Abstract
Background
Gains in life expectancy through optimal control of HIV infection with antiretroviral therapy (ART) may be threatened if other comorbidities, such as diabetes, are not optimally managed.
Methods
We analyzed cross-sectional data of the Women’s Interagency HIV Study (WIHS) from 2001, 2006, and 2015. We estimated the proportions of HIV-positive and HIV-negative women with diabetes who were engaged in care and achieved treatment goals (hemoglobin A1c [A1c] <7.0%, blood pressure [BP] <140/90 mmHg, low-density lipoprotein [LDL] cholesterol <100 mg/dL, not smoking) and viral suppression. Repeated-measures models were used to estimate the adjusted prevalence of achieving each diabetes treatment goal at each time point, by HIV status.
Results
We included 486 HIV-positive and 258 HIV-negative women with diabetes. In 2001, 91.8% visited a health care provider, 60.7% achieved the A1c target, 70.5% achieved the BP target, 38.5% achieved the LDL cholesterol target, 49.2% were nonsmokers, 23.3% achieved combined ABC targets (A1c, BP, and cholesterol), and 10.9% met combined ABC targets and did not smoke. There were no differences by HIV status, and patterns were similar in 2006 and 2015. Among HIV-positive women, viral suppression increased from 41% in 2001 to 87% in 2015 compared with 8% and 13% achieving the ABC goals and not smoking. Viral suppression was not associated with achievement of diabetes care goals.
Conclusions
Successful management of HIV is outpacing that of diabetes. Future studies are needed to identify factors associated with gaps in the HIV–diabetes care continuum and design interventions to better integrate effective diabetes management into HIV care.
Keywords: care continuum, diabetes, HIV, quality
Antiretroviral therapy (ART) has transformed HIV into a chronic disease, leading to life expectancies nearing that of the general population [1]. However, as people living with HIV (PLHIV) age, it is predicted that up to 84% will have at least 1 noncommunicable chronic disease (eg, diabetes) by the year 2030 [2]. Of note, the HIV and type 2 diabetes mellitus (DM) epidemics are converging in the United States, as PLHIV have a 1.6 times adjusted prevalence of DM compared with the general population [3]. Globally, noncommunicable chronic diseases (NCDs), including DM, are increasing and are projected to account for a growing proportion of costs in care for PLHIV [4]. Although older age and obesity are associated with the development of DM [5, 6], data show that PLHIV are more likely to develop DM at younger ages and without obesity compared with the general population [3]. The dual diagnosis of HIV and DM is troubling given their independent association with higher risk of cardiovascular disease (CVD), the leading cause of mortality in PLHIV [7]. Among PLHIV, the gains in life expectancy through control of HIV with ART may be threatened if other comorbidities, such as DM, are not optimally managed.
The HIV care continuum approach offers lessons for the management of NCDs such as DM. The HIV care continuum was introduced in 2011 to conceptualize and measure key stages of diagnosis and treatment necessary to achieve and maintain viral suppression [8]. The continuum assesses the proportion of HIV-positive patients aware of their condition who are engaged in care, retained in care, and achieving viral suppression. It is now the standard for measuring the quality of HIV care and assessing overall progress in controlling the HIV epidemic [9]. The care continuum serves as a critical tool to understand where gaps in care exist and where to target both clinical and population-based interventions [10]. More recently, the care continuum was applied to DM in the United States, showing a decline in each step from diagnosis of diabetes to being engaged/retained in diabetes care to achieving important clinical benchmarks for diabetes control [11]. Both the HIV and DM care continua are characterized by suboptimal disease control and significant health disparities in age, gender, and race/ethnicity [11, 12].
Few studies have reported on the achievement of DM treatment goals among PLHIV [13, 14], and no study has yet comprehensively described the DM care continuum among PLHIV. Motivated by the impact of the HIV care continuum on informing HIV quality improvement interventions, we characterized and compared being at target for DM care goals among HIV-positive and matched HIV-negative women from the Women’s Interagency HIV Study (WIHS) over the past 15 years. Gaps identified along the HIV–DM care continuum can guide further research and programs to improve DM care among PLHIV.
