Abstract
Although the majority of studies on mate choice focus on female mate choice, there is growing recognition of the role of male mate choice too. Male mate choice is tightly linked to 2 other phenomena: female competition for males and ornamentation in females. In the current article, I review the existing literature on this in a group of fishes, Poeciliidae. In this group, male mate choice appears to be based on differences in female quality, especially female size, which is a proxy for fecundity. Some males also have to choose between heterospecific and conspecific females in the unusual mating system of the Amazon molly. In this case, they typically show a preference for conspecific females. Whereas male mate choice is relatively well documented for this family, female ornamentation and female competition are not.
Keywords: binary choice test, fecundity, female choice, female size, Gambusia, guppy, Poecilia, preference function, sexual selection, Xiphophorus
Introduction
Within sexual selection, mate choice is especially important. Selecting a mating partner might be the most critical decision any individual makes. Mate choice is thought to drive the evolution of ornamental traits, including courtship, and can induce competition for mates in the opposite sex. Based on work by Darwin (1859, 1871), Bateman (1948), Trivers (1972), and Lehtonen et al. (2016), it is generally agreed that the sex that invests more into the offspring evolves to be the more selective one. In the majority of species this is the female, which invests strongly into eggs, as compared with a very small investment of males into sperms. This ecological differential in investment sets the stage for sexual selection and the 2 mechanisms proposed by Darwin: typically females choose partners and males compete over reproductive opportunities. In species with post-copulatory paternal investment into offspring, males can compensate for the lack of early investment, sometimes leading to a reversal in roles and the evolution of male mate choice and female competition for males. This is well understood in some of the few species that show this pattern, like pipefish (Vincent et al. 1992) and Jacanas (Temrin and Sillentullberg 1994). In the vast majority of species, however, the pattern is much more subtle, and one question arising from male compensatory investment is whether this can lead to the evolution of choosiness in males (Edward and Chapman 2011). In addition to male investment, environmental stochasticity may flip the balance between limiting and limited sex, for example, when males become exceedingly rare locally (Heubel et al. 2009), thus potentially increasing their choosiness. More importantly, can choosiness evolve in species where there is no compensatory investment? Essentially, we have to ask what the adaptive benefits, the mechanisms, and the evolutionary consequences of male mate choice might be for such males. We need to explore if male mate choice could induce the evolution of female ornaments, and also lead to female competition over males (Figure 1).
Figure 1.
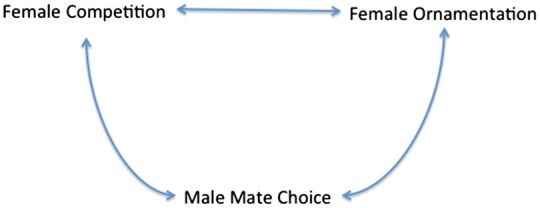
Hypothesized relationship between male mate choice, female competition, and female ornamentation.
To investigate this, I am reviewing our knowledge of a group of fishes that are on one extreme of the continuum of male investment. Males of livebearing fishes of the family Poeciliidae show no paternal investment into offspring after copulation. They only invest into ejaculates, pre-copulatory behavior, including courtship, as well as sexually selected ornaments. Although clearly unlikely to evolve due to male investment, empirically, male preferences have been documented in several species within this family. However, even males that invest very little can be selected to respond to differences in female quality (Edward and Chapman 2011) and most authors assume that male preferences in this group evolved due to significant differences in female fecundity. Alternatively, this may simply be the easiest hypothesis to test, with other ideas still awaiting attention. Also, males should be able to distinguish between females of different quality, but preferences can also be based on sensory or cognitive bias (Rosenthal 2017). Furthermore, choosiness is typically expressed with the cost of foregoing some mating opportunities.
Fecundity is tightly linked to size in most fishes (Helfman et al. 2009). In livebearing fishes, male growth rates slow down once they reach maturity (Snelson 1984; Morris and Ryan 1990). Females, however, continue to grow throughout their lives. Because larger females typically can carry more eggs, males that prefer larger females should have increased fitness. To my knowledge this direct link has not been experimentally demonstrated, although many studies have found male preferences for larger females. However, in Poeciliids a few factors complicate the picture. First, females store sperm and can use stored sperm for several months to fertilize eggs (Greven 2011). It is not clear that males can directly assess how many eggs a female carries (although in a related family, Goodeidae, a male preference for females with wider bellies was reported [Méndez-Janovitz and Macías Garcia 2017], and in 2 Livebearers, females are known to prefer well-fed males with an extended belly [Fisher and Rosenthal 2006, Plath et al. 2005]) or how many may be available for fertilization. Second, in most species, females cannot conceal pregnancies due to a significant change in body shape. Hence, body shape is probably not an ornament, and it is not clear if females evolved to honestly advertise fecundity. Third, females go through a sexual cycle of roughly 30 days and appear to be fully capable of receiving sperm from males only during a few days during the cycle, or right after parturition (Parzefall 1973). This means that the operational sex ratio is almost always male biased, with many more males available to inseminate females than females being receptive for males. Fourth, the relationship between female body size and fecundity is not uniform across all species. In other words, the slope of the regression line describing the relationship between fecundity and size is not the same for all species. How this might influence the evolution and potentially the strength of male preference is not well understood (Arriaga and Schlupp 2013). In addition, this relationship of fecundity and size can be confounded by superfetation (Pollux and Reznick 2011), which evolved several times independently within the family. Superfetation means that females carry broods of different stages at any given time. This provides a fertile ground for asking how males actually judge female fecundity based on female size. Finally, very large females may not be of high value to males because they may be senescing, and not reproducing anymore. Senescence in fishes in general (Reznick et al. 2002), and livebearing fishes in particular, is well documented (Reznick et al. 2006), but any effect this may have on male choice is unknown.
Nonetheless, almost all studies that looked at male preferences for size did find a preference for larger females (Dosen and Montgomerie 2004b), with a notable exception in the Least Killifish, Heterandria formosa (Ala-Honkola et al. 2010). This study seems to be the only one that found a preference for smaller males in a binary choice test, and a lack of preference in an open field test. The authors suggest that the absence of a preference may be driven by the strong first male precedence found in sperm competition. It is also possible, however, that publication bias exists and that studies reporting no preference are less likely to be published (e.g., Scherer U., Tiedemann R., Schlupp I., submitted for publication). A majority of studies did document a clear preference for larger females in multiple taxa (Herdman et al. 2004; Guevara-Fiore et al. 2010; Arriaga and Schlupp 2013; Head et al. 2015). Fundamentally, this predominance of male preference for larger females is matched with similar preferences in females, in other taxa (Ryan and Keddy-Hector 1992) beyond livebearing fishes. As we discuss the potential evolution of male mate choice in response to differences in female quality (e.g., as differential fecundity) or via other pathways (Edward and Chapman 2011), a very commonly invoked explanation is that male preferences are expressed via pleiotropy and may simply be due to the existence of evolved female preferences. They would not have evolved independently and might not even be adaptive. Interestingly, males and females can show preferences—seemingly for the same trait—body size, but likely for very different reasons. Male preferences seem to be related to a direct benefit, via increased fecundity (although a direct link to fitness remains to be shown [Dosen and Montgomerie 2004a, 2004b]), whereas female preferences for large males are thought to be due to indirect genetic benefits (Reynolds and Gross 1992).
Relevant Theoretical Treatment of Male Mate Choice
As male mate choice has moved more into the mainstream of sexual selection research (Clutton-Brock 2007; Edward and Chapman 2011), additional theoretical analysis of male mate choice has been published (for a recent review, see Fitzpatrick and Servedio, this volume; Fitzpatrick and Servedio 2018; Servedio 2007; Fitzpatrick and Servedio 2017). In a somewhat simplified view, male mate choice can evolve when mate availability is larger than the capacity to mate with, due to which there is recognizable variation in female quality and the benefit of choice is larger than the cost (Edward and Chapman 2011). A classical view in population ecology was that males did not matter much, but this has been corrected (Rankin and Kokko 2007). It needs to be acknowledged, again, that male mate choice (just like female ornaments; see Section on Female ornaments), might not be adaptive, but may be expressed due to pleiotropy. Recently, relative searching time (RST), the proportion of lifetime invested into mate search has been suggested as an important factor shaping the evolution of choosiness (Etienne et al. 2014; Courtiol et al. 2016). This approach emphasizes the role of direct benefits in the evolution of mate choice. For example, one study (Head et al. 2015) argues that males should be choosier when encountering females simultaneously, as compared with sequential encounters, because there is no opportunity cost. Empirically, preferences were indeed—as predicted—stronger during simultaneous encounters, but the number of sperms transferred and insemination success were unaffected. Theory also predicts that male mate choice will not easily evolve under sequential mate choice conditions (Barry and Kokko 2010). These authors rightly call for rigorous tests of male mate choice, going beyond just describing the existence of male preferences.
Overall, one could argue that there is still a mismatch between theoretical predictions and empirical evidence, as models often argue that male mate choice will evolve only under a limited set of conditions (Barry and Kokko 2010), yet, empirically, an increasing number of studies in a variety of taxa other than fishes (e.g., insects [Bonduriansky 2001] or amphibians [Krupa 1995]) do report the widespread presence of male mate choice.
