is associated with adaptive strengths like creativity75,76 and with better functioning in other psychiatric illness such as bipolar disorder.77 High “negative” schizotypy has been shown to share considerable variance with neuroticism, a personality trait linked to other affective or anxiety disorders.55 As recently argued by Grant,42 the ability of the dimensional model of schizotypy to represent the dimensionality of schizophrenia on a continuous scale makes it a valuable candidate endophenotype which is likely to add power to genetic analyses and to aid investigation into the aetiology of schizophrenia and other psychotic disorders. The SPQ can be interpreted within the 3-factor model framework of schizotypy74,78–80 with each of the 3 factors being individually heritable.81
The psychobiological model proposed by Cloninger and colleagues45,82,83 is based on 4 temperament and 3 character dimensions and accounts for most of the variance in personality, both in the general population and in psychiatric patients.83 The 4 temperament dimensions—Novelty Seeking (NS), Harm Avoidance (HA), Reward Dependence (RD), and Persistence (P) are hypothesized to be closely connected with neurotransmitter systems82 and are described as heritable biases in learning which lead to variation in responses to danger, novelty, and reward.84 They have been shown to be relatively stable over lifetime and to be universal across different cultures and various political and ethnic groups.45
The 3 character dimensions—self directedness (SD), cooperativeness (C) and self transcendence (ST)—are related to self-concepts about values and goals that impact the meaning of what is experienced.83 Although character has been shown to be influenced by heritable/biological factors,85 environmental factors such as sociocultural pressures and random life events are thought to impact more on character than on temperament—character traits are dynamic and mature in response to learning and life experiences. Nevertheless, what little data are available suggest that both are moderately heritable (h2~0.24–0.45, with HA, ST and C being significantly heritable86,87). Schizophrenia has consistently been associated with abnormal temperament (especially increased HA and decreased RD40,88–90) and character (especially low SD, low C, and high ST40,88–90) dimensions, reviewed in Ohi et al91. Some studies have shown character differences between unaffected relatives of people with schizophrenia and controls. Unaffected relatives have shown lower C,37,40,86SD,37,40 and RD37 and higher HA40,86 and ST40,86 compared to healthy controls. Other studies did not find significant differences between relatives and controls however,90 and some studies have shown group differences in the opposite direction, with healthy individuals at high genetic risk for schizophrenia demonstrating lower ST,90,92 and higher SD and C35, indicating a more mature personality profile than healthy controls. These differences were more pronounced in individuals with less schizotypal features,35 indicating that temperament and character profile may depend on the schizotypy of the relatives. Very few studies have examined genetic correlation between the character domains and schizophrenia using co-segregation analysis, although one recent study of the TCI did not find significant genetic correlations.86 Positive and negative schizotypy has been associated with different character and temperament features. In unaffected relatives of people with schizophrenia and in healthy controls, negative schizotypy is associated with high HA, and positive and disorganized schizotypy has been associated with low SD and high ST.35 This supports Cloninger’s theory that schizotypy is characterized by the character traits; low SD and C, and high ST.83 Similarly, looking at schizophrenia symptomatology, high HA is associated with negative symptoms and high ST with positive features.90 Character dimensions may help to separate those who benefit from high schizotypy from those in whom it becomes pathological.40,84 For example, although high ST is correlated with schizotypal and paranoid symptoms, when coupled with high SD and C, high ST indicates maturity, spirituality, and creativity rather than psychopathology.84
Given the promise of personality measures to be informative endophenotypes, the aim of this study was to evaluate them against endophenotype criteria and to compare them to the more well-established cognitive measures. Specifically, the study had 3 aims: (a) to comprehensively evaluate for the first time both cognitive and personality measures as candidate endophenotypes against 4 of Gottesman and Gould’s 5 criteria, (b) to rank the strength of the evidence for cognitive and personality measures as candidate endophenotypes in relation to each other using the ERV, and (c) to examine the relationship between cognitive and personality measures by conducting a factor analysis to identify the composition of any latent variables.
Methods
The Western Australian Family Study of Schizophrenia
The WAFSS study has been described in detail elsewhere.17,93 It was initiated in 1996 with the aim of comprehensively assessing families with ≥1 member affected with a disorder within the ICD-10 and DSM-IV schizophrenia spectrum (schizophrenia, schizoaffective disorder, schizotypal disorder and acute transient psychosis). The majority of probands were recruited from consecutive admissions to a psychiatric hospital. Full pedigree descriptions and family histories were collected using the National Institute for Mental Health Family Interview for Genetic Studies.94 Clinical assessment included the Diagnostic Interview for Psychosis (DIP95) and a best-estimate diagnosis, established by consensus of 2 senior clinicians blinded to family relatedness. Control families were screened for psychopathology and excluded if they or a first-degree relative had been diagnosed with schizophrenia/schizophrenia spectrum disorder or bipolar affective disorder. Written informed consent was obtained from all participants. The study complied with the ethics guidelines of the institutions involved.
All WAFSS participants were administered a battery of tests by research psychologists that assessed performance across 6 domains of cognitive function and personality. The battery of tests was chosen on the basis of showing evidence of heritability21 and reasonable effect sizes of test measures18 at the time of the study design. For this study, all tests for which data were available for a reasonable number of study participants were included.
The cognitive and personality tests are summarized below:
-
1. General cognitive ability:
-
2. Verbal learning and memory:
Rey Auditory Verbal Learning Test immediate (RAVLT-IW) and delayed (RAVLT-DW) word recall98
-
3. Sustained attention:
-
4. Executive function:
Controlled Oral Word Association Test (COWAT100) of phonetic verbal fluency, FAS letters
-
5. Speed of information processing:
Visual Inspection Time (IT) Tasks,101 block A and B
-
6. Laterality:
Edinburgh Handedness Inventory (EHI102), laterality quotient
-
7. Schizotypal traits:
SPQ44—3-factor model of Cognitive-Perceptual Deficits, Interpersonal Deficits, and Disorganization
-
8. Temperament and character:
TCI45
In the present study, we included all individuals for whom cognitive and personality measures were available, with at least one documented relative in the study for whom measures were also available. Thus, the study cohort comprised 127 “affected” families with at least 1 member with schizophrenia (n = 535 family members, including 160 schizophrenia cases) and a separate set of 30 control families (n = 121, total n = 656). The median family size was 4 (range 3–9, supplementary figure 1). Affected families had a median of 1 case per family, although 26 families were multiplex (2–5 cases per family, supplementary figure 2). Data were available in both the multiply affected families and the control families for all measures apart from the IT tasks, for which insufficient data were available in the control families.
Aim (a) to Comprehensively Evaluate for the First Time Both Cognitive and Personality Measures as Candidate Endophenotypes Against 4 of Gottesman and Gould’s 5 Criteria
Data Analysis.
All analyses were performed in R version 3.3.1.103 Pedigree analyses were performed using the “gap” package version 1.1-16.104 The kinship matrix was directly estimated from recorded pedigrees. All cognitive and personality measures were adjusted for age, sex and years of formal education; linear regression of each of the candidate endophenotypes on all 3 covariates was performed and the resulting residual statistics were each transformed to an (approximately) normal distribution using the “boxcox” function in the R package MASS version 7.3-45105 prior to analysis. Additional correction for NART and medication use in cases (chlorpromazine equivalence) was performed as a sensitivity analysis.
Multiple Testing Corrections.
Benjamini and Hochberg’s False Discovery Rate (FDR) correction106 was applied to account for multiple testing within each of Gottesman and Gould’s criteria (a–d), with q values <.05 considered statistically significant.
Post hoc Power Calculations.