METHODS
Participants
The WIHS was established in 1994 and is the largest multicenter prospective cohort study of comparable HIV-positive and HIV-negative women aimed to investigate the natural history of women with HIV and those at risk for HIV infection in the United States [15]. From 1994 to 2012, the WIHS was comprised of 6 sites (Bronx/Manhattan, NY; Brooklyn, NY; Chicago, IL; Washington, DC; San Francisco, CA; and Los Angeles, CA). Since 2012, the WIHS has been comprised of 5 of the 6 original sites (the Los Angeles, CA, site is no longer included), and 5 southern sites have been added (Miami, FL; Atlanta, GA; Jackson, MS; Birmingham, AL; and Chapel Hill, NC). We analyzed cross-sectional data from all WIHS sites at 3 time points: 2001 (6 sites), 2006 (6 sites), and 2015 (10 sites). The years were selected based on availability of A1c data in the cohort. A total of 4982 women (n = 3678 HIV-positive, n = 1304 HIV-negative) were enrolled in 4 waves, 1994–1995 (n = 2623), 2001–2002 (n = 1143), 2011–2012 (n = 371), and 2013–2015 (n = 845). The WIHS site and recruitment wave were considered for each participant.
In the current analysis, we included participants with confirmed diabetes, defined as having at least 1 of the following: (1) self-reported use of antidiabetic medication; (2) a fasting glucose (FG) ≥126 mg/dL, confirmed by A1c ≥6.5% or a subsequent FG ≥126 mg/dL; (3) a hemoglobin A1c ≥6.5%, confirmed by a FG ≥126 mg/dL; (4) a self-report of diabetes, confirmed by 2 FG ≥126 mg/dL; or (5) a hemoglobin A1c ≥6.5% concurrent with an FG ≥126 mg/dL [6]. Duration of DM represents the period in which a woman had DM while enrolled in the WIHS cohort.
Data Collection
WIHS participants completed semi-annual study visits consisting of a comprehensive physical exam, collection of serum and plasma for laboratory analyses, and an interviewer-administered survey collecting information such as demographics, social characteristics, disease characteristics, and medication-related information. Study design, survey instruments, and data collection methods were previously described [15].
Care Goal Measurements
Measures to define diabetes (A1c, blood pressure [BP], cholesterol, smoking status) and HIV care goals (viral load) were collected using standardized techniques that have been described in detail elsewhere [16]. All laboratory measures were conducted annually, whereas BP measurements and smoking behavior were measured every 6 months. Some clinical data were only collected on alternating visits, and those data were obtained from the next consecutive visit.
Covariate Definitions
HIV-positive and -negative participants were classified as having seen any health care provider if they provided a positive response to the question “Have you seen a health care provider (HCP) since your last WIHS visit?” This question was asked at every WIHS visit, or every 6 months across all years for both HIV-positive and -negative participants. This question was used as a surrogate for engagement in care. Other covariates included age, race/ethnicity, self-reported health insurance status (ie, present or absent), income, education level, and body mass index (BMI; kg/m2). Waist circumference was measured in centimeters. Medication use related to diabetes, hypertension, hyperlipidemia, and HIV was self-reported. The HIV viral load was measured using TaqMan HIV-1 RNA quantitative polymerase chain reaction.
Diabetes and Treatment Goal Definitions
We used an A1c goal <7.0% and BP goal of systolic blood pressure (SBP) <140 mmHg and diastolic blood pressure (DBP) <90 mmHg, based on the American Diabetes Association 2017 standards [17]. The cholesterol goal was low-density lipoprotein (LDL) <100 mg/dL, consistent with primary prevention in the American College of Cardiology/American Heart Association (ACC/AHA) 2013 guidelines and the older Adult Treatment Panel (ATP) III Guidelines [18]. Viral suppression was considered the last HIV-1 RNA being <200 copies/mL or undetectable. ABC control was defined as achieving control of A1c, BP, and cholesterol. ABC + nonsmoking was defined as achieving control of A1c, BP, cholesterol, and not smoking.
Statistical Analysis
Descriptive statistics (counts and percentages for categorical variables, means and SDs for continuous variables, and medians and interquartile ranges for continuous non–normally distributed variables) were assessed by study year (2001, 2006, 2015) and HIV status. Univariate analyses, including chi-square or Fisher exact tests for categorical variables and t tests or Wilcoxon rank-sum tests for continuous variables, were used to compare demographic and clinical characteristics by HIV status and by study year. Care continua were developed for each study year and included A1c control, BP control, cholesterol control, smoking, engagement in care, viral suppression, ABC control, and ABC control + nonsmoking. For viral suppression, A1c, BP, and cholesterol control, we determined the proportion of participants treated with medication.