Natural History of Livebearing Fishes
Species in the family Poeciliidae are ideally suited to test the fecundity hypothesis of male mate choice as outlined above. As there are likely no or few benefits to male mate choice other than increased fecundity, this seems to be the default explanation for the existence of male mate choice. Poeciliids are generally small, freshwater fishes that tend to be ecological generalists. The family is widespread from the United States of America to South America, with a center of diversity in Mexico. Roughly 200 species in 29 genera are currently recognized (Hrbek et al. 2007, Meredith et al. 2010). Members of the family are widely used in biological research, including ecology, evolution, and animal behavior, but also genetics, genomics, and cancer research (Evans et al. 2011). The group is characterized by internal fertilization and ovoviviparity, where females give live birth to a relatively small number of offspring that have developed in the female. Males have a modified anal fin, called the gonopodium, which is used to transfer sperm (Greven 2011) and plays a role in evolution and speciation (Langerhans et al. 2005, 2007). Other important traits, however, like superfetation or courtship, have evolved several times within the family (Parenti and Rauchenberger 1989; Meredith et al. 2011). Females show lifelong growth, while male growth slows down significantly after they mature (Snelson 1984). Consequently, females are often larger than males (Bisazza and Pilastro 1997). It is not entirely clear what the evolutionary benefit of this is, but females might grow too big for some of their gape-limited predators. Generally, the mating system is characterized by promiscuity, with males either trying to court females or force copulations. Courtship evolved multiple times independently within the family, and genera can be polymorphic for this trait (Plath et al. 2007). Even within some species, such as sailfin mollies (Travis and Woodward 1989), males can be polymorphic and some size classes will show courtship whereas others may not (Rios-Cardenas and Morris 2011). Courtship displays usually involve males presenting themselves in front of a female, or showing elaborate motion patterns either in front or sideways of the female (Rios-Cardenas and Morris 2011). Coloration and courtship has been implicated in increased mortality rates for males (Garcia et al. 1998, Godin and McDonough 2003), but males from non-courting species can also experience high sex-specific mortality (Tobler et al. 2008), so that not only courtship can be blamed for this pattern. Females, especially because they are larger and more profitable prey, may also be at higher risk by size-selective predators (Trexler et al. 1994). Generally, it should be taken into account that most of our knowledge of mating behavior in Poeciliids stems from relatively few, well-studied species, such as the guppy Poecilia reticulata, some swordtails Xiphophorus sp., and several mollies Poecilia sp., while other genera and species are far less well studied.
In the sailfin molly Poecilia latipinna, for example, males of intermediate size can show courtship behavior when accompanied by small males and sneaky copulation attempts when accompanied by large males (Travis and Woodward 1989). These sneaky copulation attempts and the associated sexual harassment (Magurran and Seghers 1994a, 1994b; Clutton-Brock and Parker 1995) are very common and can be the only male mating behavior in some species (Plath et al. 2007). They are best understood as male strategies to circumvent female choice in the context of sexual conflict. Males in some species can switch from courtship to sneaking dynamically; in other species, the trait is genetically fixed. Often larger males show courtship (and other ornamentation), whereas smaller males rely on sneaky copulation attempts, and consequently being around larger males is less costly for females (Schlupp et al. 2001; Makowicz and Schlupp 2013). Probably the best understood example is a swordtail, Xiphophorus nigrensis, where a balanced polymorphism for 2 male morphs has been documented (Ryan et al. 1992).
Also, in many species males can be very colorful. Many colors are in the red and orange, but black spots are known from many species. The red and orange ornaments are produced by carotenoids or pteridines in chromatophores (Grether 2001). Black spots are generated by melanocytes, and are thought of as enhancers (Brooks 1996). Interestingly, there is a widespread parasitic disease, named Black Spot Disease that also results in black spots (Tobler and Schlupp 2008a, 2008b). Occasionally, white ornaments are observed, for example, in Poecilia gillii. Finally, males and females can have structural colors, often as iridescent blues. In many species, males have exaggerated dorsal fins, which are often displayed to females during courtship (MacLaren and Rowland 2006). In one group, swordtails of the genus Xiphopohorus, males of some species have evolved extended rays of the tail fin (Rosenthal and Evans 1998), in at least one species Xiphophorus montezumae, exceeding the length of the body of the male. These appendages are thought to mimic large male body size (Rosenthal 2017). Color and black spots are found in females of many species (see Section on Female ornaments for a discussion), but no exaggerated fins (MacLaren and Fontaine 2013). It might be worthwhile to point out here that color and spotting patterns may arise also under natural selection, not just by sexual selection.
Definitions
Before I begin to review male mate choice in livebearing fishes, I want to provide an operational definition of “mate choice.” I am using the definition recently suggested by Rosenthal (2017): “Mate choice can be defined as any aspect of an animal’s phenotype that leads to it being more likely to engage in sexual activity with certain individuals than with others.” Note that this definition parts elegantly from the problematic traditional usage of sex roles (Ah-King and Ahnesjö 2013). Consequently, Rosenthal (2017) replaces female and male with the terms chooser and courter, which can be of any sex. I fully agree with this definition, but for the purpose of this review I retain the usage of male and female as a heuristic tool, to reflect the existing difference in the ecology of early investment into gametes, without acknowledging specific sex roles. I think that we eventually have to realize that mate choice is best understood as a continuum with the traditional sex roles of male and female confined to the extreme ends. I suggest that in reality in most mating systems, females and males both have preferences, exercise choice, and resolve the underlying sexual conflict in some form of mutual mate choice.
Another term that needs to be defined is “preference.” Again, I use a definition by Rosenthal (2017): “a chooser’s internal representation of courter traits that predisposes it to mate with some phenotypes over others.” The difference between choice and preference is that we can assess choice by measuring actual sexual behaviors, while preferences can also be measured indirectly, for example, using association times (Wagner 1998). One thing that can dictate how we measure preference or choice is the obviously interactive nature of actual mating, which involves behaviors from both individuals. Ironically, this sometimes requires that we separate individuals in a choice test, because we are interested in their “pure” preferences, not the outcome of an interaction between 2 partners.
Finally, an ornament is a trait that is likely to have arisen via sexual or social selection, and plays a role in mate choice by making the bearer attractive to choosers, often at a cost to survival. Ornaments are often sexually dimorphic, but they do not have to be. They do not have to have a function outside of social interactions.
Historical Studies on Interspecific Male Choice
In the 1960s and 1970s, the seminal papers by Hamilton (1964a, 1964b), triggered a Kuhnian paradigm shift (Kuhn 1962), which led to recognizing the gene as unit of selection in biology providing a new framework for biology, including mate choice. However, there was already considerable interest in male mate choice, including in livebearing fishes prior to this paradigm shift. Consequently, very early, livebearing fishes emerged as important model organisms in the study of mate choice. This early work was focused on questions of species recognition and isolating mechanisms; historically, female choice had not yet been recognized as very important (Milam 2010). Very importantly, with more female scientists conducting and publishing research on Sexual Selection beginning in the 1990s, more studies on female choice appeared (Zuk 1993). In a broader context, this provides a cautionary tale of how societal conditions influence and often hinder scientific work. Early on, Haskins and Haskins (1949, 1950) published their studies of male mate choice in guppies and some close relatives and reported evidence for male preferences for conspecific females and also provided a first comment on the role of size in male mate choice: “It is well known that males of Lebistes, when exposed to several females of their own species, tend to pay most attention to the largest individuals …” [note: Lebistes reticulatus was the recognized name for the guppy at the time] (Haskins and Haskins 1949). Another early account of male mate choice in the context of species recognition was offered by Hubbs and Delco (1960). In this article, the authors describe a conspecific preference in 4 species of Gambusia. They conclude that most species indeed show the predicted species preference, but that G. affinis does not. They note that this may explain why G. affinis is involved in many interspecific hybridization events. A recent study revisits this topic and found strong male preferences for conspecifics (Espinedo et al. 2010) in sympatric G. affinis and G. geiseri.
Male Choice within Populations
Generally based on the notion that larger females would provide a direct fecundity benefit to males, later studies started investigating male choice. Virtually all studies used binary choice tests. In such a test, a male is simultaneously exposed to (typically) 2 females that differ in the trait under investigation and can reveal his preference by approaching the females. The measured variable is typically association time, which is generally a good proxy for preference (Bischoff et al. 1985; Berglund 1993; Kodric-Brown 1993; Witte 2006), especially in male mate choice (Jeswiet and Godin 2011). Very few published studies have used preference functions (Wagner 1998), in which 2 or more females are presented singly in random order (Arriaga and Schlupp 2013, Spikes and Schlupp, manuscript in preparation).
One of the few studies directly comparing male and female choice was conducted by Ptacek and Travis (1997) looking at mate preferences in sailfin mollies P. latipinna. The study also stands out because it used multiple populations, investigating population variation in the traits under consideration. This seems especially relevant in species that have a wide range. This is the case in the sailfin molly, which occurs from Wilmington, NC southward to roughly Tuxpan in Mexico (Schlupp et al. 2002). The study by Ptacek and Travis (1997) reported that both males and females generally prefer larger partners and that, larger males showed stronger preferences for female size.