As this sample was used in different ways to test each of the criteria (a–d), some tests were more powerful than others. To aid in the interpretation of the results of each test, we report post hoc power calculated for each test (at α = .05). The probability of estimating h2 (criterion b) and the genetic correlation (ρg, criterion c) greater than zero in this sample was estimated using the GCTA-GREML power calculator.107 The variance of the genetic relationships was set as the empirical variance of the off-diagonals of the GRM (in this case, 0.000356).
Power for detecting a difference between schizophrenia cases and their unaffected relatives (criterion a) and for difference between unaffected relatives and healthy controls (criterion d) was estimated using the R package “simr,” which conducts simulation-based power analysis for mixed models. Power was estimated using the existing family structures and sample sizes, for a range of effect size estimates.
Test of Criterion (a)—Significantly Associated With Schizophrenia.
We examined association with schizophrenia by testing for differences between schizophrenia cases and their unaffected family members for each cognitive and personality measure. Groups were compared using a maximum likelihood estimation model including the kinship matrix (the null model, describing only the relationship between the measure and genetic relatedness) to the same model including a variable differentiating cases from controls using log likelihood ratio tests. We had approximately 5% power to detect a between-group difference in the candidate endophenotypes of 0.01 SD, 27% power to detect a change of 0.05 SD, and 100% power to detect a change of 0.3 SD and above. Although the primary analysis for this criterion was a comparison of cases and their unaffected family members, a comparison of cases and control families is also reported for completeness.
Test of Criterion (b)—Significantly Heritable.
Total additive genetic (narrow sense) heritability is an estimate of the proportion of variability of a trait attributable to the additive effect of genes. The underlying variance-component model asserts that variation in the trait can be partitioned into genetic, known covariate, and environmental components. Each component can then be estimated. In this study, heritability estimation was performed for schizophrenia () and all cognitive and personality measures () using maximum likelihood variance-component estimation.108 The null hypothesis of no heritability was tested by comparing 2 maximum likelihood models: the sporadic model, which assumes no genetic effects (h2 = 0) and the polygenic model, which assumes that some fraction of the phenotypic variation is explained by genetic factors (in this case, the kinship matrix), using likelihood ratio tests. The heritability of schizophrenia was estimated on the continuous liability scale under the assumption of a normal threshold model from all affected families and corrected for ascertainment bias,109 as the families were recruited through a proband and are not representative of the general population (the proportion of schizophrenia cases among WAFSS family members was 24% compared to a lifetime morbid risk in the general population of 1%). For the candidate endophenotypes, this sample provided 68% power to detect ≠ 0 assuming a true of 0.3, 99% power assuming a true of 0.4, and 90% power assuming a true of 0.5.
Test of Criterion (c)—Significantly Genetically Correlated With Schizophrenia.
The proportion of the phenotypic correlation between schizophrenia and each of the cognitive or personality measures which was attributable to shared genetic effects (ρg) can be estimated by decomposing Pearson’s r into ρg and ρe, where ρg is the proportion of variability due to shared genetic effects, and ρe is the proportion of variability due to shared environmental effects. Genetic correlation with schizophrenia was calculated for all measures which were significantly heritable using maximum likelihood variance-component estimation, comparing the sporadic model (assuming no genetic effects, ρg = 0) and the polygenic model (maximized without constraint of ρg) using likelihood ratio tests.
Power to detect ρg ≠ 0 between schizophrenia (binary) and the quantitative trait candidate endophenotypes, assuming = 0.5 and = 0.8 (on the liability scale; the actual estimate in this sample) was as follows: 41% power to detect genetic correlation of 0.2, 78% power to detect genetic correlation of 0.3 and 98% power to detect genetic correlation of 0.4.
Test of Criterion (d)—Significantly Different Between Unaffected Relatives and Control Families.
Group differences between unaffected relatives and control families were examined using the same methods as criterion (a) above. As the comparison group (control families) was slightly smaller than for criterion (a) (schizophrenia cases), power was lower for this analysis, with approximately 1% power to detect a between-group difference in the candidate endophenotypes of 0.01 SD, 5% power to detect a change of 0.05 SD, 81% power to detect a change of 0.5 SD and 100% power to detect a change of 0.6 SD and above.
Aim (b) to Rank the Strength of the Evidence for Cognitive and Personality Measures as Candidate Endophenotypes in Relation to Each Other Using the ERV
The ERV was developed by Glahn et al10 to rank candidate endophenotypes using both heritability and genetic correlation, therefore incorporating both the strength of the genetic signal for the endophenotype and its relationship to the disorder of interest into a quantitative measure of the strength of the evidence for the candidate endophenotype. The ERV describes the standardized genetic covariance with values between 0 and 1, where higher values indicate that the candidate endophenotype and the illness are more strongly influenced by shared genetic factors. It is calculated as the absolute value of the square-root of the heritability of schizophrenia (), multiplied by the square-root of the heritability of the candidate endophenotype () multiplied by the genetic correlation between them: .
Aim (c) to Examine the Relationship Between Cognitive and Personality Measures; Correlations and Factor Analysis
The phenotypic and genetic correlations between candidate endophenotypes with significant heritability and genetic correlation were calculated from the entire cohort. Phenotypic correlations were adjusted for relatedness using maximum likelihood estimation to incorporate the kinship matrix. Genetic correlations between candidate endophenotypes were calculated using maximum likelihood variance-component estimation, as described above.
Very Simple Structure and Parallel analysis were used to determine the optimal number of factors to extract using the “vss” and “factpar” commands in the R package “psych.”110 Factor analysis was performed using Maximum Likelihood Factor Analysis entering raw data and extracting the user-specified number of factors, with varimax rotation using the “factanal” command in the R package “stats.”103
Results
Aim (a) to Comprehensively Evaluate for the First Time Both Cognitive and Personality Measures as Candidate Endophenotypes Against 4 of Gottesman and Gould’s 5 Criteria
Test of Criterion (a)—Significantly Associated With Schizophrenia.
Cases were significantly younger, more likely to be male and had significantly lower educational attainment compared to their unaffected family members and healthy control families, and all of the above were included as covariates in all analyses. Cases showed significant impairment compared to their unaffected family members across all measures (q < .05, Table 1) apart from P, which did not differ significantly between groups.
Table 1.