To account for repeated measures in longitudinally collected WIHS data, logistic generalized estimating equation (GEE) models with a compound symmetry covariance structure were used to estimate the prevalence of achieving the control outcome of interest in each of the 3 years (2001, 2006, 2015) and by HIV status. Covariates included insurance status, income, age, WIHS site and recruitment wave, race/ethnicity, education, BMI, waist circumference, and duration of DM. In addition to the aforementioned covariates, models were adjusted for other variables clinically relevant to the specific situations. The A1c control model included use of DM medication, the BP control model included use of BP medication, the LDL control model included use of cholesterol medication, and the ABC model included use of DM, BP, and cholesterol medications. The viral suppression model included any ART use and was restricted to HIV-positive women. As a sensitivity analysis, to evaluate the effect of repeated measures, all GEE models were re-analyzed assuming independent observations. Model fit was assessed using Hosmer-Lemeshow, diagnostic plots, and/or predictive ability.
RESULTS
Population Characteristics
Of 681 HIV-positive and HIV-negative women with DM during the study period, 605 were included in the current analysis. Supplementary Figure 1 shows the criteria for DM that each participant met. We excluded women with missing information about health care providers (n = 20), A1c (n = 46), BP (n = 1), and LDL (n = 9). Characteristics of the analytical subsample in 2001, 2006, and 2015 are reported in Table 1. The HIV-positive and HIV-negative women were similar in demographic characteristics, except for the proportion with a high school degree in 2006, insurance status in 2006 and 2015, and BMI and waist circumference in 2001 and 2006.
Table 1.
Baseline Characteristics of HIV-Positive and HIV-Negative Women With Diabetes Who Attended at Least a Single WIHS Study Visit During the Indicated Years
| 2001 | 2006 | 2015 | ||||
|---|---|---|---|---|---|---|
| HIV + | HIV - | HIV + | HIV - | HIV + | HIV - | |
| n = 86 | n = 36 | n = 222 | n = 92 | n = 282 | n = 130 | |
| Age, mean (SD), y | 45.7 (7.7) | 43.5 (7.5) | 47.8 (8.1) | 45.7 (8.8) | 52.8 (8.1) | 52.2 (7.8) |
| Race, % | ||||||
| White, NH | 13.9 | 5.6 | 14.9 | 7.6 | 9.9 | 4.6 |
| AA, NH | 62.8 | 66.7 | 58.1 | 57.6 | 69.9 | 69.2 |
| Hispanic | 19.8 | 27.7 | 23.8 | 32.6 | 17.0 | 20.8 |
| Other | 3.5 | 0 | 3.2 | 2.2 | 3.2 | 5.4 |
| Education, % | ||||||
| <HS | 31.4 | 41.7 | 34.7 | 42.4 | 35.5 | 36.2 |
| HS | 36.1 | 38.9 | 30.6 | 36.9 | 30.9 | 32.3 |
| >HS | 32.5 | 19.4 | 34.7 | 20.7a | 33.7 | 31.6 |
| Income, % | ||||||
| <$12 000 | 72.1 | 63.9 | 52.5 | 53.9 | 59.6 | 54.4 |
| $12 001–24 000 | 22.1 | 16.7 | 24.7 | 23.6 | 23.6 | 23.2 |
| >$24 000 | 5.8 | 19.4 | 22.8 | 22.5 | 16.7 | 22.4 |
| WIHS site, % | ||||||
| NY | 31.4 | 44.4 | 36.9 | 47.8 | 34.0 | 33.9 |
| DC | 8.1 | 8.3 | 13.5 | 9.8 | 11.7 | 16.9 |
| CA | 38.4 | 33.3 | 32.4 | 32.6 | 11.7 | 13.1 |
| Chicago | 22.1 | 13.9 | 17.1 | 9.8 | 14.2 | 5.4 |
| Southern | 0 | 0 | 0 | 0 | 28.4 | 30.8 |
| Uninsured, % | 7.0 | 16.7 | 5.4 | 15.2a | 3.2 | 13.9a |
| BMI, mean (SD), kg/m2 | 32.0 (8.5) | 36.4 (8.8)a | 30.8 (8.1) | 35.7 (8.9)a | 35.3 (9.6) | 35.2 (8.1) |
| Waist circum., mean (SD), cm | 98.7 (16.5) | 109.5 (18.3)a | 98.5 (15.2) | 105.3 (19.1)a | 110.4 (17.1) | 109.5 (16.4) |
| Duration diabetes, median (IQR),b y | 2.7 (0.5–6.1) | 1.1 (0.2–5.3) | 3.9 (2.1–6.4) | 4.0 (2.2–5.5) | 5.9 (1.5–12.2) | 3.9 (1.4–11.6) |
| CD4 count, mean (SD), cells/µL | 521 (354) | NA | 542 (316) | NA | 735 (374) | NA |
Abbreviations: AA, African American; BMI, body mass index; IQR, interquartile range; NH, non-Hispanic; WIHS, Women’s Interagency HIV Study.
aStatistically significant differences between the HIV-positive and -negative populations are indicated: P < .05.
bDuration of diabetes indicates the duration of diabetes while the participant was enrolled in WIHS.