Male mate choice is particularly well researched in guppies, often combined with studying the role of social influences on mate choice (Auld and Godin 2015; Auld et al. 2015, 2016, 2017; Jeswiet et al. 2011, 2012) (see also Section on Male mate choice and social information). In general, male preferences for larger females have been found many times (Dosen and Montgomerie 2004b; Herdman et al. 2004), often, but not always, using just visual information. In a study by Herdman et al. (2004), visual information was not sufficient for males to show a preference, but males did show a preference when allowed to access other information as well. Another study using guppies documented that results from open field tests and binary choice tests are correlated and yield comparable results (Jeswiet and Godin 2011). Mosquitofish, G. holbrooki, were also found to have a preference for larger females (Hoysak and Godin 2007).
Females can differ in quality in many different ways, and virgin females might be of very high value, especially in systems with first male sperm precedence. In this case, mating with a virgin female might secure a large number of offspring for the male that inseminates a female first. In guppies, males do not distinguish visually between virgin and mated females, but in an open field test, where males and females could fully interact, males directed more sexual behaviors toward virgin females. However, they showed more coercive, sneaky copulations toward previously mated females (Guevara-Fiore et al. 2009). Males also invested more effort into mating with females that were in the receptive phase of their sexual cycle (Guevara-Fiore et al. 2010). Finally, males of Brachyrhaphis episcopi, a species from Panama, preferred familiar females, but this preference was modulated by predation risk (Simcox et al. 2005). Another trait that may be used in male choice is a brood spot (or gravid spot) that is found in many livebearing fishes. A recent study found that size and intensity of the gravid spot are correlated with clutch size (Norazmi-Lokman et al. 2016), which may potentially be used by males in mate choice.
Cave mollies are a special population of the Atlantic molly, which has colonized a hydrogensulfide (H2S) rich, toxic cave in Tabasco, Mexico (Tobler and Plath 2011). This population is widely used to study effects of both toxicity and darkness on mollies, often addressing ecological speciation (Riesch et al. 2011). Cave mollies are capable of mate choice both in darkness and in light. One study found that males of both the surface and cave form have a preference for larger females, but only cave mollies show the preference in darkness (Plath et al. 2006). Males of the surface form, but not males of the cave form can deceive other males relative to their mate choice (Plath et al. 2010; see also Section on Male mate choice and social information).
Mechanisms of Male Mate Choice
Documenting male mate choice would be incomplete without looking at the mechanisms (see Section on Male mate choice and social information) that are used in male mate choice. Turbidity, for example, was found to slow down decision-making in sailfin mollies P. latipinna (Heubel and Schlupp 2006). A separate study found that male choice is also affected by seasonality (Heubel and Schlupp 2008). A study using G. affinis documented that males rejected females that were parasitized with nematodes, presumably because the infection reduces fecundity (Deaton 2009; Cureton et al. 2011). Furthermore, in the Atlantic molly, personality affects male mate choice and bolder males respond more strongly to the presence of an audience (Bierbach et al. 2015). Interestingly, in G. holbrooki, dominant females were preferred by males, whereas size had no significant effect (Chen et al. 2011). This is an important finding, because it shows that other factors—not only size—likely play an important role in male mate choice. MacLaren and Fontaine (2013) explored a potential female ornament in X. variatus, a species of swordtail without a sword. They found that males preferred larger body size in females, but not larger fins. Larger fins in males are often preferred by females and could serve as an indicator trait for females (McLaren et al. 2004). Apparently this is not the case for males. Finally, a general concern with mate choice studies is how reliable the data collected are. This has been addressed in a few studies investigating how repeatable male mate choice is, finding very low repeatability (Gabor and Aspbury 2008). By contrast, a study on guppies (Godin and Auld 2013) reported that male mate choice is fairly consistent, and a study on the swordtail X. nigrensis also found relatively good repeatability (Cummings and Mollaghan 2006). Clearly more studies on this topic are needed. Low repeatability between individuals may reflect many different things, including problematic experimental design. But it may also reflect true changes in a chooser’s preferences, especially when responding to conditional traits.
Very little is known about the many other factors that are recognized in female choice, including preferences for Major Histocompatibility Complex (MHC) compatibility and inbreeding avoidance. It is well known that learning plays a role in mate choice (Verzijden et al. 2012) (see Section on Male mate choice and social information for discussion of social influences), and that there are sex differences in learning. In guppies, for example, females are twice as efficient in reversal learning (Petrazzini et al. 2017), possibly indicating that females have a generally higher cognitive flexibility.
Cost to Males and Cryptic Male Choice
Females make strong investments into their eggs. By comparison, sperm and mating are less costly. It is important to realize, however, that sperm is not free. There is growing evidence that males can be sperm depleted and that the costs of mating (viewed inclusively, and counting, e.g. cost for sperm, ejaculates, courtship behavior, predation risk, and lost opportunities) can be high for some males as compared with other males (Anthes et al. 2014, Hardling et al. 2008). Consequently, males may evolve mechanisms to exercise cryptic mate choice and allocate ejaculates and sperm strategically (Matthews et al. 1997; Schlupp and Plath 2005; Riesch et al. 2008; Robinson et al. 2008, 2011), and also prime sperm relative to species identity (Aspbury and Gabor 2004b) and female size (Aspbury and Gabor 2004a). Sperm priming is a mechanism that makes sperm ready to be ejaculated. Furthermore, there is growing evidence—at least in guppies—that males differ in sperm and ejaculate characteristics based on age (Gasparini et al. 2010), and that they can adjust to changes in the social environment very quickly (Boschetto et al. 2011; Barrett et al. 2014; Cattelan et al. 2016). Females appear to respond to these changes by modulating the environment for sperm in their ovaries (Gasparini and Pilastro 2011; Gasparini et al. 2012). These interactions seem to reflect an ongoing sexual conflict (Parker 2006).
Clearly, mating in livebearing fishes is often characterized by intense sexual conflict (Chapman et al. 2003, Schärer et al. 2012) in which male or female preferences may be undermined or thwarted by the behavior of their mate. Forced copulations (Magurran 2001) and sexual harassment (Plath et al. 2007; Heubel and Plath 2008) are common throughout the family and probably lead to significant differences between measurable mate preferences and actual reproductive outcomes (Rosenthal 2017).
Male Choice for Correct Female Species: The Amazon Molly as an Example
The ecology of male investment relative to the mating value of the female can drive the evolution of male mate choice. In pipefish, male investment is very high and they have evolved to be selective. In other cases, the mating value of certain females may be so low that males evolve to reject them. The latter is the case in males facing a choice between heterospecific Amazon mollies P. formosa and their conspecific females. Amazon mollies are an all-female, clonal species of fish of hybrid origin (Hubbs and Hubbs 1932, Schlupp and Riesch 2011). The maternal ancestor is the Atlantic molly P. mexicana and the paternal ancestor is the sailfin molly P. latipinna. The single, original hybridization apparently took place about 100,000 generations ago in an area near present-day Tampico (Stöck et al. 2010; Warren et al. 2018), but see Alberici da Barbiano et al. (2013). Amazon mollies reproduce by gynogenesis, where sperm simply serves as stimulus for embryonic development, but is typically not incorporated into the offspring (Schlupp 2005). Based on this, the sperm-providing males are generally predicted to prefer conspecifics to heterospecifics. The Amazon molly uses at least 3 species as sperm donors: its 2 parental species, P. latipinna and P. mexicana, and P. latipunctata (Tamesi molly), an endemic species found near Ciudad Mante. Sailfin and Atlantic mollies not only show populations that occur in sympatry with Amazon mollies, but also populations that occur in allopatry. This creates an opportunity for work comparing characters, including male mate choice between allopatric and sympatric populations (Gabor and Ryan 2001, Gabor et al. 2005).
More importantly, this situation can be used to make very clear predictions relative to male mate choice. For males the fitness return for mating with Amazon mollies is very low. Even if the cost of mating is low or moderate, males should evolve to prefer conspecific females, or lower their cost by investing less into heterospecific copulations. Via mate copying, a process of using social information in mate choice (Witte et al. 2015; Varela et al. 2018), males gain an indirect fitness benefit offsetting some of the cost of heterospecific matings: the interactions of a sexual male and an Amazon molly are observed by conspecific females and make that male more attractive to conspecific females. Interestingly, males have also been shown to copy the mate choice of other males (Schlupp and Ryan 1997; Bierbach et al. 2011).
Male mate choice in this complex has been intensively studied (reviewed in Schlupp 2009; Schlupp and Riesch 2011). Often the “wrong” mating decisions are viewed as mistakes, and several studies looked into potential mechanisms for the mistakes. Interestingly, theory does not predict the evolution of perfect male choice (Heubel et al. 2009), and it seems that evolving very strong preferences is costly to the sexual males. Nonetheless, an older study, for example, found that male Atlantic mollies show species recognition when choosing between visually presented conspecific and Amazon molly females, but that females undermine this probably with chemical signals when they are post-partum (Schlupp et al. 1991). In this case, females seem to win the underlying evolutionary arm-race. Chemical information alone, however, is insufficient for species recognition (Aspbury et al. 2010). A study of sailfin mollies (Muraco et al. 2014), documented the existence of distinct male behavioral phenotypes or personalities, but found no strong correlation with male preferences. A unique feature of this mating system highlights the complexity of mating interactions: Amazon mollies are known to actively intervene in conspecific mating attempts (Schlupp et al. 1991; Foran and Ryan 1994). They sometimes approach mating pairs of sailfin mollies and maneuver themselves into the position of the sexual female, thereby redirecting the mating to them.