Cognitive and Personality Traits Distribution and Heritability in WAFSS Participants
| Characteristics | Cases (n = 160, 25%) | Relatives (n = 375, 57%) | Controls (n = 121, 18%) | P (Cases vs Rels) | P (Cases vs Controls) | P (Rels vs Control) | ||
|---|---|---|---|---|---|---|---|---|
| Male sex (n, %) | 121 (75%) | 176 (47%) | 61 (50%) | 1.0E-08 | 7.3E-04 | .57 | ||
| Age at assessment (years) (mean, SD) | 34.0 (10.8) | 42.5 (22.2) | 39.8 (15.6) | 4.7E-15 | 2.6E-05 | .16 | ||
| Education (years formal) (mean, SD) | 11.2 (2.2) | 11.8 (3.0) | 12.8 (2.7) | .03 | 7.20E-09 | 2.2E-03 | ||
| Cognitive and personality traits | Cases (n = 160, 25%) | Relatives (n = 375, 57%) | Controls (n = 121, 18%) | q (cases vs rels) | q (cases vs controls) | h 2 | q | q (rels vs control) |
| NART IQ (mean, SD) | 99.5 (11.1) | 104.7 (10.8) | 106.6 (8.7) | 3.1E-02 | 1.9E-03 | 0.48 | 3.4E-03 | .043 |
| SILS IQ (mean, SD) | 93.4 (14.8) | 104.5 (11.8) | 108.9 (8.3) | 5.3E-07 | 1.0E-10 | 0.44 | 0.012 | 5.4E-03 |
| RAVLT-IW (mean, SD) | 21.5 (6.6) | 25.9 (6.0) | 27.2 (5.2) | 7.7E-09 | 4.7E-09 | 0.60 | 1.3E-03 | .105 |
| RAVLT-DW (mean, SD) | 6.2 (3.1) | 8.3 (3.2) | 9.06 (2.8) | 4.7E-08 | 1.2E-08 | 0.41 | 5.4E-03 | .094 |
| IT block A (mean, SD) | 69.6 (107.9) | 47.9 (28.0) | — | 1.2E-05 | — | 0.55 | 6.3E-03 | — |
| IT block B (mean, SD) | 62.4 (96.7) | 42.5 (34.1) | — | 1.0E-05 | — | 0.60 | 3.1E-03 | — |
| CPT-IP (mean, SD) | 3.13 (1.7) | 3.99 (1.5) | 4.88 (1.6) | 2.7E-08 | 9.0E-11 | 0.43 | .011 | .022 |
| CPT-DS (mean, SD) | 4.50 (1.5) | 5.31 (1.2) | 5.62 (1.19) | 1.8E-10 | 2.2E-04 | 0.14 | .574 | — |
| COWAT (FAS version) (mean, SD) | 30.7 (10.4) | 37.0 (11.9) | 38.0 (9.9) | 2.3E-04 | 4.8E-05 | 0.16 | .307 | — |
| EHI (lq) (mean, SD) | 51.7 (53.0) | 62.7 (53.9) | 72.0 (37.8) | 4.9E-03 | 8.6E-05 | 0.24 | .211 | — |
| SPQ cognitive-perceptual (mean, SD) | 12.4 (9.2) | 2.8 (4.4) | 2.5 (4.3) | 6.1E-18 | 7.4E-11 | 0.59 | 5.9E-04 | .094 |
| SPQ interpersonal (mean, SD) | 9.9 (7.1) | 3.9 (4.9) | 3.6 (4.7) | 2.3E-13 | 5.2E-09 | 0.47 | 6.9E-03 | .102 |
| SPQ disorganization (mean, SD) | 5.8 (4.8) | 1.8 (2.8) | 2.0 (3.2) | 9.4E-12 | 3.4E-07 | 0.52 | 5.2E-03 | .119 |
| TCI cooperativeness (mean, SD) | 30.5 (7.1) | 36.0 (4.5) | 34.0 (5.4) | 1.1E-06 | 0.023 | 0.73 | .012 | .023 |
| TCI self-directedness (mean, SD) | 26.5 (7.6) | 35.9 (6.7) | 33.9 (6.0) | 3.7E-13 | 7.9E-05 | 0.28 | .168 | — |
| TCI persistance (mean, SD) | 4.4 (1.6) | 4.4 (1.9) | 4.0 (2.0) | .276 | .089 | — | — | — |
| TCI self-transcendence (mean, SD) | 18.4 (7.7) | 11.2 (6.3) | 10.4 (6.9) | 4.1E-11 | 6.9E-06 | 0.31 | .149 | — |
| TCI reward dependence (mean, SD) | 14.0 (3.9) | 16.1 (3.7) | 16.1 (3.5) | 7.0E-04 | 4.3E-03 | 0.21 | .223 | — |
| TCI novelty seeking (mean, SD) | 18.9 (5.3) | 17.9 (5.9) | 18.1 (6.2) | .015 | .024 | 0.16 | .322 | — |
| TCI harm avoidance (mean, SD) | 18.4 (7.5) | 13.3 (6.5) | 14.1 (5.8) | 4.6E-09 | 6.0E-05 | 0.07 | .689 | — |
Note: NART, National Adult Reading Test IQ; SILS, Shipley Institute of Living Scale IQ; RAVLT-IW, Rey Auditory Verbal Learning Test immediate word recall; RAVLT-DW, Rey Auditory Verbal Learning Test delayed word recall; IT, inspection time; CPT-IP, Continuous Performance Task identical pairs; CPT-DS Continuous Performance Task degraded stimulus; COWAT, controlled oral word association test; EHI, Edinburgh Handedness Index; SPQ, Schizotypal Personality Questionnaire; TCI, Temperament and Character Inventory. Trait distribution in schizophrenia cases, their unaffected relatives and control families free of psychopathology. Residuals of cognitive and personality measures after regression of age, sex, and years of formal education were used and transformed to an (approximately) normal distribution prior to analysis. q values are analogous to P values that incorporate FDR-based multiple testing correction. q values which are significant after FDR-correction at α = .05 are shown in bold. Only significantly heritable traits were assessed for genetic correlation (comparison between relatives and healthy controls).
To control for the possibility that the between-group differences in the cognitive tests were simply reflective of higher IQ in the controls, the tests for group differences were repeated adjusting for NART (supplementary table 1). All of the significant differences shown in Table 1 were recapitulated in this sensitivity analysis.
Test of Criterion (b)—Significantly Heritable.
The additive heritability of schizophrenia after correction for ascertainment was estimated at = 0.80). Eleven of the candidate endophenotypes showed significant heritability in this sample, and some measures of both cognition (IT tests and RAVLT-IW) and personality (SPQ disorganization, SPQ cognitive-perceptual and C) had particularly high estimates ( > 0.5, Table 2). Only measures which were significantly heritable and associated with schizophrenia were assessed for genetic correlation in the following 2 sections.
Table 2.
Endophenotype Criteria Met
| Associated With Schizophrenia | Heritable | Co-segregates With Schizophrenia | Relatives Intermediate | |
|---|---|---|---|---|
| NART IQ | ✓ | ✓ | ✓ | ✓ |
| SILS IQ | ✓ | ✓ | ✓ | ✓ |
| RAVLT-IW | ✓ | ✓ | ✓ | ✗ |
| RAVLT-DW | ✓ | ✓ | ✓ | ✗ |
| IT block A | ✓ | ✓ | ✓ | — |
| IT block B | ✓ | ✓ | ✓ | — |
| CPT-IP | ✓ | ✓ | ✓ | ✓ |
| CPT-DS | ✓ | ✗ | — | — |
| COWAT (FAS version) | ✓ | ✗ | — | — |
| EHI (lq) | ✓ | ✗ | — | — |
| SPQ cognitive-perceptual | ✓ | ✓ | ✓ | ✗ |
| SPQ interpersonal | ✓ | ✓ | ✓ | ✗ |
| SPQ disorganization | ✓ | ✓ | ✓ | ✗ |
| TCI cooperativeness | ✓ | ✓ | ✓ | ✗ |
| TCI self-directedness | ✓ | ✗ | — | — |
| TCI persistence | ✗ | ✗ | — | — |
| TCI self-transcendence | ✓ | ✗ | — | — |
| TCI reward dependence | ✓ | ✗ | — | — |
| TCI novelty seeking | ✓ | ✗ | — | — |
| TCI harm avoidance | ✓ | ✗ | — | — |
Note: Check marks indicate that FDR-corrected significance was achieved and the direction of effect was as hypothesized. Measures which were not significantly both associated with schizophrenia and heritable were not assessed against the 2 measures of genetic correlation (indicated by “—”). As no data were available for the inspection time task in the control families, the assessment of whether relatives were intermediate between cases and controls was not available for the IT tasks. Abbreviations as shown in Table 1.
Test of Criterion (c)—Significantly Genetically Correlated With Schizophrenia.