Care Continuum Outcomes Over Time
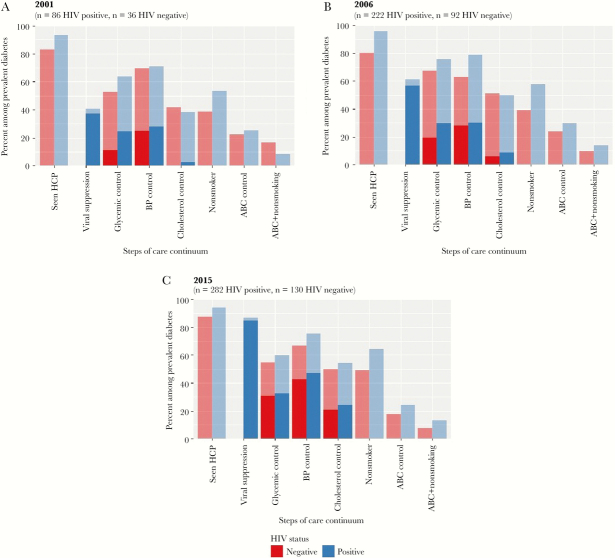
Figure 1 shows the HIV–DM care continuum by HIV status among women with DM in 2001 (A), 2006 (B), and 2015 (C). The values plotted in Figure 1 are reported in Supplementary Table 1. In 2001, 122 HIV-positive and -negative women had DM, 314 in 2006, and 412 in 2015. Most women had visited a health care provider since their last study visit across the 3 time points (91.8%, 91.4%, and 92.2%, respectively). The proportion of women achieving the A1c goal was 60.7% in 2001, 73.2% in 2006, and 58.0% in 2015. The proportion achieving the BP goal was similar across 2001, 2006, and 2015 (70.5%, 74.5%, and 72.8%, respectively). There was a small but steady increase in the proportion of women achieving the cholesterol goal: 38.5% in 2001, 47.5% in 2006, and 53.2% in 2015. The proportion of women not smoking also increased, from 49.2% in 2001 to 52.2% in 2006 and 59.6% in 2015. The proportion of women achieving combined ABC goals was low across the 3 time points (23.3% in 2001, 26.4% in 2006, and 22.3% in 2015), and even fewer women achieved combined ABC goals and did not smoke (10.9% in 2001, 12.5% in 2006, and 11.4% in 2015). We also explored DM care goals continuously (unadjusted) and report means for HIV-positive and HIV-negative women in Supplementary Table 2. The overall means during the 3 time points ranged as follows: mean A1c (range, 6.6–7.4); mean BP (SBP range, 127–128 mmHg; DBP range, 76–81 mmHg); and mean LDL (range, 100–113 mg/dL).
Figure 1.
Care continuum for HIV-positive and HIV-negative adult women with diabetes, Women’s Interagency HIV Study (A, 2001; B, 2006; C, 2015). Data are presented as a percentage of the prevalent cases of diabetes in each cross-section. In columns with dark and light shading, the column represents those at goal. The lighter shading represents the proportion of patients not on medications, and the darker shading represents patients who self-report taking medications for that diagnosis. Seen HCP: defined by self-report of visiting a health care provider in the prior 12 months. Viral suppression: defined by last viral load of the year being <200 copies/mL. Glycemic control: defined by hemoglobin A1c target of <7.0%. BP control: defined by systolic BP <140 mmHg and Diastolic BP <90 mmHg. Cholesterol control: defined by low-density lipoprotein (LDL) <100 mg/dL. Nonsmoker: defined by self-report of not smoking. ABC control: combined control of hemoglobin A1c level, blood pressure, LDL cholesterol level. ABC + nonsmoker: ABC control plus being nonsmoker. Abbreviations: ABC, A1c, BP, and cholesterol; BP, blood pressure; HCP, health care provider.
Care Continuum Outcomes by HIV Status
In 2001, there were no differences in achieving any of the DM care goals in HIV-positive compared with HIV-negative women (Figure 1). In 2006, more HIV-positive compared with HIV-negative women saw a health care provider (96.0% vs 80.4%, P < .0001), achieved BP control (79.3% vs 63.0%, P = .003), and were nonsmokers (57.7% vs 39.13%, P = .003). In 2015, more HIV-positive compared with HIV-negative women saw a health care provider (94.3% vs 87.7%, P = .02) and were nonsmokers (64.4% vs 49.2%, P = .004). Notably, there were no differences among HIV-positive and HIV-negative women in terms of achieving A1c goals, cholesterol goals, ABC, or the ABC + nonsmoking goals in any year.