Most importantly, in this system, male mate choice has been hypothesized to drive the system and play an important role in the apparent ecological stability of the coexistence of Amazon mollies and its hosts (Schlupp 2009), essentially via frequency-dependent male mate choice. This coexistence is an ecological puzzle because the Amazon mollies should quickly outcompete their sexual host. The role of male mate choice in the stability has been explored in a series of papers presenting evidence that both in the laboratory and in the field, female Amazon mollies receive fewer sperm from males of one of their hosts, the sailfin molly (Aspbury and Gabor 2004b, schlupp and Plath 2005; Riesch et al. 2008, 2012; Robinson et al. 2008. Furthermore, male mate choice changes over the season, potentially in response to changing frequencies of Amazon mollies in nature (Heubel and Schlupp 2008).
Male Mate Choice and Social Information
Mating is by nature an interactive process. Mating decisions are increasingly viewed as interactions that take in a public realm, and often other individuals observe these interactions (Danchin et al. 2004). Male mosquitofish G. holbrooki, for example, are attracted to all-female groups (Agrillo et al. 2008) and the authors conclude that males are capable of recognizing important properties of the presented groups.
The general question of how an audience (known to the focal individual) or eavesdropping (audience unknown to the focal individual) might alter sexual preferences is a relatively young line of inquiry. It should be noted that for social species, a situation where mating happens in public is more likely to be the default, not a more private situation, which is often assumed in laboratory choice tests. In addition to studying effects on female choice, there is also a strong emerging literature on social influences on male mate choice. This includes mate copying (see above for examples using the Amazon molly system) in guppies (Auld and Godin 2015), but also general audience effects (Jordan et al. 2006; Plath et al. 2008a; Auld et al. 2015). Responses by males to another male as an audience are surprisingly fine-tuned. For example, several studies documented that the response of a focal male guppy is influenced by the size of the audience male, potentially minimizing sperm-competition risk (Jeswiet et al. 2012; Nöbel and Witte 2013; Auld et al. 2017). One mechanism for mediating this might be to manipulate their chances of obtaining copulations by selectively associating with less attractive individuals and also reduce sperm competition this way. This is indeed what a study on guppies found: males preferred females that were surrounded by drab males, presumably because those pose a lesser threat in sperm competition (Gasparini et al. 2013).
In guppies, male mate choice can also be modified based on the perceived difference between self and the value of an opponent (Yoshikawa et al. 2016): dull males abandoned approaches to females in the presence of bright males. This shows the importance of including information about the tested subjects into our interpretation of male mate choice. In this context, more studies on the role of learning in male mate choice would be very useful.
Finally, deceptive behavior is relatively rare. Therefore, one of the more striking recent findings is that males of the Atlantic molly seem to be able to deceive other males by interacting with females they initially did not prefer in the presence of other males. This was documented in the surface form of the Atlantic mollies (Plath et al. 2008b), but not in the Cave molly (Plath et al. 2010) or in guppies (Makowicz et al. 2010). A theoretical model of this process (Castellano et al. 2016) indicated that this kind of deceptive behavior is not very likely to evolve.
Female Competition
Another important question in this context is if males are choosy, do females start competing over males? Female competition is probably widespread, but documentation of direct female competition over males is relatively rare (Rosvall 2013; Cain and Rosvall 2014). Most female competition seems to be relative to resources other than males (Scharnweber et al. 2011a, 2011b), but at least 2 studies (Schlupp et al. 1991; Foran and Ryan 1994), found that Amazon molly females will actively compete for males. Furthermore, female sailfin mollies appear to be suppressing the feeding efficiency of Amazon mollies (Alberici da Barbiano et al. 2010). A field study in Atlantic mollies (Heubel and Plath 2008), pointed toward intensive between species competition over males and other resources. This view is supported by recent experimental work on female aggression and competition (Makowicz and Schlupp 2013, 2015, Makowicz et al. 2016). This research can be a template for more work on within species competition, as we seem to know relatively little about within species female competition. Theoretically, females might compete over males if they show signs of sperm depletion.
Female Ornaments
In parallel to the effects of female choice on males, does male choice have the potential to drive the evolution of female ornaments? Logically, if males are choosy this could induce sexual selection on females and lead to female ornamentation. It should be noted, however, that traits that are detrimental to female fitness are not likely to evolve under male mate choice (Fitzpatrick and Servedio 2018). Male ornaments are typically under selection by females, which have preferences for elaborate, and often costly, ornaments (Andersson 1994). Whether preferring ornamented males confers a fitness advantage to females is not always clear, especially when indirect benefits are invoked. One also has to keep in mind that not all dimorphic traits are automatically ornamental and under sexual selection. To complicate things further, we are very likely to miss important traits because they are difficult for humans to assess. Recent work has highlighted the role of visual ornaments that are in the UV wavelengths that we can measure, but not see, in mate choice and predation avoidance (Cummings et al. 2003, 2006). Beyond that there are aspects of chemical communication, or lateral line communication that we cannot fathom. Even acoustical communication, although very unlikely (Schulz-Mirbach et al. 2010; Schulz-Mirbach et al. 2011), should not be completely ruled out. One example would be the role of chemical information in species recognition and female mate preference (McLennan and Ryan 1997; Fisher et al. 2006; Plath and Tobler 2007; Rosenthal et al. 2011; Rüschenbaum and Schlupp 2013).
Based on this, it is not clear if female Poeciliids have ornaments. Restricting my argument to coloration, clearly females in many species have color, but I am not aware of any coloration that would be easily interpreted as a female ornament. In all cases the males seem to have similar coloration, which means the trait is either not an ornament and has evolved under natural selection (e.g., black spots might contribute to crypsis) or could be expressed due to pleiotropy as a result of a genetic correlation with males. One example would be the black spots and orange coloration in the Cuban Limia, Limia vittata. Both sexes seem to have equal amounts of black spotting, but orange is less common in females than in males. The black spots could make the fishes cryptic in their environment by dissolving the body outline, and the expression of orange could be some form of evolutionary byproduct, due to pleiotropie, or beneficial to females due to the general advantages often ascribed to carotenoids, such as anti-parasite properties (Olson and Owens 1998; Martin and Johnsen 2007). In the black-finned goodeid, Girardinichthys viviparus, a preference for orange hue in females was described (Méndez-Janovitz and Macías Garcia 2017), but interestingly, female coloration was not associated with fecundity and negatively associated with offspring survival. Furthermore, in Salmon, male preferences for red have been documented (Foote et al. 2004) and extensive studies in birds on the species level have recently shown that the degree of ornamentation in females is often correlated to the ornamentation found in males, but that sexual selection and also life-history characteristics can influence the degree of dimorphism (Rubenstein and Lovette 2009; Dale et al. 2015). In livebearing fishes, a similar analysis would be very useful.
For the green swordtail X. hellerii, a very interesting potential female signal has been suggested. Females of that species (and others) are known to perform “headstands” and males prefer this behavior to females showing regular swimming (Fernandez et al. 2008). Without further investigation, it is difficult to say if this behavior is any kind of advertisement, but the possibility is intriguing. In other groups of fishes, female ornaments have been suggested, such as female eye color, which can indicate readiness to spawn (Olsson et al. 2017) in sand gobies.
Same-sex Behavior
Many species show same-sex behavior, but almost nothing is known about this in livebearing fishes. Yet, clearly if we discuss mate choice in general, and male mate choice in particular, potential preferences for members of the same sex needs to be considered (Poiani 2010). In one study (Field and Waite 2004), using guppies, the authors found that males can show same-sex behavior after long times of isolation from females. Interestingly, male sexual behaviors toward males persisted even after exposure to females. Another study, conducted on Atlantic mollies, suggests that same-sex behavior is beneficial to males as it makes them more attractive to females via the use of social information (Bierbach et al. 2013). It is apparent from the lack of studies that much more work is needed on this topic.
Conclusion
Intuitively, the evolution of male mate choice in livebearing fishes seems an unlikely proposition: males make no investment into their offspring after copulation, and most mating systems seem to be strongly characterized by sexual conflict. Nonetheless, male mate choice has been documented in several species within the family, mainly for female size, but also for female species.
Outlook
Male mate choice, female competition, and female ornamentation are tightly connected. While male mate choice has been surprisingly well documented in livebearing fishes, the other 2 elements are poorly understood. Nonetheless, this provides an excellent basis for future research.
More work is needed to document and understand female competition for males. Right now it is not very clear if this even exists.
Females have a number of traits that might be considered ornamental, but how they evolved and if they are preferred by males, is less studied.
More interaction between theoretical and empirical studies would be beneficial.
So far, variability in female fecundity is viewed as the driver of male mate choice, but there might be many more traits in which females differ and that might be used in male mate choice.
Better evidence for the adaptive benefit of choosing larger females is needed. The large variability in fecundity found in livebearing fishes, should allow for comparative tests.
In recent decades, much progress has been made understanding the perceptual and cognitive aspects of female mate choice (Ryan and Cummings 2013), without similar attention to male mate choice.
