Abstract
Schizophrenia (SZ) is a devastating mental disease caused by complex genetic and environmental factors. The pathological process and clinical manifestation of SZ are heterogeneous among patients, which hampers precise diagnosis and treatment of the disease. Since no objective marker for SZ has been established today, to identify a subgroup of the patients with homogeneous biochemical traits will provide a new angle for both researchers and clinicians to understand and manage the disease. In this study, we employed the niacin skin-flushing test in Chinese population and confirmed a niacin-blunted subgroup of SZ patients distinguishable from mood disorders (MD) and normal individuals. This subgroup accounted for 30.67% of the total SZ patients with a specificity of 88.37% in male subjects and 83.75% in female subjects. We support the notion that bluntness in niacin skin test might reflect abnormalities in membrane fatty acid composition, which could be induced by increased PLA2 enzyme activity, in vivo oxidative stress or lipid metabolism imbalance in SZ. Further studies are encouraged to clarify the molecular origins of niacin-bluntness in SZ, which would provide extra clues for etiological research in schizophrenia and for new targeted treatment.
Keywords: niacin skin test, schizophrenia, patient subclassing, personalized medicine, mood disorders
Introduction
The lack of objective diagnostic markers has long been a challenge in the clinical management of schizophrenia (SZ). The 1980 study by Horrobin1 found that patients with SZ required much larger doses of niacin to produce skin flushing in the niacin-flushing test than normal subjects. Niacin-induced skin flushing is mediated by a G-protein-coupled receptor called hydroxyl-carboxylic acid receptor 2 (HCA2, GPR109A or HM74a), which is widely expressed on keratinocytes and epidermal immune cells, such as Langerhans cells. When niacin binds to HCA2, phospholipase A2 is activated and hydrolyses membrane phospholipids, leading to the release of arachidonic acid (AA). Free AA is then transformed into prostaglandin D2 (PGD2) and prostaglandin E2 (PGE2) by cyclooxygenase (COX) enzymes and subsequently relaxes vascular smooth muscle, inducing vasodilation and skin redness.
This intuitional and biochemically based test for SZ has been successively replicated in various cohorts. In 1991, Rybakowski and Weterle2 administered an oral niacin treatment to patients with SZ and depression and found that all of the depression patients exhibited discernible skin flushing, but a subgroup of patients with SZ did not exhibit flushing. As oral administration of niacin always took hours to fully exhibit the flushing and too large area of the body would be affected by this treatment. In 1998, Ward et al3 improved the niacin test by using topical aqueous methyl nicotinate (AMN) treatment on the forearm skin instead and conducted a semi-quantitative measurement of the skin flushing using a 4-point scale. They found that the SZ cohort exhibited significantly reduced sensitivity to niacin compared with healthy individuals. There are other forms of optimized niacin tests as well, such as using Doppler flowmetry4 or optical reflection spectroscopy5 to measure the skin redness more quantitatively. Diminished flush responses in patients with SZ were consistently revealed by these studies, confirming potential clinical utility of this test. However, due to different procedures and different criteria used in these studies, the reported proportion of the niacin-blunted subgroup of SZ patients varied among studies.6 For example, Horrobin1 found 80% of the SZ patients were niacin-blunted, while Rybakowski and Weterle2 found only 24% of their patients were niacin-blunted, even their doses of niacin were 20% less than Horrobin’s. Liu et al7 used the skin flush score at 0.01 M at 10 min as an indicator to identify niacin-bluntness. They found 49.2% of their SZ patients were niacin-blunted, while using the same criteria, Lin et al8 only found 24.2% of their patients were niacin-blunted. Given these discrepancies, more studies are required to further clarify the characteristics of the niacin-blunted subgroup of SZ.
Mood disorders (MD) constitute a group of illnesses that are featured by a serious change in mood. They are categorized into 3 basic types, ie, elevated mood (mania), depressed mood (depression), and bipolar disorder. SZ and MDs are closely related. They share common symptoms,9 such as delusions and negative symptoms, and common biochemical and genetic traits.10,11 Studies have been conducted to improve the clinical discrimination of these 2 distinct diseases.12,13 Any diagnosis criterion on a pathophysiological basis for distinguishing the 2 diseases is particularly desirable.14,15 It has been reported that diminished responses to niacin also existed in patients with bipolar disorder.7,16 Compared to studies on SZ and controls, very few niacin skin test studies have included MD patient group for comparison,17–19 and for the studies that have considered MD, only bipolar or depression patients were usually included. Therefore, in this study, we try to compare a comprehensive MD group (with all 3 types of MDs included) with SZ in order to find more typical differences between these 2 disease groups.