Standard multivariable logistic regression models did not differ appreciably from the repeated-measures model (Table 2). HIV-negative women experienced improvement in glycemic control from 2001 to 2015 with prevalence estimates of 0.34 (95% confidence interval [CI], 0.16–0.59) in 2001, 0.56 (95% CI, 0.40–0.70) in 2006, and 0.66 (95% CI, 0.54–0.77) in 2015 (P = .033), whereas the HIV-positive women did not. There were no gains in BP, cholesterol, ABC control, or ABC + nonsmoking in either HIV-positive or -negative women (Table 2).
Table 2.
Adjusted Prevalence Estimatesa for Glycemic Control, BP Control, Cholesterol Control, ABC Control, ABC + Nonsmoking, and Viral Suppression, by Year of Analysis
| Adjusted Prevalence Estimatesa,b (95% CI) | P Values | ||||||
|---|---|---|---|---|---|---|---|
| 2001 | 2006 | 2015 | Difference Between Years | Difference in Trend | Difference in Trend Between HIV- Positive and -Negativec | ||
| Glycemic controld | HIV-positive | 0.53 (0.33–0.73) | 0.68 (0.54–0.79) | 0.69 (0.58–0.78) | .167 | .139 | .448 |
| HIV-negative | 0.34 (0.16–0.59) | 0.56 (0.40–0.70) | 0.66 (0.54–0.77) | .084 | .033 | ||
| BP controle | HIV-positive | 0.73 (0.58–0.84) | 0.82 (0.74–0.89) | 0.85 (0.77–0.90) | .151 | .077 | .238 |
| HIV-negative | 0.70 (0.51–0.84) | 0.64 (0.50–0.76) | 0.78 (0.68–0.86) | .110 | .348 | ||
| Cholesterol controlf | HIV-positive | 0.34 (0.19–0.54) | 0.45 (0.32–0.58) | 0.51 (0.40–0.62) | .229 | .092 | .271 |
| HIV-negative | 0.42 (0.24–0.63) | 0.48 (0.33–0.64) | 0.44 (0.32–0.56) | .749 | .915 | ||
| ABC controlg | HIV-positive | 0.21 (0.10–0.40) | 0.21 (0.13–0.34) | 0.27 (0.17–0.39) | .656 | .507 | .760 |
| HIV-negative | 0.16 (0.06–0.38) | 0.16 (0.08–0.29) | 0.16 (0.09–0.26) | .993 | .993 | ||
| ABC + nonsmoking | HIV-positive | 0.06 (0.02–0.22) | 0.11 (0.05–0.23) | 0.12 (0.07–0.22) | .516 | .287 | .189 |
| HIV-negative | 0.13 (0.03–0.40) | 0.06 (0.02–0.17) | 0.06 (0.02–0.12) | .431 | .250 | ||
| Viral suppressionh | HIV-positive | 0.50 (0.29–0.71) | 0.60 (0.41–0.77) | 0.79 (0.65–0.89) | .002 | <.001 | |
Abbreviations: ABC, A1c, BP, and cholesterol; BP, blood pressure; CI, confidence interval.
aRepeated-measures adjusted prevalence estimates were performed for each step of the care continuum across the 3 time points. Repeated measures were used because the same woman could contribute data to multiple time points.
bAdjusted for study site, study year, age, race, education, income, insurance, diabetes duration, HIV status, and study year*HIV status interaction.
cNo statistically significant difference between HIV-positive and -negative outcomes in any of the years.
dGlycemic control was also adjusted for use of diabetes medications.
eBP control was also adjusted for use of antihypertensive medications.
fCholesterol control was also adjusted for cholesterol medications.
gCombined control of hemoglobin A1c level, blood pressure, and low-density lipoprotein cholesterol.
hViral suppression was also adjusted for use of antiretroviral therapy.
Viral Suppression
The unadjusted proportion of HIV-positive women with diabetes who achieved viral suppression steadily increased from 2001 (35/86, 40.7%) to 2006 (136/222, 61.5%) to 2015 (245/282, 86.9%). Of those who were virologically suppressed, 91.4%, 92.6%, and 97.6% self-reported being on antiretroviral therapy during the respective years. In the logistic regression models, the viral suppression prevalence estimates were 0.50 (95% CI, 0.29–0.71), 0.60 (95% CI, 0.41–0.77), and 0.79 (95% CI, 0.65–0.89) in 2001, 2006, and 2015, respectively, demonstrating improvement from 2001 to 2015 (P = .0022) (Table 2). The proportion of HIV-positive women achieving any of the single or combined DM care goals did not differ between women achieving and not achieving viral suppression.