Finally, there is significant taxonomic bias, even within the livebearing fishes. A majority of studies conducted use guppies; clearly more diversity would be important.
Acknowledgments
I am very grateful for the many people that helped me understand mate choice, including colleagues and students, but especially to the participants (both speakers and discussants) of the symposium “Integrating Male Mate Choice, Female Competition, and Female Ornaments” and the organizers of the Behaviour conference in Estoril, Portugal. You were all very generous with your time and knowledge. I have tried to be as inclusive as time and space allowed, but most likely I have missed some important published work. For that I apologize. I am grateful for the very helpful reviews provided by 3 anonymous reviewers. All wrong opinions, of course, are solely mine. No external funding was received for this paper. I acknowledge generous support from the University of Oklahoma.
References
- Agrillo C, Dadda M, Serena G, 2008. Choice of female groups by male mosquitofish Gambusia holbrooki. Ethology 114:479–488. [Google Scholar]
- Ah-King M, Ahnesjö I, 2013. The “Sex Role” concept: an overview and evaluation. Evol Biol 40:461–470. [Google Scholar]
- Ala–Honkola O, Saila L, Lindström K, 2010. Males prefer small females in a dichotomous choice test in the Poeciliid fish Heterandria formosa. Ethology 116:736–743. [Google Scholar]
- Alberici da Barbiano L, Gompert Z, Aspbury AS, Gabor CR, Nice CC, 2013. Population genomics reveals a possible history of backcrossing and recombination in the gynogenetic fish Poecilia formosa. Proc Natl Acad Sci USA 110:13797–13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberici da Barbiano L, Waller J, Gabor CR, 2010. Differences in competitive efficiency between a sexual parasite and its host provide insight into the maintenance of a sperm-dependent vertebrate species. J Freshw Ecol 25:523–530. [Google Scholar]
- Andersson M, 1994. Sexual Selection. Princeton: Princeton University Press. [Google Scholar]
- Anthes N, Werminghausen J, Lange R, 2014. Large donors transfer more sperm, but depletion is faster in a promiscuous hermaphrodite. Behav Ecol Sociobiol 68:477–483. [Google Scholar]
- Arriaga LR, Schlupp I, 2013. Poeciliid male mate preference is influenced by female size but not by fecundity. PeerJ 1:e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspbury AS, Espinedo CM, Gabor CR, 2010. Lack of species discrimination based on chemical cues by male sailfin mollies Poecilia latipinna. Evol Ecol 24:69–82. [Google Scholar]
- Aspbury AS, Gabor CR, 2004a. Differential sperm priming by male sailfin mollies Poecilia latipinna: effects of female and male size. Ethology 110:193–202. [Google Scholar]
- Aspbury AS, Gabor CR, 2004b. Discriminating males alter sperm production between species. Proc Natl Acad Sci USA 101:15970–15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld HL, Godin JGJ, 2015. Sexual voyeurs and copiers: social copying and the audience effect on male mate choice in the guppy. Behav Ecol Sociobiol 69:1795–1807. [Google Scholar]
- Auld HL, Jeswiet SB, Godin JGJ, 2015. Do male Trinidadian guppies adjust their alternative mating tactics in the presence of a rival male audience? Behav Ecol Sociobiol 69:1191–1199. [Google Scholar]
- Auld HL, Pusiak RJP, Godin JGJ, 2016. Independent mating preferences for male body size and coloration in female Trinidadian Guppies. Ethology 122:597–608. [Google Scholar]
- Auld HL, Ramnarine IW, Godin JGJ, 2017. Male mate choice in the Trinidadian guppy is influenced by the phenotype of audience sexual rivals. Behav Ecol 28:362–372. [Google Scholar]
- Barrett LT, Evans JP, Gasparini C, 2014. The effects of perceived mating opportunities on patterns of reproductive investment by male Guppies. PloS ONE 9:e93780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry KL, Kokko H, 2010. Male mate choice: why sequential choice can make its evolution difficult. Anim Behav 80:163–169. [Google Scholar]
- Bateman AJ, 1948. Intra-sexual selection in Drosophila. Heredity 2:349–368. [DOI] [PubMed] [Google Scholar]
- Berglund A, 1993. Risky sex: male pipefishes mate at random in the presence of a predator. Anim Behav 46:169–175. [Google Scholar]
- Bierbach D, Jung CT, Hornung S, Streit B, Plath M, 2013. Homosexual behaviour increases male attractiveness to females. Biol Lett 9:20121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbach D, Kronmarck C, Hennige-Schulz C, Stadler S, Plath M, 2011. Sperm competition risk affects male mate choice copying. Behav Ecol Sociobiol 65:1699–1707. [Google Scholar]
- Bierbach D, Sommer-Trembo C, Hanisch J, Wolf M, Plath M, 2015. Personality affects mate choice: bolder males show stronger audience effects under high competition. Behav Ecol 26:1314–1325. [Google Scholar]
- Bisazza A, Pilastro A, 1997. Small male mating advantage and reversed size dimorphism in poeciliid fishes. J Fish Biol 50:397–406. [Google Scholar]
- Bischoff RJ, L GJ, I RD, 1985. Tail size and female choice in the guppy Poecilia reticulata. Behav Ecol Sociobiol 17:253–256. [Google Scholar]
- Bonduriansky R, 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339. [DOI] [PubMed] [Google Scholar]
- Boschetto C, Gasparini C, Pilastro A, 2011. Sperm number and velocity affect sperm competition success in the guppy Poecilia reticulata. Behav Ecol Sociobiol 65:813–821. [Google Scholar]
- Brooks R, 1996. Melanin as a visual signal amplifier in male Guppies. Naturwissenschaften 83:39–41. [Google Scholar]
- Cain KE, Rosvall KA, 2014. Next steps for understanding the selective relevance of female-female competition. Front Ecol Evol 2:1–3. [Google Scholar]
- Castellano S, Friard O, Pilastro A, 2016. The audience effect and the role of deception in the expression of male mating preferences. Anim Behav 115:273–282. [Google Scholar]
- Cattelan S, Evans JP, Pilastro A, Gasparini C, 2016. The effect of sperm production and mate availability on patterns of alternative mating tactics in the guppy. Anim Behav 112:105–110. [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L, 2003. Sexual conflict. Trends Ecol Evol 18:41–47. [Google Scholar]
- Chen T, Beekman M, Ward AJW, 2011. The role of female dominance hierarchies in the mating behaviour of mosquitofish. Biol Lett 7:343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T, 2007. Sexual selection in males and females. Science 318:1882–1885. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Parker GA, 1995. Sexual coercion in animal societies. Anim Behav 49:1345–1365. [Google Scholar]
- Courtiol A, Etienne L, Feron R, Godelle B, Rousset F, 2016. The evolution of mutual mate choice under direct benefits. Am Nat 188:521–538. [DOI] [PubMed] [Google Scholar]
- Cummings M, Mollaghan D, 2006. Repeatability and consistency of female preference behaviours in a northern swordtail Xiphophorus nigrensis. Anim Behav 72:217–224. [Google Scholar]
- Cummings ME, Garcia De León FJ, Mollaghan DM, Ryan MJ, 2006. Is UV ornamentation an amplifier in swordtails? Zebrafish 3:91–100. [DOI] [PubMed] [Google Scholar]
- Cummings ME, Rosenthal GG, Ryan MJ, 2003. A private ultraviolet channel in visual communication. Proc R Soc Lond Ser B Biol Sci 270:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton JC, Martin RE, Lewis RL, Stoops SB, Deaton R, 2011. Effects of a trematode infestation on body condition, reproduction and mating behaviors in a livebearing fish. Behaviour 148:967–984. [Google Scholar]
- Dale J, Dey CJ, Delhey K, Kempenaers B, Valcu M, 2015. The effects of life history and sexual selection on male and female plumage colouration. Nature 527:367. [DOI] [PubMed] [Google Scholar]
- Danchin E, Giraldeau LA, Valone TJ, Wagner RH, 2004. Public information: from nosy neighbors to cultural evolution. Science 305:487–491. [DOI] [PubMed] [Google Scholar]
- Darwin C, 1859. The Origin of Species. London: John Murray. [Google Scholar]
- Darwin C, 1871. The Descent of Man. London: John Murray. [Google Scholar]
- Deaton R, 2009. Effects of a parasitic nematode on male mate choice in a livebearing fish with a coercive mating system (western mosquitofish, Gambusia affinis). Behav Processes 80:1–6. [DOI] [PubMed] [Google Scholar]
- Dosen LD, Montgomerie R, 2004a. Female size influences mate preferences of male guppies. Ethology 110:245–255. [Google Scholar]
- Dosen LD, Montgomerie R, 2004b. Mate preferences by male guppies Poecilia reticulata in relation to the risk of sperm competition. Behav Ecol Sociobiol 55:266–271. [Google Scholar]