In addition, except for Lin’s and Liu’s study, most of the studies mentioned above were conducted in small cohorts of Caucasian subjects. In this study, we employed a larger sample set (163 subjects with SZ, 63 subjects with MD, and 63 healthy controls [HC]) from the Chinese Han population and used the 4-point scale measurement to investigate the niacin flush responses in different disease groups, with the aim of identifying indicators that can properly diagnose and discriminate a niacin-blunted subgroup of patients with SZ from subjects with MD and healthy controls. The identification of such SZ subgroup would hopefully lead to the development of a method for the precise diagnosis and personalized treatment of SZ in the future.
Methods
Participants
Patients with SZ and MD were recruited from inpatients at the Fourth People’s Hospital of Wuhu in Anhui province of China. The inclusion criteria for SZ and MD group were (1) a diagnosis of SZ or MD (including mania, depressive disorders, and bipolar disorder) according to ICD-10 (International Classification of Diseases, 10th Revision) and (2) 15–75 years old. Age-matched HCs were recruited from the staff in the same hospital, including doctors and nurses. The inclusion criteria for HC group were (1) with no current or past symptoms of psychiatric illness and (2) 15–75 years old. The general exclusion criteria for SZ, MD, and HC group were (1) the presence of a neurological disease, such as epilepsy, cerebral tumor, or severe head injury; (2) substance dependence or with scores greater than 10 on the Alcohol Use Disorders Identification Test (AUDIT-C) or greater than 6 on the Fagerstrom Test for Nicotine Dependence (FTND); (3) have taken nonsteroidal or steroidal anti-inflammatory drug within the previous 14 days; (4) severe skin diseases or diseases that would induce significant immune responses, such as lupus and asthma; and (5) pregnancy. Written informed consent was obtained from all participants. All data were collected following the guidelines of the local ethics committee.
Assessment of Niacin Sensitivity
A round filter paper patch (1.29 cm in diameter, Bio-Rad) was used to apply niacin in the form of aqueous methylnicotinate (AMN, C7H7NO2, 99%, Sigma–Aldrich). Four concentrations (0.1, 0.01, 0.001, and 0.0001 M) of AMN solution were freshly prepared. Before the test, a sticky ruler was attached to the inner side of the subject’s forearm in order to better locate the paper patches. Four wetted paper patches from each of the 4 AMN solutions were applied to 4 neighboring sites on the forearm skin for 1 min and then removed. The skin flush response was photographed from a fixed vertical view at 5, 10, 15, and 20 min after the patches were removed. The responses were rated according to the photos presented in Adobe Photoshop (Version CS6, Adobe) on a 4-point scale: 0 indicates no erythema, 1 indicates incomplete erythema, 2 indicates complete erythema within the area covered by the patch, and 3 indicates erythema beyond the definite area of the patch.3
The flush responses of each subject were rated twice by 2 research assistants trained by a same senior researcher. Spearman correlation coefficient of the scores rated by the 2 assistants was 0.92, indicating good inter-rater reliability.
Data Analysis
The scores rated by the 2 research assistants were averaged to obtain a final score for each flush response. Multivariate analysis of variance (MANOVA) was conducted in SPSS software (Version 22, IBM Corp.) for group comparisons and to adjust for confounding factors. The dependent variables include the 16 raw scores obtained at different AMN concentrations (0.0001, 0.001, 0.01, and 0.1 M) and at different time points (5, 10, 15, and 20 min), 4 sum scores for 4 AMN concentrations, 4 sum scores for4 time points and 1 total flush response score (supplementary table 1). Body mass index (BMI), education level, and FTND scores for nicotine dependence (which differed between groups) were used as covariates and the gender and disease groups were assigned as between-subject factors. Since this study aimed to use the niacin test to discriminate patients with SZ from HC or subjects with MD, simple contrasts were conducted in the univariate analysis with the SZ group as the reference category. Partial eta2 values were calculated to evaluate the effect sizes of the independent variables.20 For quasi-meta-analysis, we searched Pubmed (https://www.ncbi.nlm.nih.gov/pubmed) with the keywords of “schizophrenia,” “skin,” “niacin,” and “methylnicotinate,” and selected the studies with experimental data of niacin skin test in SZ. Review Manager 5.3 was employed to integrate the results in fixed effect model and generate forest plots.