DISCUSSION
We aimed to comprehensively assess and compare the DM care process between HIV-positive and HIV-negative women from the WIHS over the past 15 years. We found no differences in the proportion of HIV-positive and HIV-negative women achieving glycemic control or optimal DM control (ie, ABC goals), nor did we find improvements in either group for optimal DM control from 2001 to 2015. Among HIV-positive women, we found that HIV control (ie, viral suppression) has improved over time, but DM control has not. HIV-negative women, however, did have significant improvement in glycemic control from 2001 to 2015. Overall, these findings reinforce the importance of considering HIV a chronic infectious disease, for which the aggressive management of comorbid cardio-renal risk factors, such as diabetes, will be important.
We observed increases in the prevalence of HIV-positive women who achieved viral suppression over 15 years (from 51% in 2001 to 81% in 2015), which aligns with findings from other cohort studies [19, 20]. These improvements in viral suppression reflect a combination of changing guidelines, making more patients eligible for antiretroviral therapy; increased tolerability, potency, and durability of ART; and a shift in clinical and public health programs, to focus more on care continuum metrics and medication adherence. Despite these positive gains in control of HIV, we found that DM control was not optimal (ie, ABC goals) and did not improve from 2001 to 2015. Among HIV-negative women, only glycemic control improved (from 34% in 2001 to 66% in 2015), whereas neither BP nor cholesterol control improved over time in either the HIV-positive or HIV-negative women. Overall, less than 15% of HIV-positive and -negative women achieved ABC goals and did not smoke, meaning most women did not achieve the targets to avoid cardio-renal complications of DM. However, despite disappointing numbers achieving these care goals, the mean levels of A1c, BP, and cholesterol were not far from goal.
A greater proportion of HIV-positive compared with HIV-negative women were nonsmokers, which is contrary to national estimates, where 83% of the general population and 66% of the HIV-positive population were nonsmoking [21]. The difference is mainly that the HIV-negative population in our cohort has much higher smoking rates than the general HIV-negative population for reasons that are not yet clear.
The trends in the HIV–DM care continuum presented here mirror those observed in the US diabetes care continuum for the general adult population, where only 25% achieved ABC control and 21% combined ABC control plus nonsmoking [11]. Achievement of DM care goals in this study using data from the WIHS was either similar to or better than that previously reported in retrospective cross-sectional studies, yet it was still suboptimal [13, 14]. As the WIHS cohort study was conducted in academic health care settings where guideline-concordant care may have been more prevalent compared with community settings, our findings may be conservative. Together, these results demonstrate a need to better understand disparities across and within the HIV–DM care continuum.
In contrast to findings from previous studies [22], viral suppression was not associated with improved glycemic control or any other DM care continuum outcome among HIV-positive women in this study. Our study included HIV-positive participants from earlier eras of antiretroviral therapies known to have dysglycemic effects, which may have contributed to this finding. Although the association between viral suppression and DM control remains unclear, a qualitative study suggests that poor DM and/or hypertension control in PLHIV may stem from knowledge gaps in disease processes or the importance of medication adherence for the non-HIV condition, the complexity of the medication regimens, and the need to incorporate lifestyle changes in addition to taking pills [23]. Another factor contributing to why viral suppression may not coincide with DM control is that providers may not be comfortable optimizing diabetes treatment regimens [24]. Together, these factors contribute to the challenge of DM control among PLHIV, even once viral suppression is achieved.
Strategies to improve medication adherence and achievement of care goals are available for both HIV and DM. In HIV care, 1-on-1 adherence education, pill boxes, reminder alarms, and SMS tools have been shown to improve adherence to ART [25]. Similarly, meta-analyses have shown that quality improvement interventions in patients with DM can improve A1c by 0.37% and BP control by 3.13/1.88 mmHg [26]. Further, a recent pragmatic trial demonstrated the effectiveness of using care coordinators and electronic clinical decision support software to improve DM management in outpatient low- to middle-income country settings [27]. In settings where HIV is already being successfully managed as a chronic disease, overlaying proven DM care strategies with existing chronic care models should be feasible. In a setting where the vast majority of patients with HIV and DM achieve viral suppression, optimally managing both comorbidities and HIV infection is imperative. As such, future studies should focus on identifying barriers to quality DM care, testing strategies to close gaps identified in the HIV–DM care continuum, and determining, longitudinally, if achieving DM targets is associated with fewer complications of DM.