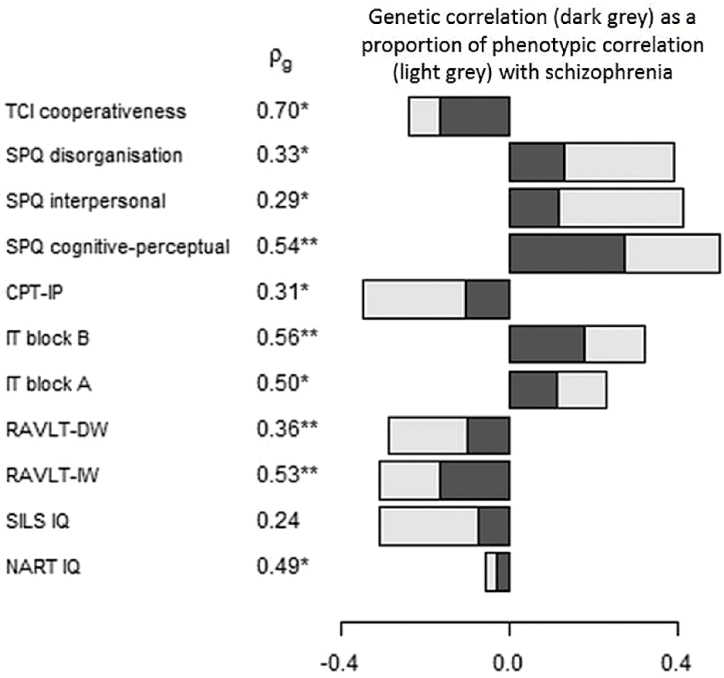
Ten of the 11 significantly associated and heritable traits also showed significant genetic correlation with schizophrenia liability in the co-segregation analysis (q < .05, figure 1) and the magnitude of genetic correlation largely mirrored that of heritability.
Fig. 1.
Genetic correlation (ρg) between candidate endophenotypes and schizophrenia. Only significantly heritable traits were assessed for genetic correlation. The genetic correlation (dark grey) as a proportion of the phenotypic correlation (Pearson’s r) with schizophrenia (light grey) is shown. *q < .05, **q < .01. Other abbreviations as shown in Table 1.
Test of Criterion (d)—Significantly Different Between Unaffected Relatives and Control Families.
Unaffected family members were intermediate between cases and controls for all of the cognitive traits, and showed significant impairment compared to the control families in the CPT-IP, NART, and SILS IQ (q < .05, Table 1). For the personality measures, unaffected family members and controls were only significantly different for C, where unaffected family members had higher mean scores than both their affected family members and control families.
Summary of Endophenotype Criteria Met.
Two of the candidate endophenotypes, CPT-IP and NART, met all 4 criteria for being an endophenotype (Table 2). Eight measures, including many of the traits with the highest ERV scores, met 3 of the 4 criteria but did not differ significantly between unaffected family members and healthy controls in the expected direction.
Aim (b) to Rank the Strength of the Evidence for Cognitive and Personality Measures as Candidate Endophenotypes in Relation to Each Other Using the ERV
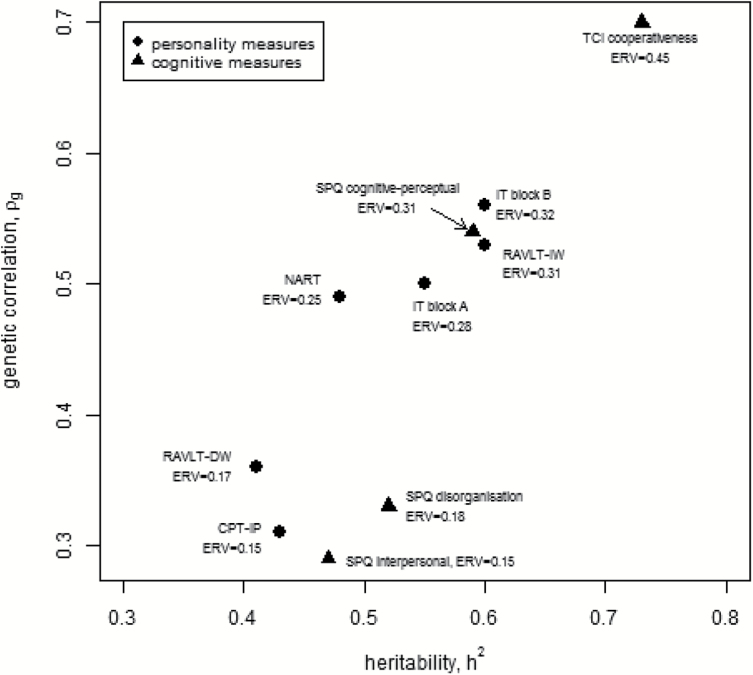
When traits were ranked according to their ERV, RAVLT-IW, both IT tasks, NART premorbid IQ, SPQ cognitive-perceptual and C had the highest scores (ERV > 0.20, figure 2).
Fig. 2.
Traits with significant heritability and genetic correlation with schizophrenia with their endophenotype ranking variable (ERV). Personality traits are shown as triangles, cognitive traits as circles. TCI, Temperament and Character Inventory; SPQ, Schizotypal Personality Questionnaire; CPT-IP, Continuous Performance Task identical pairs; IT, inspection time; RAVLT-DW, Rey Auditory Verbal Learning Test delayed word recall; RAVLT-IW, Rey Auditory Verbal Learning Test immediate word recall; NART, National Adult Reading Test IQ.
Aim (c) To Examine the Relationship Between Cognitive and Personality Measures; Correlations and Factor Analysis
The phenotypic and genetic correlations between all traits with significant heritability and genetic correlation with schizophrenia are shown in supplementary figure 3. There were moderate-strong positive phenotypic correlations among most of the cognitive measures (although less consistent for IT and EHI), and within the SPQ factors. Phenotypic correlations between the cognitive and the personality measures were low, and high genetic correlations indicate that what phenotypic correlations exist are largely driven by a shared genetic component. Genetic correlations between most of the cognitive traits were substantial, especially for premorbid IQ.
Factor analysis was performed for all individuals in the affected families (unaffected families were not included due to lack of IT measures) with complete data across the 10 traits which had significant association with schizophrenia, heritability and genetic correlation (n = 253, including 82 schizophrenia cases). Very Simple Structure and Parallel analysis suggested that the optimal number of factors to extract from this dataset was 3. Maximum Likelihood Factor Analysis was then performed specifying the number of factors as 3 (Table 3). The cumulative variability explained by the 3-factor model is modest, at 0.50. Factor 1, which explains the most variance, is heavily loaded on the 3 SPQ domains, whereas Factors 2 and 3 are driven by cognitive measures—primarily the RAVLT scores (Factor 2), and the IT scores (Factor 3). All 3 factors were strongly and significantly heritable (h2~0.6–0.90, P < .01, Table 3). Phenotypic correlations among factors indicated that the 3 factors were independent of each other and what little correlation was found between them was largely due to shared genetic factors (supplementary table 2).
Table 3.