Results
Demographic Characteristics
We recruited 163 patients with SZ, 63 patients with MD, and 63 healthy control volunteers as HC. The basic demographic and clinical characteristics of the participants are shown in table 1. Most of the items were appropriately matched between the SZ and MD groups, but the HC group consisted of more females, thinner, shorter and more educated subjects, and no smokers. Therefore, items such as gender, education level, BMI (the integrative indicator of weight and height), and FTND scores (nicotine dependence) failed to pass the statistical tests for homogeneity of the 3 groups (P-value < .05) and were adjusted as covariates in the subsequent analyses as described in the Methods section. In the SZ group, only 4 of the patients were first episode (less than 3%), and others were recurrent. In the MD group, there were 23 individuals diagnosed as mania (F30 in ICD-10), 32 diagnosed as bipolar disorder (F31 in ICD-10) and 5 diagnosed as depressive disorder (F32/33 in ICD-10). In the 32 patients with bipolar disorder, 30 of them were type 1 and 2 were type 2. Six of the 63 MD patients (less than 10%) were first episode. Other MD patients were recurrent.
Table 1.
Demographic Characteristics of the 3 Study Groups
| Schizophrenia (SZ) | Mood Disorders (MD) | Healthy Controls (HC) | |
|---|---|---|---|
| Total number | 163 | 63a | 63 |
| Male/female# | 93/70 | 34/29 | 10/53 |
| Age (year, mean ± SEM) | 37.02 ± 0.995 | 37.97 ± 1.752 | 34.84 ± 1.503 |
| Weight (kg, mean ± SEM)* | 66.80 ± 1.000 | 69.87 ± 1.470 | 59.57 ± 1.256 |
| Height (m, mean ± SEM)* | 1.65 ± 0.007 | 1.65 ± 0.009 | 1.62 ± 0.007 |
| BMI (kg/m2, mean ± SEM)* | 24.39 ± 0.344 | 25.72 ± 0.537 | 22.60 ± 0.448 |
| AUDIT-C score (alcohol-dependence) | 0.82 ± 0.145 | 0.90 ± 0.232 | 0.27 ± 0.091 |
| FTND score (nicotine-dependence)* | 0.42 ± 0.087 | 0.63 ± 0.174 | <0.01 ± <0.001 |
| Education levelb,* | 2.09 ± 0.075 | 2.16 ± 0.108 | 3.20 ± 0.169 |
| Temperature (°C, mean ± SEM) | 36.54 ± 0.026 | 36.48 ± 0.026 | — |
| Heart rate (beats/min, mean ± SEM) | 84.54 ± 0.761 | 85.16 ± 1.105 | — |
| Respiratory rate (breaths/min, mean ± SEM) | 19.71 ± 0.053 | 19.74 ± 0.069 | — |
| Systolic pressure (mmHg, mean ± SEM) | 120.68 ± 0.913 | 118.82 ± 1.346 | — |
| Diastolic pressure (mmHg, mean ± SEM) | 78.10 ± 0.639 | 78.73 ± 1.060 | — |
| Disease duration (year, mean ± SEM) | 10.76 ± 0.716 | 10.62 ± 1.227 | — |
| Number of hospitalizations (n, mean ± SEM) | 3.59 ± 0.385 | 4.07 ± 0.776 | — |
| Atypical antipsychotics used [n (%)] | 159 (97.5%) | 59 (93.7%) | — |
aFive patients with depression, 32 with bipolar disease, 23 with mania, and 3 without subclassified data.
b0 for illiteracy, 1 for elementary school, 2 for junior high school, 3 for senior high school, and 4 for college or university.
# P < 0.05 when comparing the 3 groups by the chi-square test.
*P < 0.05 when comparing the 3 groups by one-way ANOVA.