As with all research studies and analyses, the present study has some limitations. First, although the WIHS cohort includes major urban centers in the United States affected by the HIV epidemic, a broader geographic representation of the United States is lacking and our samples sizes are relatively small. Our reported DM control estimates may be underestimates as the southeastern sites were recruited at a later stage, and the southeastern United States is an area where there are more DM-related complications [28]. Second, the repeated cross-sectional nature of the analyses resulted in some women being included in more than 1 of the cross-sectional time points. To account for this potential limitation, we used both logistic regression models (treating each encounter as 1) and generalized estimating equations (accounting for individuals with multiple visits), and the results were similar. Third, we were unable to determine true DM duration, as our baseline sample included existing DM cases. Fourth, we did not capture undiagnosed diabetes; therefore, our continuum begins with the presumption of diagnosed diabetes and excludes the step of diagnosis. Fifth, it was not possible to assess visit frequency for HIV or DM appointments (outside of study visits), a traditional measure of the HIV continuum, so a surrogate was used (eg, self-reported health care provider visits since the last WIHS study visit). Sixth, the type of health care provider was unknown. This makes it difficult to ascertain if poor outcomes may be due to lack of medical expertise/interest vs other systems-level or patient-level factors. PLHIV may receive HIV care in a primary care setting, subspecialty setting, or through a combined approach. Therefore, PLHIV may have their HIV managed by a provider who may or may not be simultaneously addressing DM or other chronic illness, where expertise affects quality indicators [29, 30]. A recent survey among infectious diseases physicians found that the majority of those providing HIV care also acted as the patient’s primary care provider, but primary care screening by this group was suboptimal [31]. Barriers, cited in the survey, to completing these screening tests included time constraints in the clinic and financial/insurance limitations, and the same barriers may also contribute to suboptimal management of comorbidities in HIV care settings [31]. Finally, the cross-sectional approach to a care continuum for chronic diseases limits our ability to know how the diseases are being managed longitudinally over time, which may provide a more accurate view of the state of care for diabetes and HIV [32]. Future studies should strive to create longitudinal measurements for care continua outcomes.
There are several strengths of our current analysis, which outweigh limitations. First, we were able to evaluate 15 years of data collected using a consistent measurement approach. Second, the HIV viral suppression variable was comparable with that observed in US national care continua. Third, measurement of glycemic control, cholesterol control, BP control, ABC, and ABC + nonsmoking was equivalent to the methods used in national estimates [11]. Finally, inclusion of HIV-positive and HIV-negative matched controls allows for valuable comparisons from both patient-level and systems-level perspectives.
CONCLUSIONS
This study is the first to apply the care continua approach for both HIV and DM and provides a comprehensive assessment of the diabetes care process (ie, engagement in health care, HIV viral suppression, and diabetes care goals) in people living with HIV. Though there were no differences in DM control between HIV-positive and HIV-negative women, DM control was poor among both groups. In contrast, HIV control (ie, viral suppression) did improve from 2001 to 2015 among HIV-positive women, though viral suppression was not associated with better DM control. These findings reinforce the importance of considering HIV a chronic infectious disease for which management of comorbid cardio-renal risk factors, such as diabetes, is important. Identifying the barriers and possible innovations for how to optimize management of these comorbidities is a priority across all aspects of the health care and research continuum.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. J.C., K.I.G., V.C.M., and M.K.A. designed the study, provided guidance for statistical analyses, provided interpretation of study findings, and drafted the manuscript. C.C.M. conducted the statistical analyses, contributed to interpretation of findings, critically revised the manuscript, and provided approval for submission. K.P. and M.F.S. contributed to study design, provided guidance for statistical analyses and study conduct, contributed to interpretation of findings, critically revised the manuscript, and provided approval for submission. P.T. and I.O. contributed to interpretation of findings, provided guidance for study conduct, critically revised the manuscript, and provided approval for submission. A.A.A., M.A., M.H.C., D.G., R.K., D.M., A.S., and G.W. contributed to interpretation of findings, critically revised the manuscript, and provided approval for submission.
WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I–WIHS IV).
Disclosures. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), and P30-AI-050410 (UNC CFAR).
Financial support. This study was funded by the National Institute of Allergy and Infectious Diseases through the WIHS (U01AI103408-04). M.K.A. was partially supported by the Georgia Center for Diabetes Translation Research (P30DK111024).