Factor Analysis of Cognitive and Personality Traits
| Factor 1 | Factor 2 | Factor 3 | |
|---|---|---|---|
| NART IQ | 0.013 | 0.441 | 0.004 |
| CPT-IP | 0.219 | 0.272 | 0.148 |
| RAVLT-IW | 0.110 | 0.854 | 0.211 |
| RAVLT-DW | 0.149 | 0.857 | 0.204 |
| IT block A | 0.144 | 0.206 | 0.581 |
| IT block B | 0.141 | −0.054 | 0.986 |
| SPQ cognitive-perceptual | 0.838 | 0.073 | 0.112 |
| SPQ interpersonal | 0.749 | 0.038 | 0.140 |
| SPQ disorganization | 0.832 | −0.072 | 0.072 |
| TCI cooperativeness | 0.347 | 0.196 | 0.058 |
| SS loadings | 2.197 | 1.881 | 1.460 |
| Proportion Var | 0.200 | 0.171 | 0.133 |
| Cumulative Var | 0.200 | 0.371 | 0.504 |
| Heritability | 0.826 | 0.687 | 0.675 |
Note: TCI, Temperament and Character Inventory; SPQ, Schizotypal Personality Questionnaire; CPT-IP, Continuous Performance Task identical pairs; IT, inspection time; RAVLT-DW, Rey Auditory Verbal Learning Test delayed word recall; RAVLT-IW, Rey Auditory Verbal Learning Test immediate word recall; NART, National Adult Reading Test IQ. Loadings >0.1 are shown in bold. All heritabilities were significant at P < .01. Chi-square test of the hypothesis that 3 factors are sufficient: 49.59 on 25 degrees of freedom, P = .00239.
Discussion
This study reports for the first time a thorough assessment of cognitive and personality measures as candidate endophenotypes against 4 of the criteria suggested by Gottesman and Gould; a systematic prioritizing of the strongest endophenotypes using the ERV and an examination of the underlying structure of this group of candidate endophenotypes using factor analysis.
One of the strengths of this study was in the use of a large sample of families multiply affected by schizophrenia, which allows us to directly examine the co-segregation (genetic correlation) of schizophrenia and candidate endophenotypes (criteria c), in addition to implying genetic correlation by comparing unaffected relatives with healthy controls (criteria d). Previous studies have largely relied on group comparisons (criteria d) to infer genetic correlation. In addition, our family sample meant that we could infer the degree of genetic similarity between individuals in its entirety from the pedigree structure, meaning that our estimates of heritability of the candidate endophenotypes and their genetic correlation with schizophrenia will be more complete than studies which use SNP-based genetic relationships, representing only part of the genetic similarity between individuals.
Most of the cognitive and personality measures in this study have previously been shown to be associated with schizophrenia; therefore it was unsurprising that group differences between cases and unaffected relatives were significant for all but P in this study. The direction of associations were all in line with previous studies—cognition was impaired in cases, cases scored higher in all 3 SPQ domains and the group differences in TCI domains were in concordance with those previously reported,40,88–91 with particularly significant increased HA and ST and decreased SD in cases. Adjustment of group differences in specific cognitive tests for a generalized cognitive deficit was not performed in the main analysis because this generalized cognitive deficit is a core feature of schizophrenia. As noted by Miller and Chapman,111 adjustment for a covariate which is closely related to the independent variable of interest is inappropriate, as the removal of variance due to the covariate would remove considerable variance in the independent variable of interest. We did perform a sensitivity analyses which showed that the between-group differences were robust to correction for NART and medication use in cases. A limitation of these analyses is that we had insufficient data to adjust for some potential confounders, such as drug and alcohol misuse, although in the limited data available these potential confounders were not correlated with the candidate endophenotypes measured.
Heritability estimations for the cognitive traits in the WAFSS were in keeping with previous reports (h2~0.4–0.8). We show for the first time substantial heritability estimates for all 3 SPQ factors in a clinical cohort, in keeping with a previous report in healthy adolescents.81 Our heritability estimates for the TCI were in a similar range to those reported previously (h2~0.3–0.45).86–87 Heritability estimates of C, HA, and ST were significant in Korean families,86 whereas only C was significantly heritable in the WAFSS.
The proportion of phenotypic correlation between schizophrenia and the candidate endophenotypes which was due to shared genetic effects (the genetic correlation with schizophrenia) calculated using variance components analysis was particularly high for NART IQ, RAVLT-IW, and IT, in keeping with a previous variance components analysis showing high genetic correlation with schizophrenia for measures of memory and IQ.29 All 3 SPQ factors showed significant genetic correlation with schizophrenia using variance components analysis. To our knowledge, this represents the first finding of genetic correlation between SPQ measures and schizophrenia using co-segregation analysis. Within the TCI domains, only the negative phenotypic correlation between C and schizophrenia exhibited a significant genetic component, although this is difficult to interpret in the context of the mean C for unaffected relatives not being intermediate between patients and controls (discussed below).
Group differences in the candidate endophenotypes between unaffected relatives of people with schizophrenia and controls, indicative of the measure being genetically correlated with schizophrenia, were significant for NART, SILS, CPT-IP, and C. Previous studies have shown differences in most of the cognitive measures examined in this study.30 Previous reports of differences in schizotypy62 between unaffected relatives and controls were not replicated, although the interpersonal and cognitive-perceptual factors showed trends in the expected direction, consistent with previous findings of elevation of these factors among unaffected relatives, with less consistent results for disorganized symptoms.62 Differences between unaffected relatives and healthy controls in HA, ST, and C40,86 were not replicated in this study. There was a significant difference in C between unaffected relatives and healthy controls; unaffected relatives had higher scores than both patients and healthy controls which has been shown previously.35,40 It is likely to be an environmental rather than a genetic effect, as the unaffected relatives are not, in this case, intermediate between their affected relatives and healthy controls.
Only CPT-IP, a measure of sustained attention, and NART, a measure of general cognitive ability, met all 4 criteria proposed by Gottesman and Gould assessed in this study. However, all 3 SPQ domains, both RAVLT measures and both IT measures showed evidence for genetic correlation with schizophrenia in the co-segregation analysis. Group comparisons between unaffected relatives and controls were not available for the IT measures as the IT tests were not performed in the control families.
A limitation of this study was the modest size of the sample of control families, which meant that the different methods employed in this study to assess the endophenotype criteria did not have equal power, as outlined in the methods section. For the effect sizes observed in this study, the power for detection of genetic correlation using co-segregation analysis in all the affected families was higher than the power to detect group differences between unaffected family members and healthy controls, which is slightly underpowered compared to the other tests due to the relatively small number of healthy control families available for inclusion in this study. The differences in power may account for the fact that the genetic correlation assessed by variance components analysis was more pronounced in this study than the genetic correlation suggested by group differences, although most of the traits show a non-significant trend towards unaffected family members being intermediate between patients with schizophrenia and healthy controls. Despite the small differences in power between the different tests of the endophenotype criteria, we had good power to assess all 4 of the different criteria for being an endophenotype in this study for all but very small effects, meaning that we can presume candidate endophenotypes which did not show evidence for genetic correlation with schizophrenia in the group comparison do not have particularly high genetic correlation with schizophrenia in this sample.
Both NART and the CPT-IP have been consistently shown to share a genetic basis with schizophrenia25–27,29,30,112 and in a recent meta-analysis, both NART and CPT-IP measures had substantial effect sizes when comparing unaffected relatives with controls. A recent paper from the Consortium on the Genetics of Schizophrenia (COGS) study113 reported that CPT-IP deficits in schizophrenia could be reliably detected across 5 sites in the COGS study despite significant site differences in participant age, sex, education, and racial distribution; deficits were relatively independent of current symptom severity but rather, related to functional capacity. Although cognitive measures are by no means the only promising endophenotypes for schizophrenia, within the neurocognitive literature there is increasing evidence that the CPT-IP is one of the strongest candidate endophenotypes. A recent study of 16 endophenotypes (15 neurocognitive, 1 neurophysiological) in the COGS study reported a model including 4 important endophenotypes: CPT-IP, the California Verbal Learning Test, emotion identification and the antisaccade task, had the same power to discriminate between schizophrenia cases and healthy controls as the model including all 16 endophenotypes (84% vs 85% accuracy14).