- Edward DA, Chapman T, 2011. The evolution and significance of male mate choice. Trends Ecol Evol 26:647–654. [DOI] [PubMed] [Google Scholar]
- Espinedo CM, Gabor CR, Aspbury AS, 2010. Males, but not females, contribute to sexual isolation between two sympatric species of Gambusia. Evol Ecol 24:865–878. [Google Scholar]
- Etienne L, Rousset F, Godelle B, Courtiol A, 2014. How choosy should I be? The relative searching time predicts evolution of choosiness under direct sexual selection. Proc R Soc B Biol Sci 281, doi:10.1098/rspb.2014.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP, Pilastro A, Schlupp I, 2011. Ecology and Evolution of Poeciliid Fishes. Chicago and London: University of Chicago Press. [Google Scholar]
- Fernandez AA, Fernandez LR, Toth L, 2008. Head over heels: an examination of a possible mating signal in female swordtails Xiphophorus cortezi. Anim Behav 76:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KL, Waite TA, 2004. Absence of female conspecifics induces homosexual behaviour in male guppies. Anim Behav 68:1381–1389. [Google Scholar]
- Fisher HS, Rosenthal GG, 2006. Female swordtail fish use chemical cues to select well-fed mates. Anim Behav 72:721–725. [Google Scholar]
- Fisher HS, Wong BBM, Rosenthal GG, 2006. Alteration of the chemical environment disrupts communication in a freshwater fish. Proc R Soc B Biol Sci 273:1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CL, Servedio MR, 2017. Male mate choice, male quality, and the potential for sexual selection on female traits under polygyny. Evolution 71:174–183. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick C, Servedio M, 2018. The evolution of male mate choice and female ornamentation; a review of mathematical models. Current Zoology in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote CJ, Brown GS, Hawryshyn CW, 2004. Female colour and male choice in sockeye salmon: implications for the phenotypic convergence of anadromous and nonanadromous morphs. Anim Behav 67:69–83. [Google Scholar]
- Foran CM, Ryan MJ, 1994. Female - female competition in a unisexual/bisexual complex of mollies. Copeia 2:504–508. [Google Scholar]
- Gabor CR, Aspbury AS, 2008. Non-repeatable mate choice by male sailfin mollies Poecilia latipinna, in a unisexual-bisexual mating complex. Behav Ecol 19:871–878. [Google Scholar]
- Gabor CR, Ryan MJ, 2001. Geographical variation in reproductive character displacement in mate choice by male sailfin mollies. Proc R Soc B Biol Sci 268:1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CR, Ryan MJ, Morizot DC, 2005. Character displacement in sailfin mollies Poecilia latipinna: allozymes and behavior. Environ Biol Fish 73:75–88. [Google Scholar]
- Garcia CM, Saborio E, Berea C, 1998. Does male-biased predation lead to male scarcity in viviparous fish? J Fish Biol 53:104–117. [Google Scholar]
- Gasparini C, Andreatta G, Pilastro A, 2012. Ovarian fluid of receptive females enhances sperm velocity. Naturwissenschaften 99:417–420. [DOI] [PubMed] [Google Scholar]
- Gasparini C, Marino IA, Boschetto C, Pilastro A, 2010. Effect of male age on sperm traits and sperm competition success in the guppy Poecilia reticulata. J Evol Biol 23:124–135. [DOI] [PubMed] [Google Scholar]
- Gasparini C, Pilastro A, 2011. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc R Soc B Biol Sci 278:2495–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini C, Serena G, Pilastro A, 2013. Do unattractive friends make you look better? Context-dependent male mating preferences in the guppy. Proc R Soc B 280:20123072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin JGJ, Auld HL, 2013. Covariation and repeatability of male mating effort and mating preferences in a promiscuous fish. Ecol Evol 3:2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin JGJ, McDonough HE, 2003. Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behav Ecol 14:194–200. [Google Scholar]
- Grether GF, 2001. Carotenoid scarcity, synthetic pteridine pigments and the evolution of sexual coloration in guppies Poecilia reticulata. Proc R Soc B Biol Sci 268:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven H, 2011. Gonads, genitals, and reproductive biology In: Evans JP, Pilastro A, Schlupp I, editors. Ecology and Evolution of Poeciliid Fishes. 5–17, Chicago: University of Chicago Press. [Google Scholar]
- Guevara-Fiore P, Skinner A, Watt PJ, 2009. Do male guppies distinguish virgin females from recently mated ones? Anim Behav 77:425–431. [Google Scholar]
- Guevara-Fiore P, Stapley J, Watt PJ, 2010. Mating effort and female receptivity: how do male guppies decide when to invest in sex? Behav Ecol Sociobiol 64:1665–1672. [Google Scholar]
- Hamilton WD, 1964a. The genetical evolution of social behaviour I. J Theor Biol 7:1–16. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, 1964b. The genetical evolution of social behaviour II. J Theor Biol 7:17–52. [DOI] [PubMed] [Google Scholar]
- Hardling R, Gosden T, Aguilee R, 2008. Male mating constraints affect mutual mate choice: prudent male courting and sperm-limited females. Am Nat 172:259–271. [DOI] [PubMed] [Google Scholar]
- Haskins CP, Haskins EF, 1949. The role of sexual selection as an isolating mechanism in 3 species of Poeciliid fishes. Evolution 3:160–169. [DOI] [PubMed] [Google Scholar]
- Haskins CP, Haskins EF, 1950. Factors governing sexual selection as an isolating mechanism in the Poeciliid fish Lebistes reticulatus. Proc Natl Acad Sci USA 36:464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head ML, Jacomb F, Vega-Trejo R, Jennions MD, 2015. Male mate choice and insemination success under simultaneous versus sequential choice conditions. Anim Behav 103:99–105. [Google Scholar]
- Helfman G, Collette BB, Facey DE, Bowen BW, 2009. The Diversity of Fishes: Biology, Evolution, and Ecology. 2nd edn.Chichester, UK: Wiley-Blackwell. [Google Scholar]
- Herdman EJE, Kelly CD, Godin JGJ, 2004. Male mate choice in the guppy Poecilia reticulata: do males prefer larger females as mates? Ethology 110:97–111. [Google Scholar]
- Heubel KU, Plath M, 2008. Influence of male harassment and female competition on female feeding behaviour in a sexual - asexual mating complex of mollies (Poecilia mexicana, P. formosa). Behav Ecol Sociobiol 62:1689–1699. [Google Scholar]
- Heubel KU, Rankin DJ, Kokko H, 2009. How to go extinct by mating too much: population consequences of male mate choice and efficiency in a sexual - asexual species complex. Oikos 118:513–520. [Google Scholar]
- Heubel KU, Schlupp I, 2006. Turbidity affects association behaviour in male Poecilia latipinna. J Fish Biol 68:555–568. [Google Scholar]
- Heubel KU, Schlupp I, 2008. Seasonal plasticity in male mating preferences in sailfin mollies. Behav Ecol 19:1080–1086. [Google Scholar]
- Hoysak DJ, Godin JGJ, 2007. Repeatability of male mate choice in the mosquitofish, Gambusia holbrooki. Ethology 113:1007–1018. [Google Scholar]
- Hrbek T, Seckinger J, Meyer A, 2007. A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Mol Phylogenet Evol 43:986–998. [DOI] [PubMed] [Google Scholar]
- Hubbs C, Delco EA, 1960. Mate preference in males of 4 species of gambusiine fishes. Evolution 14:145–152. [Google Scholar]
- Hubbs CL, Hubbs LC, 1932. Apparent parthenogenesis in nature in a form of fish of hybrid origin. Science 76:628–630. [DOI] [PubMed] [Google Scholar]
- Jeswiet SB, Godin JGJ, 2011. Validation of a method for quantifying male mating preferences in the guppy Poecilia reticulata. Ethology 117:422–429. [Google Scholar]
- Jeswiet SB, Lee-Jenkins SSY, Godin JGJ, 2012. Concurrent effects of sperm competition and female quality on male mate choice in the Trinidadian guppy Poecilia reticulata. Behav Ecol 23:195–200. [Google Scholar]
- Jeswiet SB, Lee-Jenkins SSY, Ramnarine IW, Godin JGJ, 2011. Sperm competition risk and mate choice in male Trinidadian guppies Poecilia reticulata. Anim Behav 81:639–644. [Google Scholar]
- Jordan RC, Howe DV, Beavers A, Dean A, Gould JL, 2006. Female associative behavior accompanies morphological distinction in two Panamanian populations of the molly Poecilia gilli (Kner). J Freshw Ecol 21:47–52. [Google Scholar]
- Kodric-Brown A, 1993. Female choice of multiple male criteria in guppies: interacting effects dominance, coloration and courtship. Behav Ecol Sociobiol 32:415–420. [Google Scholar]
- Krupa JJ, 1995. How likely is male mate choice among Anurans. Behaviour 132:643–664. [Google Scholar]
- Kuhn TS, 1962. The Structure of Scientific Revolutions. Chicago: University of Chicago Press. [Google Scholar]