Potential conflicts of interest. The authors declare no competing interests. A.A. reports grants from Gilead and personal fees from Merck outside the submitted work. All other authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Antiretroviral Therapy Cohort C. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4:e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smit M, Brinkman K, Geerlings S et al. ATHENA observational cohort Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez-Romieu AC, Garg S, Rosenberg ES et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res Care 2017; 5:e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smit M, Cassidy R, Cozzi-Lepri A et al. Projections of non-communicable disease and health care costs among HIV-positive persons in Italy and the U.S.A.: a modelling study. PLoS One 2017; 12:e0186638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown TT, Cole SR, Li X et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005; 165:1179–84. [DOI] [PubMed] [Google Scholar]
- 6. Tien PC, Schneider MF, Cox C et al. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr 2012; 61:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith CJ, Ryom L, Weber R et al. D:A:D Study Group Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 8. Gardner EM, McLees MP, Steiner JF et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: an ambitious treatment target to help end the AIDS epidemic 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. Accessed 18 September 2017. [PubMed]
- 10. Hall HI, Gray KM, Tang T et al. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr 2012; 60:77–82. [DOI] [PubMed] [Google Scholar]
- 11. Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med 2014; 161:681–9. [DOI] [PubMed] [Google Scholar]
- 12. Hall HI, Frazier EL, Rhodes P et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013; 173:1337–44. [DOI] [PubMed] [Google Scholar]
- 13. Bury JE, Stroup JS, Stephens JR, Baker DL. Achieving American Diabetes Association goals in HIV-seropositive patients with diabetes mellitus. Proc (Bayl Univ Med Cent) 2007; 20:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Satlin MJ, Hoover DR, Glesby MJ. Glycemic control in HIV-infected patients with diabetes mellitus and rates of meeting American Diabetes Association management guidelines. AIDS Patient Care STDS 2011; 25:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barkan SE, Melnick SL, Preston-Martin S et al. The Women’s interagency HIV study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 16. Bacon MC, von Wyl V, Alden C et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes 2017. J Diabetes 2017; 9:320–4. [DOI] [PubMed] [Google Scholar]
- 18. Goff DC Jr, Lloyd-Jones DM, Bennett G et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheth AN, Ofotokun I, Buchacz K et al. Antiretroviral regimen durability and success in treatment-naive and treatment-experienced patients by year of treatment initiation, United States, 1996-2011. J Acquir Immune Defic Syndr 2016; 71:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanna DB, Buchacz K, Gebo KA et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiologic Databases to Evaluate AIDS Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001-2009. Clin Infect Dis 2013; 56:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frazier EL, Sutton MY, Brooks JT et al. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009-2014. Prev Med 2018; 111:231–4. [DOI] [PubMed] [Google Scholar]
- 22. Zuniga J, Nguyen ML, Holstad M. Predictors of dual control of HIV and diabetes. AIDS Care 2016; 28:1124–7. [DOI] [PubMed] [Google Scholar]
- 23. Monroe AK, Rowe TL, Moore RD, Chander G. Medication adherence in HIV-positive patients with diabetes or hypertension: a focus group study. BMC Health Serv Res 2013; 13:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williamson C, Glauser TA, Burton BS et al. Health care provider management of patients with type 2 diabetes mellitus: analysis of trends in attitudes and practices. Postgrad Med 2014; 126:145–60. [DOI] [PubMed] [Google Scholar]
- 25. Thompson MA, Mugavero MJ, Amico KR et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012; 156:817–33, W–284, W–285, W–286, W–287, W–288, W–289, W–290, W–291, W–292, W–293, W–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tricco AC, Ivers NM, Grimshaw JM et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012; 379:2252–61. [DOI] [PubMed] [Google Scholar]
- 27. Ali MK, Singh K, Kondal D et al. CARRS Trial Group Effectiveness of a multicomponent quality improvement strategy to improve achievement of diabetes care goals: a randomized, controlled trial. Ann Intern Med 2016; 165:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention. National Diabetes Surveillance System Atlanta, GA. 2015 Available at: http://www.cdc.gov/diabetes/data/national.html. Accessed 27 March 2018.
- 29. Cheung A, Stukel TA, Alter DA et al. Primary care physician volume and quality of diabetes care: a population-based cohort study. Ann Intern Med 2017; 166:240–7. [DOI] [PubMed] [Google Scholar]
- 30. O’Neill M, Karelas GD, Feller DJ et al. The HIV workforce in New York state: does patient volume correlate with quality?Clin Infect Dis 2015; 61:1871–7. [DOI] [PubMed] [Google Scholar]
- 31. Lakshmi S, Beekmann SE, Polgreen PM et al. HIV primary care by the infectious disease physician in the United States - extending the continuum of care. AIDS Care 2018; 30(5):569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colasanti J, Kelly J, Pennisi E et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis 2016; 62:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.