A novel aspect of this study was the examination of personality measures as endophenotypes and in ranking them compared to cognitive measures using the ERV. We show for the first time that ERVs for the top ranked personality traits and cognitive traits are similar in magnitude. The top ranked cognitive measures in this study were verbal learning and memory, sustained attention and premorbid IQ. This is in keeping with ERV scores reported by Glahn et al15 for a different cognitive battery which showed that measures of verbal learning and memory, sustained attention, speed of information processing, and general IQ had high ERV scores. The fact that SPQ cognitive-perceptual had an ERV score equivalent to these top cognitive measures suggests that it should be considered an equivalently promising endophenotype for schizophrenia.
In addition, our dataset enabled us to examine the relationship between cognition and personality traits for the first time. There were no significant phenotypic correlations between personality and cognitive measures, reaffirming previous reports63,64,66 including previous work in the WAFSS17 showing that these measures are largely independent of each other. Genetic correlations between personality and cognitive traits however were high; indicating that what little phenotypic correlation exists between them is largely due to a shared genetic contribution. The substantial genetic correlation we observed among most of the cognitive measures, especially with IQ, has been shown previously.25,27,28,31 Previous data suggest that the positive and negative facets of schizotypy are influenced genetically by 2 distinct latent genetic factors,58 which is supported by the moderate genetic correlations between the 3 domains in this study (rg < .37–.45, supplementary figure 3).
The results of the factor analysis incorporating personality measures extend previous work showing that cognitive deficits are separable into distinct factors. Factor analysis in the COGS cognitive battery23 showed distinct factors with moderate phenotypic and high genetic correlation with each other. By comparison, we demonstrate much lower phenotypic correlation between factors, likely due to the fact that we included both cognitive and personality factors. This study also confirmed the finding that verbal memory and processing speed are largely uncorrelated and contribute to 2 distinct factors among cognitive batteries.114 Data from the Dunedin cohort study have shown varying longitudinal trajectories in those who go on to develop schizophrenia, with functions which reflect processing speed deteriorating over time, but little evidence of a decline in functions reflecting verbal memory.115
In summary, the richly phenotyped, familial WAFSS cohort is one of the few suitable for a comprehensive analysis of cognitive and personality traits as candidate endophenotypes for schizophrenia. We demonstrate that the strength of genetic support for personality traits as endophenotypes is broadly equivalent to that of cognitive traits, and factor analysis showed that both personality and cognitive traits contribute to independent latent factors. The recent development of the ERV facilitated a systematic ranking of these traits for the first time. Future genetic studies incorporating highly ranked cognitive and personality endophenotypes will hopefully aid in identifying latent genetic variants previously missed due to the heterogeneity of the neurobiological disorders subsumed under the clinical diagnosis of schizophrenia. However, sufficiently powered studies including large samples with these measures will be necessary to perform these analyses.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
National Institutes of Mental Health Co-operative Research Grant (1U01MH105632 to D.C.G. and J.B., 2015–2019); National Health and Medical Research Council Australia (APP1064582 to A.J., E.M., V.A.M., J.C.B., N.S.M., and P.M., 2014–2016); Society for Mental Health Research (SMHR) Postdoctoral Fellowship (to N.S.M., 2016).
Supplementary Material
Acknowledgments
We would like to thank all of the participants in the Western Australian Family Study of Schizophrenia. Support from the Cooperative Research Centre for Mental Health Australia and the Medical Research Foundation, Royal Perth Hospital is gratefully acknowledged. All authors declare that they have no conflicts of interest.
References
- 1. Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. [DOI] [PubMed] [Google Scholar]
- 3. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 5. Glahn DC, Knowles EE, McKay DR, et al. Arguments for the sake of endophenotypes: examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. Am J Med Genet. 2001;105:42–44. [PubMed] [Google Scholar]
- 7. Blangero J. Localization and identification of human quantitative trait loci: king harvest has surely come. Curr Opin Genet Dev. 2004;14:233–240. [DOI] [PubMed] [Google Scholar]
- 8. Blangero J, Williams JT, Almasy L. Novel family-based approaches to genetic risk in thrombosis. J Thromb Haemost. 2003;1:1391–1397. [DOI] [PubMed] [Google Scholar]
- 9. Williams JT, Blangero J. Power of variance component linkage analysis-II. Discrete traits. Ann Hum Genet. 2004;68:620–632. [DOI] [PubMed] [Google Scholar]
- 10. Glahn DC, Curran JE, Winkler AM, et al. High dimensional endophenotype ranking in the search for major depression risk genes. Biol Psychiatry. 2012;71:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams JT, Van Eerdewegh P, Almasy L, Blangero J. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. I. Likelihood formulation and simulation results. Am J Hum Genet. 1999;65:1134–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Pei Y, Papasian CJ, Deng HW. Bivariate association analyses for the mixture of continuous and binary traits with the use of extended generalized estimating equations. Genet Epidemiol. 2009;33:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weickert CS, Weickert TW, Pillai A, Buckley PF. Biomarkers in schizophrenia: a brief conceptual consideration. Dis Markers. 2013;35:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Millard SP, Shofer J, Braff D, et al. Prioritizing schizophrenia endophenotypes for future genetic studies: an example using data from the COGS-1 family study. Schizophr Res. 2016;174:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glahn DC, Williams JT, McKay DR, et al. Discovering schizophrenia endophenotypes in randomly ascertained pedigrees. Biol Psychiatry. 2015;77:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiat. 2012;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hallmayer JF, Kalaydjieva L, Badcock J, et al. Genetic evidence for a distinct subtype of schizophrenia characterized by pervasive cognitive deficit. Am J Hum Genet. 2005;77:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 19. Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–691. [DOI] [PubMed] [Google Scholar]
- 20. Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophr Res. 2005;78:35–43. [DOI] [PubMed] [Google Scholar]
- 21. Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiat. 2004;56:301–307. [DOI] [PubMed] [Google Scholar]
- 23. Seidman LJ, Hellemann G, Nuechterlein KH, et al. Factor structure and heritability of endophenotypes in schizophrenia: findings from the Consortium on the Genetics of Schizophrenia (COGS-1). Schizophr Res. 2015;163:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. [DOI] [PubMed] [Google Scholar]
- 25. Edmonds CJ, Isaacs EB, Visscher PM, et al. Inspection time and cognitive abilities in twins aged 7 to 17 years: age-related changes, heritability and genetic covariance. Intelligence. 2008;36:210–225. [Google Scholar]
- 26. Lee SH, DeCandia TR, Ripke S, et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012;44:247–U235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luciano M, Smith GA, Wright MJ, et al. On the heritability of inspection time and its covariance with IQ: a twin study. Intelligence. 2001;29:443–457. [Google Scholar]