- Langerhans RB, Gifford ME, Joseph EO, 2007. Ecological speciation in Gambusia fishes. Evolution 61:2056–2074. [DOI] [PubMed] [Google Scholar]
- Langerhans RB, Layman CA, DeWitt TJ, 2005. Male genital size reflects a tradeoff between attracting mates and avoiding predators in two live-bearing fish species. Proc Natl Acad Sci USA 102:7618–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen J, Parker GA, Scharer L, 2016. Why anisogamy drives ancestral sex roles. Evolution 70:1129–1135. [DOI] [PubMed] [Google Scholar]
- MacLaren RD, Fontaine A, 2013. Incongruence between the sexes in preferences for body and dorsal fin size in Xiphophorus variatus. Behav Processes 92:99–106. [DOI] [PubMed] [Google Scholar]
- MacLaren RD, Rowland WJ, 2006. Female preference for male lateral projection area in the shortfin molly Poecilia mexicana: evidence for a pre-existing bias in sexual selection. Ethology 112:678–690. [Google Scholar]
- Magurran AE, 2001. Sexual conflict and evolution in Trinidadian guppies. Genetica 112:463–474. [PubMed] [Google Scholar]
- Magurran AE, Seghers BH, 1994a. A cost of sexual harassment in the guppy Poecilia reticulata. Proc R Soc Lond Ser B Biol Sci 258:89–92. [Google Scholar]
- Magurran AE, Seghers BH, 1994b. Sexual conflict as a consequence of ecology: evidence from guppy Poecilia reticulata, populations in Trinidad. Proc R Soc Lond Ser B Biol Sci 255:31–36. [Google Scholar]
- Makowicz AM, Plath M, Schlupp I, 2010. Male guppies Poecilia reticulata adjust their mate choice behaviour to the presence of an audience. Behaviour 147:1657–1674. [Google Scholar]
- Makowicz AM, Schlupp I, 2013. The direct costs of living in a sexually harassing environment. Anim Behav 85:569–577. [Google Scholar]
- Makowicz AM, Schlupp I, 2015. Effects of female - female aggression in a sexual/unisexual species complex. Ethology 121:903–914. [Google Scholar]
- Makowicz AM, Tiedemann R, Steele RN, Schlupp I, 2016. Kin recognition in a clonal fish Poecilia formosa. Plos ONE 11:e0158442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH, Johnsen S, 2007. A field test of the Hamilton - Zuk hypothesis in the Trinidadian guppy Poecilia reticulata. Behav Ecol Sociobiol 61:1897–1909. [Google Scholar]
- Matthews IM, Evans JP, Magurran AE, 1997. Male display rate reveals ejaculate characteristics in the Trinidadian guppy Poecilia reticulata. Proc R Soc Lond Ser B Biol Sci 264:695–700. [Google Scholar]
- McLaren RD, Rowland WJ, Morgan N, 2004. Female preferences for sailfin and body size in the sailfin molly Poecilia latipinna. Ethology 110:363–379. [Google Scholar]
- McLennan DA, Ryan MJ, 1997. Responses to conspecific and heterospecific olfactory cues in swordtail Xiphophorus cortezi. Anim Behav 54:1077–1088. [DOI] [PubMed] [Google Scholar]
- Méndez-Janovitz M, Macías Garcia C, 2017. Do male fish prefer them big and colourful? Non–random male courtship effort in a viviparous fish with negligible paternal investment. Behav Ecol Sociobiol 71:160. [Google Scholar]
- Meredith RW, Pires MN, Reznick DN, Springer MS, 2010. Molecular phylogenetic relationships and the evolution of the placenta in Poecilia (Micropoecilia) (Poeciliidae: Cyprinodontiformes). Mol Phylogenet Evol 55:631–639. [DOI] [PubMed] [Google Scholar]
- Meredith RW, Pires MN, Reznick DN, Springer MS, 2011. Molecular phylogenetic relationships and the coevolution of placentotrophy and superfetation in Poecilia (Poeciliidae: Cyprinodontiformes). Mol Phylogenet Evol 59:148–157. [DOI] [PubMed] [Google Scholar]
- Milam EL, 2010. Looking for a Few Good Males. Baltimore: The Johns Hopkins University Press. [Google Scholar]
- Morris MR, Ryan MJ, 1990. Age at sexual maturity of male Xiphophorus nigrensis in nature. Copeia 1990:747–751. [Google Scholar]
- Muraco JJ, Aspbury AS, Gabor CR, 2014. Does male behavioral type correlate with species recognition and stress? Behav Ecol 25:200–205. [Google Scholar]
- Nöbel S, Witte K, 2013. Public information influences sperm transfer to females in sailfin molly males. Plos One 8, https://doi.org/10.1371/journal.pone.0053865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norazmi-Lokman NH, Purser GJ, Patil JG, 2016. Gravid spot predicts developmental progress and reproductive output in a Livebearing fish Gambusia holbrooki. Plos One 11, https://doi.org/10.1371/journal.pone.0147711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VA, Owens IPF, 1998. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514. [DOI] [PubMed] [Google Scholar]
- Olsson KH, Johansson S, Blom E-L, Lindström K, Svensson O. et al. , 2017. Dark eyes in female sand gobies indicate readiness to spawn. PLoS One 12, https://doi.org/10.1371/journal.pone.0177714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti LR, Rauchenberger M, 1989. Systematic overview of the poeciliines In: Meffe GK, Snelson FF, editors. Ecology and Evolution of Livebearing Fishes (Poeciliidae ) Englewood Cliffs: Prentice Hall; 3–12. [Google Scholar]
- Parker GA, 2006. Sexual conflict over mating and fertilization: an overview. Philos Trans R Soc B: Biol Sci 361:235–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parzefall J, 1973. Attraction and sexual cycle of poeciliids In: Schröder JH, editor. Genetics and Mutagenesis of Fish. Berlin: Springer; 177–183. [Google Scholar]
- Petrazzini MEM, Bisazza A, Agrillo C, Lucon-Xiccato T, 2017. Sex differences in discrimination reversal learning in the guppy. Anim Cogn 20:1081–1091. [DOI] [PubMed] [Google Scholar]
- Plath M, Blum D, Schlupp I, Tiedemann R, 2008a. Audience effect alters mating preferences in a livebearing fish, the Atlantic molly Poecilia mexicana. Anim Behav 75:21–29. [Google Scholar]
- Plath M, Heubel KU, Garcia de Leon FJ, Schlupp I, 2005. Cave molly females (Poecilia mexicana, Poeciliidae, Teleostei) like well-fed males. Behav Ecol Sociobiol 58:144–151. [Google Scholar]
- Plath M, Makowicz AM, Schlupp I, Tobler M, 2007. Sexual harassment in live-bearing fishes (Poeciliidae): comparing courting and noncourting species. Behav Ecol 18:680–688. [Google Scholar]
- Plath M, Richter S, Schlupp I, Tiedemann R, 2010. Misleading mollies: surface- but not cave-dwelling Poecilia mexicana males deceive competitors about mating preferences. Acta Ethologica 13:49–56. [Google Scholar]
- Plath M, Richter S, Tiedemann R, Schlupp I, 2008b. Male fish deceive competitors about mating preferences. Curr Biol 18:1138–1141. [DOI] [PubMed] [Google Scholar]
- Plath M, Seggel U, Burmeister H, Heubel KU, Schlupp I, 2006. Choosy males from the underground: male mating preferences in surface- and cave-dwelling Atlantic mollies Poecilia mexicana. Naturwissenschaften 93:103–109. [DOI] [PubMed] [Google Scholar]
- Plath M, Tobler M, 2007. Sex recognition in surface- and cave-dwelling Atlantic molly females (Poecilia mexicana, Poeciliidae, Teleostei): influence of visual and non-visual cues. Acta Ethologica 10:81–88. [Google Scholar]
- Poiani A, 2010. Animal Homosexuality. Cambridge: Cambridge University Press. [Google Scholar]
- Pollux BJA, Reznick DN, 2011. Matrotrophy limits a female's ability to adaptively adjust offspring size and fecundity in fluctuating environments. Funct Ecol 25:747–756. [Google Scholar]
- Ptacek MB, Travis J, 1997. Mate choice in the sailfin molly Poecilia latipinna. Evolution 51:1217–1231. [DOI] [PubMed] [Google Scholar]
- Rankin DJ, Kokko H, 2007. Do males matter? The role of males in population dynamics. Oikos 116:335–348. [Google Scholar]
- Reynolds JD, Gross MR, 1992. Female mate preference enhances offspring growth and reproduction in a fish Poecilia reticulata. Proc R Soc Lond Ser B Biol Sci 250:57–62. [Google Scholar]
- Reznick D, Bryant M, Holmes D, 2006. The evolution of senescence and post-reproductive lifespan in guppies Poecilia reticulata. PLoS Biol 4:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick D, Ghalambor C, Nunney L, 2002. The evolution of senescence in fish. Mech Ageing Dev 123:773–789. [DOI] [PubMed] [Google Scholar]
- Riesch R, Plath M, Makowicz AM, Schlupp I, 2012. Behavioural and life-history regulation in a unisexual/bisexual mating system: does male mate choice affect female reproductive life histories? Biol J Linnean Soc 106:598–606. [Google Scholar]