- 28. Greenwood TA, Braff DL, Light GA, et al. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toulopoulou T, Goldberg TE, Mesa IR, et al. Impaired intellect and memory: a missing link between genetic risk and schizophrenia?Arch Gen Psychiatry. 2010;67:905–913. [DOI] [PubMed] [Google Scholar]
- 30. Snitz BE, Macdonald AW III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aukes MF, Alizadeh BZ, Sitskoorn MM, Kemner C, Ophoff RA, Kahn RS. Genetic overlap among intelligence and other candidate endophenotypes for schizophrenia. Biol Psychiatry. 2009;65:527–534. [DOI] [PubMed] [Google Scholar]
- 32. Bleuler E. Dementia Praecox. The Group of Schizophrenias. New York, NY: International Universities Press; 1911. [Google Scholar]
- 33. Kraepelin E. Psychiatrie, vol. 3 8th ed Leipzig, Germany: Barth; 1913. (Translation: Dementia praecox and paraphrenia. Edinburgh: Livingstone (1919)). [Google Scholar]
- 34. Andersen AM, Bienvenu OJ. Personality and psychopathology. Int Rev Psychiatry. 2011;23:234–247. [DOI] [PubMed] [Google Scholar]
- 35. Bora E, Veznedaroglu B. Temperament and character dimensions of the relatives of schizophrenia patients and controls: the relationship between schizotypal features and personality. Eur Psychiatry. 2007;22:27–31. [DOI] [PubMed] [Google Scholar]
- 36. Camisa KM, Bockbrader MA, Lysaker P, Rae LL, Brenner CA, O’Donnell BF. Personality traits in schizophrenia and related personality disorders. Psychiatry Res. 2005;133:23–33. [DOI] [PubMed] [Google Scholar]
- 37. Glatt SJ, Stone WS, Faraone SV, Seidman LJ, Tsuang MT. Psychopathology, personality traits and social development of young first-degree relatives of patients with schizophrenia. Br J Psychiatry. 2006;189:337–345. [DOI] [PubMed] [Google Scholar]
- 38. Kentros M, Smith TE, Hull J, McKee M, Terkelsen K, Capalbo C. Stability of personality traits in schizophrenia and schizoaffective disorder: a pilot project. J Nerv Ment Dis. 1997;185:549–555. [DOI] [PubMed] [Google Scholar]
- 39. Kurs R, Farkas H, Ritsner M. Quality of life and temperament factors in schizophrenia: comparative study of patients, their siblings and controls. Qual Life Res. 2005;14:433–440. [DOI] [PubMed] [Google Scholar]
- 40. Smith MJ, Cloninger CR, Harms MP, Csernansky JG. Temperament and character as schizophrenia-related endophenotypes in non-psychotic siblings. Schizophr Res. 2008;104:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lönnqvist JE, Verkasalo M, Haukka J, et al. Premorbid personality factors in schizophrenia and bipolar disorder: results from a large cohort study of male conscripts. J Abnorm Psychol. 2009;118:418–423. [DOI] [PubMed] [Google Scholar]
- 42. Grant P. Is schizotypy per se a suitable endophenotype of schizophrenia? - Do not forget to distinguish positive from negative facets. Front Psychiatry. 2015;6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCreery C, Claridge G. Healthy schizotypy: the case of out-of-the-body experiences. Pers Indiv Differ. 2002;32:141–154. [Google Scholar]
- 44. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 45. Cloninger CR, Przybeck TR, Svrakic DM.. The Temperament and Character Inventory (TCI): A Guide to its Development and Use. St. Louis, MO: Center for Psychobiology of Personality, Washington University; 1994:19–28. [Google Scholar]
- 46. RADO S. Dynamics and classification of disordered behavior. Am J Psychiatry. 1953;110:406–416. [DOI] [PubMed] [Google Scholar]
- 47. Meehl PE. Toward an integrated theory of schizotaxia, schizotypy, and schizophrenia. J Pers Dis. 1990;4:1–99. [Google Scholar]
- 48. Nelson MT, Seal ML, Pantelis C, Phillips LJ. Evidence of a dimensional relationship between schizotypy and schizophrenia: a systematic review. Neurosci Biobehav Rev. 2013;37:317–327. [DOI] [PubMed] [Google Scholar]
- 49. Claridge G, Beech T. Fully and quasi-dimensional constructions of schizotypy. Schizotypal Personality 1995;29:192–216. [Google Scholar]
- 50. Linscott RJ, van Os J. Systematic reviews of categorical versus continuum models in psychosis: evidence for discontinuous subpopulations underlying a psychometric continuum. Implications for DSM-V, DSM-VI, and DSM-VII. Annu Rev Clin Psychol. 2010;6:391–419. [DOI] [PubMed] [Google Scholar]
- 51. Barrantes-Vidal N, Grant P, Kwapil TR. The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr Bull. 2015;41(suppl 2):S408–S416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cicero DC, Martin EA, Becker TM, Docherty AR, Kerns JG. Correspondence between psychometric and clinical high risk for psychosis in an undergraduate population. Psychol Assess. 2014;26:901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grant P, Kuepper Y, Mueller EA, Wielpuetz C, Mason O, Hennig J. Dopaminergic foundations of schizotypy as measured by the German version of the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE)-a suitable endophenotype of schizophrenia. Front Hum Neurosci. 2013;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mason O, Claridge G. The Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE): further description and extended norms. Schizophr Res. 2006;82:203–211. [DOI] [PubMed] [Google Scholar]
- 55. Macare C, Bates TC, Heath AC, Martin NG, Ettinger U. Substantial genetic overlap between schizotypy and neuroticism: a twin study. Behav Genet. 2012;42:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Claridge G, Hewitt JK. A biometrical study of schizotypy in a normal population. Pers Indiv Differ. 1987;8:303–312. [Google Scholar]
- 57. Kendler KS, Hewitt J. The structure of self-report schizotypy in twins. J Pers Dis Spr. 1992;6(1):1–17. [Google Scholar]
- 58. Linney YM, Murray RM, Peters ER, MacDonald AM, Rijsdijk F, Sham PC. A quantitative genetic analysis of schizotypal personality traits. Psychol Med. 2003;33:803–816. [DOI] [PubMed] [Google Scholar]
- 59. MacDonald AW III, Pogue-Geile MF, Debski TT, Manuck S. Genetic and environmental influences on schizotypy: a community-based twin study. Schizophr Bull. 2001;27:47–58. [DOI] [PubMed] [Google Scholar]
- 60. Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study. III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50:781–788. [DOI] [PubMed] [Google Scholar]
- 61. Tarbox SI, Pogue-Geile MF. A multivariate perspective on schizotypy and familial association with schizophrenia: a review. Clin Psychol Rev. 2011;31:1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Badcock JC, Clark ML, Pedruzzi RA, Morgan VA, Jablensky A. Intact speed of processing in a community-based sample of adults with high schizotypy: a marker of reduced psychosis risk?Psychiatry Res. 2015;228:531–537. [DOI] [PubMed] [Google Scholar]
- 63. Chun CA, Minor KS, Cohen AS. Neurocognition in psychometrically defined college Schizotypy samples: we are not measuring the “right stuff”. J Int Neuropsychol Soc. 2013;19:324–337. [DOI] [PubMed] [Google Scholar]
- 64. Daneluzzo E, Bustini M, Stratta P, Casacchia M, Rossi A. Schizotypal Personality Questionnaire and Wisconsin Card Sorting Test in a population of DSM-III-R schizophrenic patients and control subjects. Compr Psychiatry. 1998;39:143–148. [DOI] [PubMed] [Google Scholar]
- 65. Smoller JW, Craddock N, Kendler K, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jones HJ, Stergiakouli E, Tansey KE, et al. Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry. 2016;73:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zammit S, Hamshere M, Dwyer S, et al. A population-based study of genetic variation and psychotic experiences in adolescents. Schizophr Bull. 2014;40:1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hatzimanolis A, Bhatnagar P, Moes A, et al. Common genetic variation and schizophrenia polygenic risk influence neurocognitive performance in young adulthood. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liebers DT, Pirooznia M, Seiffudin F, Musliner KL, Zandi PP, Goes FS. Polygenic risk of schizophrenia and cognition in a population-based survey of older adults. Schizophr Bull. 2016;42:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McIntosh AM, Gow A, Luciano M, et al. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol Psychiatry. 2013;73:938–943. [DOI] [PubMed] [Google Scholar]