- Riesch R, Plath M, Schlupp I, 2011. Speciation in caves: experimental evidence that permanent darkness promotes reproductive isolation. Biol Lett 7:909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesch R, Schlupp I, Plath M, 2008. Female sperm limitation in natural populations of a sexual/asexual mating complex (Poecilia latipinna, Poecilia formosa). Biol Lett 4:266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Cardenas O, Morris MR, 2011. Precopulatory sexual selection In: Evans JP, Pilastro A, Schlupp I, editors. Ecology and Evolution of Poeciliid Fishes. Chicago: University of Chicago Press; 187–196. [Google Scholar]
- Robinson DM, Aspbury AS, Gabor CR, 2008. Differential sperm expenditure by male sailfin mollies, Poecilia latipinna, in a unisexual - bisexual species complex and the influence of spermiation during mating. Behav Ecol Sociobiol 62:705–711. [Google Scholar]
- Robinson DM, Konkin-Garcia T, Espinedo CM, Gabor CR, Aspbury AS, 2011. Seasonal effects on female fecundity and male sperm availability in a thermally stable temperate population of sailfin mollies Poecilia latipinna. Am Midl Nat 166:394–403. [Google Scholar]
- Rosenthal GG, 2017. Mate Choice. Princeton: Princeton University Press. [Google Scholar]
- Rosenthal GG, Evans CS, 1998. Female preference for swords in Xiphophorus helleri reflects a bias for large apparent size. Proc Natl Acad Sci USA 95:4431–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal GG, Fitzsimmons JN, Woods KU, Gerlach G, Fisher HS, 2011. Tactical release of a sexually-selected pheromone in a swordtail fish. PLoS One 6, https://doi.org/10.1371/journal.pone.0016994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, 2013. Proximate perspectives on the evolution of female aggression: good for the gander, good for the goose? Philos Trans R Soc B: Biol Sci 368:20130083 http://dx.doi.org/10.1098/rstb.2013.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein DR, Lovette IJ, 2009. Reproductive skew and selection on female ornamentation in social species. Nature 462:786–789. [DOI] [PubMed] [Google Scholar]
- Rüschenbaum S, Schlupp I, 2013. Non-visual mate choice ability in a cavefish Poecilia mexicana is not mechanosensory. Ethology 119:368–376. [Google Scholar]
- Ryan MJ, Cummings ME, 2013. Perceptual biases and mate choice. Annu Rev Ecol Evol Syst 44:437. [Google Scholar]
- Ryan MJ, Keddy-Hector A, 1992. Directional patterns of female mate choice and the role of sensory biases. Am Nat 139(s1):S4–S35. [Google Scholar]
- Ryan MJ, Pease CM, Morris MR, 1992. A genetic polymorphism in the swordtail Xiphophorus nigrensis: testing the prediction of equal fitnesses. Am Nat 139:21–31. [Google Scholar]
- Schärer L, Rowe L, Arnqvist G, 2012. Anisogamy, chance and the evolution of sex roles. Trends Ecol Evol 27:260–264. [DOI] [PubMed] [Google Scholar]
- Scharnweber K, Plath M, Tobler M, 2011a. Feeding efficiency and food competition in coexisting sexual and asexual livebearing fishes of the genus Poecilia. Environ Biol Fish 90:197–205. [Google Scholar]
- Scharnweber K, Plath M, Winemiller KO, Tobler M, 2011b. Dietary niche overlap in sympatric asexual and sexual livebearing fishes Poecilia spp. J Fish Biol 79:1760–1773. [DOI] [PubMed] [Google Scholar]
- Schlupp I, 2005. The evolutionary ecology of gynogenesis. Annu Rev Ecol Evol Syst 36:399–417. [Google Scholar]
- Schlupp I, 2009. Behavior of fishes in the sexual/unisexual mating system of the Amazon molly Poecilia formosa. Advances in the Study of Behavior 39:153–183. [Google Scholar]
- Schlupp I, McKnab R, Ryan MJ, 2001. Sexual harassment as a cost for molly females: bigger males cost less. Behaviour 138:277–286. [Google Scholar]
- Schlupp I, Parzefall J, Schartl M, 1991. Male mate choice in mixed bisexual unisexual breeding complexes of Poecilia (Teleostei, Poeciliidae). Ethology 88:215–222. [Google Scholar]
- Schlupp I, Parzefall J, Schartl M, 2002. Biogeography of the Amazon molly Poecilia formosa. J Biogeogr 29:1–6. [Google Scholar]
- Schlupp I, Plath M, 2005. Male mate choice and sperm allocation in a sexual/asexual mating complex of Poecilia (Poeciliidae, Teleostei). Biol Lett 1:169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlupp I, Riesch R, 2011. Evolution of unisexual reproduction In: Evans JP, Pilastro A, Schlupp I, editors. Ecology and Evolution of Poeciliid Fishes. 50–57, Chicago: University of Chicago Press. [Google Scholar]
- Schlupp I, Ryan MJ, 1997. Male sailfin mollies (Poecilia latipinna) copy the mate choice of other males. Behav Ecol 8:104–107. [Google Scholar]
- Schulz-Mirbach T, Hess M, Plath M, 2011. Inner ear morphology in the Atlantic molly Poecilia mexicana: first detailed microanatomical study of the inner ear of a Cyprinodontiform species. Plos One 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Mirbach T, Ladich F, Riesch R, Plath M, 2010. Otolith morphology and hearing abilities in cave- and surface-dwelling ecotypes of the Atlantic molly Poecilia mexicana (Teleostei: poeciliidae). Hear Res 267:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servedio MR, 2007. Male versus female mate choice: sexual selection and the evolution of species recognition via reinforcement. Evolution 61:2772–2789. [DOI] [PubMed] [Google Scholar]
- Simcox H, Colegrave N, Heenan A, Howard C, Braithwaite VA, 2005. Context-dependent male mating preferences for unfamiliar females. Anim Behav 70:1429–1437. [Google Scholar]
- Snelson FF, Jr., 1984. Seasonal maturation and growth of males in a natural population of Poecilia latipinna. Copeia 1984:252–255. [Google Scholar]
- Spikes M, Schlupp I Is Courtship Not Enough?: Courtship in Livebearing Limia (Poeciliidae) Does Not Influence Male Mate Preference. In preparation. [Google Scholar]
- Stöck M, Lampert KP, Möller D, Schlupp I, Schartl M, 2010. Monophyletic origin of multiple clonal lineages in an asexual fish Poecilia formosa. Mol Ecol 19:5204–5215. [DOI] [PubMed] [Google Scholar]
- Temrin H, Sillentullberg B, 1994. The evolution of avian mating systems: a phylogenetic analysis of male and female polygamy and length of pair bond. Biol J Linnean Soc 52:121–149. [Google Scholar]
- Tobler M, Franssen C, Plath M, 2008. Male-biased predation of a cave fish by a giant water bug. Naturwissenschaften 95:775–779. [DOI] [PubMed] [Google Scholar]
- Tobler M, Plath M, 2011. Living in extreme environments In: Evans JP, Pilastro A, Schlupp I, editors. Ecology and Evolution of Poeciliid Fishes. Chicago: University of Chicago Press; 120–127. [Google Scholar]
- Tobler M, Schlupp I, 2008a. Expanding the horizon: the Red Queen and potential alternatives. Can J Zool 86:765–773. [Google Scholar]
- Tobler M, Schlupp I, 2008b. Influence of black spot disease on shoaling behaviour in female western mosquitofish Gambusia affinis (Poeciliidae, Teleostei). Environ Biol Fish 81:29–34. [Google Scholar]
- Travis J, Woodward BD, 1989. Social context and courtship flexibility in male sailfin mollies Poecilia latipinna, Pisces, Poeciliidae. Anim Behav 38:1001–1011. [Google Scholar]
- Trexler JC, Tempe RC, Travis J, 1994. Size-selective predation of sailfin mollies by two species of heron. Oikos 69:250–258. [Google Scholar]
- Trivers R, 1972. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man. 139–179, Chicago, IL: Aldine. [Google Scholar]
- Varela SAM, Matos M, Schlupp I, 2018. The role of mate-choice copying in speciation and hybridization. Biol Rev. Online, https://doi.org/10.1111/brv.12397. [DOI] [PubMed] [Google Scholar]
- Verzijden MN, ten Cate C, Servedio MR, Kozak GM, Boughman JW. et al. , 2012. The impact of learning on sexual selection and speciation. Trends Ecol Evol 27:511–519. [DOI] [PubMed] [Google Scholar]
- Vincent A, Ahnesjö I, Berglund A, Rosenqvist G, 1992. Pipefishes and seahorses: are they all sex role reversed? Trends Ecol Evol 7:237–241. [DOI] [PubMed] [Google Scholar]
- Wagner WEJ, 1998. Measuring female mating preferences. Anim Behav 55:1029–1042. [DOI] [PubMed] [Google Scholar]
- Warren WC, García-Pérez R, Xu S, Lampert KP, Chalopin D. et al. , 2018. Clonal polymorphism and high heterozygosity in the celibate genome of the Amazon molly. Nat Ecol Evol 2:669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte K, 2006. Time spent with a male is a good indicator of mate preference in female zebra finches. Ethol Ecol Evol 18:195–204. [Google Scholar]
- Witte K, Kniel N, Kureck IM, 2015. Mate-choice copying: status quo and where to go. Curr Zool 61:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Ohkubo Y, Karino K, Hasegawa E, 2016. Male guppies change courtship behaviour in response to their own quality relative to that of a rival male. Anim Behav 118:33–37. [Google Scholar]
- Zuk M, 1993. Feminism and the study of animal behavior. Bioscience 43:774–778. [Google Scholar]