- 71. Lencz T, Knowles E, Davies G, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol Psychiatry. 2014;19:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fonseca-Pedrero E, Debbané M, Ortuño-Sierra J, et al. The structure of schizotypal personality traits: a cross-national study. Psychol Med. In press. [DOI] [PubMed] [Google Scholar]
- 73. Reynolds CA, Raine A, Mellingen K, Venables PH, Mednick SA. Three-factor model of schizotypal personality: invariance across culture, gender, religious affiliation, family adversity, and psychopathology. Schizophr Bull. 2000;26:603–618. [DOI] [PubMed] [Google Scholar]
- 74. Vollema MG, van den Bosch RJ. The multidimensionality of schizotypy. Schizophr Bull. 1995;21:19–31. [DOI] [PubMed] [Google Scholar]
- 75. Batey M, Furnham A. The relationship between measures of creativity and schizotypy. Pers Indiv Differ. 2008;45:816–821. [Google Scholar]
- 76. Mohr C, Claridge G. Schizotypy–do not worry, it is not all worrisome. Schizophr Bull. 2015;41(suppl 2):S436–S443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Abu-Akel A, Clark J, Perry A, et al. Autistic and schizotypal traits and global functioning in bipolar I disorder. J Affect Disord. 2017;207:268–275. [DOI] [PubMed] [Google Scholar]
- 78. Badcock JC, Dragovic M. Schizotypal personality in mature adults. Pers Indiv Differ. 2006;40:77–85. [Google Scholar]
- 79. Wuthrich VM, Bates TC. Confirmatory factor analysis of the three-factor structure of the schizotypal personality questionnaire and Chapman schizotypy scales. J Pers Assess. 2006;87:292–304. [DOI] [PubMed] [Google Scholar]
- 80. Fonseca-Pedrero E, Paino M, Lemos-Giráldez S, Sierra-Baigrie S, Muñiz J. Measurement invariance of the Schizotypal Personality Questionnaire-Brief across gender and age. Psychiatry Res. 2011;190:309–315. [DOI] [PubMed] [Google Scholar]
- 81. Ericson M, Tuvblad C, Raine A, Young-Wolff K, Baker LA. Heritability and longitudinal stability of schizotypal traits during adolescence. Behav Genet. 2011;41:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44:573–588. [DOI] [PubMed] [Google Scholar]
- 83. Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. [DOI] [PubMed] [Google Scholar]
- 84. Svrakic DM, Draganic S, Hill K, Bayon C, Przybeck TR, Cloninger CR. Temperament, character, and personality disorders: etiologic, diagnostic, treatment issues. Acta Psychiatr Scand. 2002;106:189–195. [DOI] [PubMed] [Google Scholar]
- 85. Ando J, Suzuki A, Yamagata S, et al. Genetic and environmental structure of Cloninger’s temperament and character dimensions. J Pers Disord. 2004;18:379–393. [DOI] [PubMed] [Google Scholar]
- 86. Lee BD, Park JM, Lee YM, et al. Heritability and familiality of temperament and character dimensions in Korean families with schizophrenic linkage disequilibrium. Clin Psychopharmacol Neurosci. 2016;14:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gillespie NA, Cloninger CR, Heath AC, Martin NG. The genetic and environmental relationship between Cloninger’s dimensions of temperament and character. Pers Individ Dif. 2003;35:1931–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guillem F, Bicu M, Semkovska M, Debruille JB. The dimensional symptom structure of schizophrenia and its association with temperament and character. Schizophr Res. 2002;56:137–147. [DOI] [PubMed] [Google Scholar]
- 89. Hori H, Noguchi H, Hashimoto R, et al. Personality in schizophrenia assessed with the Temperament and Character Inventory (TCI). Psychiatry Res. 2008;160:175–183. [DOI] [PubMed] [Google Scholar]
- 90. Cortés MJ, Valero J, Gutiérrez-Zotes JA, et al. Psychopathology and personality traits in psychotic patients and their first-degree relatives. Eur Psychiatry. 2009;24:476–482. [DOI] [PubMed] [Google Scholar]
- 91. Ohi K, Hashimoto R, Yasuda Y, et al. Personality traits and schizophrenia: evidence from a case-control study and meta-analysis. Psychiatry Res. 2012;198:7–11. [DOI] [PubMed] [Google Scholar]
- 92. Gonzalez-Torres MA, Inchausti L, Ibáñez B, et al. Temperament and character dimensions in patients with schizophrenia, relatives, and controls. J Nerv Ment Dis. 2009;197:514–519. [DOI] [PubMed] [Google Scholar]
- 93. Jablensky A. Subtyping schizophrenia: implications for genetic research. Mol Psychiatry. 2006;11:815–836. [DOI] [PubMed] [Google Scholar]
- 94. Maxwell ME. Manual for the FIGS. Maryland, Washington DC: National Institute of Mental Health; 1992. https://www.nimhgenetics.org/interviews/figs/figs_train.pdf. Accessed August 15, 2017. [Google Scholar]
- 95. Castle DJ, Jablensky A, McGrath JJ, et al. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med. 2006;36:69–80. [DOI] [PubMed] [Google Scholar]
- 96. Nelson HE, Willison JR.. The Revised National Adult Reading Test–Test Manual. Windsor, UK: NFER-Nelson; 1991. [Google Scholar]
- 97. Zachary RA, Shipley WC.. Shipley Institute of Living Scale: Revised Manual. Los Angeles, USA: WPS, Western Psychological Services; 1986. [Google Scholar]
- 98. Shapiro DM, Harrison DW. Alternate forms of the AVLT: a procedure and test of form equivalency. Arch Clin Neuropsychol. 1990;5:405–410. [PubMed] [Google Scholar]
- 99. Nuechterlein KH. Signal detection in vigilance tasks and behavioral attributes among offspring of schizophrenic mothers and among hyperactive children. J Abnorm Psychol. 1983;92:4–28. [DOI] [PubMed] [Google Scholar]
- 100. Reitan R. Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Reitan Neuropsychology; 1985. [Google Scholar]
- 101. Deary IJ, Stough C. Intelligence and inspection time - Achievements, prospects, and problems. Am Psychologist. 1996;51:599–608. [Google Scholar]
- 102. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 103. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013:104. [Google Scholar]
- 104. Zhao JH. gap: Genetic Analysis Package. R package version 1.1-17 2015.
- 105. Venables WN, Ripley BD.. Modern Applied Statistics with S. 4th ed New York, NY: Springer; 2002. [Google Scholar]
- 106. Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 107. Visscher PM, Hemani G, Vinkhuyzen AA, et al. Statistical power to detect genetic (co)variance of complex traits using SNP data in unrelated samples. PLoS Genet. 2014;10:e1004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Beaty TH, Liang KY, Seerey S, Cohen BH. Robust inference for variance components models in families ascertained through probands: II. Analysis of spirometric measures. Genet Epidemiol. 1987;4:211–221. [DOI] [PubMed] [Google Scholar]
- 110. Revelle W. psych: Procedures for Personality and Psychological Research. Version 1.6.9 Evanston, IL: Northwestern University; 2016. [Google Scholar]
- 111. Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. [DOI] [PubMed] [Google Scholar]
- 112. Michie PT, Kent A, Stienstra R, et al. Phenotypic markers as risk factors in schizophrenia: neurocognitive functions. Aust N Z J Psychiatry. 2000;34(suppl):S74–S85. [DOI] [PubMed] [Google Scholar]
- 113. Nuechterlein KH, Green MF, Calkins ME, et al. Attention/vigilance in schizophrenia: performance results from a large multi-site study of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr Res. 2015;163:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dickinson D, Goldberg TE, Gold JM, Elvevåg B, Weinberger DR. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. Schizophr Bull. 2011;37:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Meier MH, Caspi A, Reichenberg A, et al. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry. 2014;171:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.