Descriptive Statistical Analysis of Niacin Test Responses
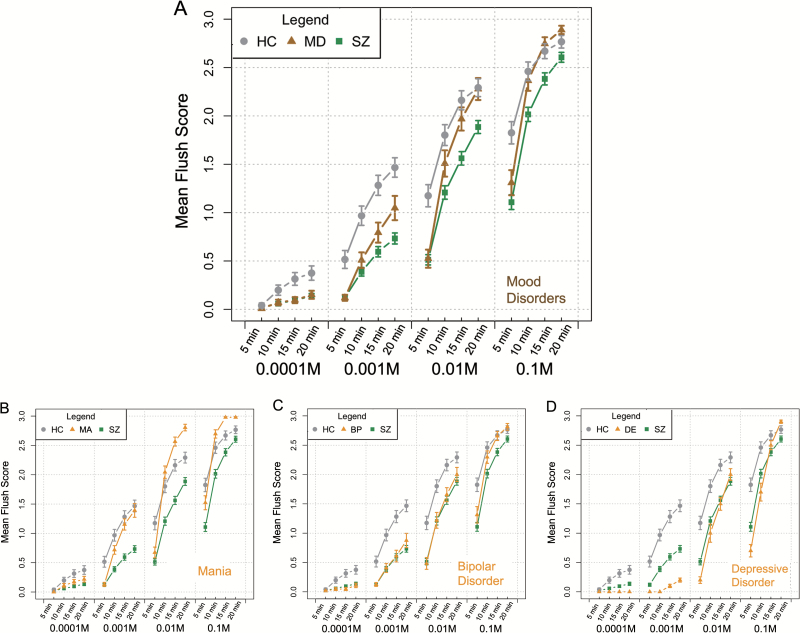
Means and SEM (standard errors of the means) of the scores in niacin skin test for different groups were calculated and are shown in figure 1A. Compared to HC group, patients with SZ exhibited lower mean flush scores under all conditions, indicating a delayed and weakened niacin flush response in patients with SZ. The flush responses in the MD group mimicked the responses observed in the SZ group at the lowest concentration (0.0001 M) or at the shortest time point (5 min), but became comparable with the responses in the HC group at 20 min or at a higher concentration (0.01 M). The MD group displayed an even more intensive skin flush than the HC group at 0.1 M AMN at 20 min, indicating that the niacin flush response in the MD group was rather delayed but not diminished. The performance of the 3 subgroups of MD patients (mania, bipolar disorder, and depressive disorder) has also been exhibited in figure 1B–D, respectively, with similar slopes at high concentrations (0.01 M or 0.1 M AMN) but different amplitudes.
Fig. 1.
A dot and line figure of means and SEMs (standard errors of the means) of the flush scores in niacin skin tests at each concentration of AMN (aqueous methyl nicotinate) and at each time point in healthy controls (HC), subjects with schizophrenia (SZ) and subjects with mood disorders (MD) (A); (B–D) performance of the 3 subgroups in MD (B for mania, C for bipolar disorder, and D for depressive disorder) in niacin skin tests.
Differential Analysis of Flush Response Scores
A MANOVA analysis was conducted to statistically evaluate the differences of flush responses among the 3 groups.20 As shown in table 2, when all scores (16 raw scores, 8 sum scores, and 1 total score, as described in the Methods section) were considered as a group of dependent variables, gender (P = .004) and disease group (P < .0001) were showed to have significant effects on the overall scores. Neither BMI (P = .458) nor education (P = .076) nor smoking status (FTND score, P = .258) was related to the flush responses, and no interaction effect (P = .189) was observed as well between gender and disease groups.
Table 2.
Multivariate Tests Resultsa
| Effect | Value | F | Hypothesis df | Error df | Sig. | Partial eta2 |
|---|---|---|---|---|---|---|
| Intercept | 0.411 | 10.494b | 16 | 241 | <0.001 | 0.411 |
| BMI | 0.062 | 1.000b | 16 | 241 | 0.458 | 0.062 |
| FTND score | 0.075 | 1.213b | 16 | 241 | 0.258 | 0.075 |
| Education | 0.095 | 1.576b | 16 | 241 | 0.076 | 0.095 |
| Gender | 0.312 | 2.386b | 16 | 241 | 0.003 | 0.156 |
| Group | 0.137 | 2.795 | 32 | 484 | <0.001 | 0.137 |
| Gender × group | 0.15 | 1.224 | 32 | 484 | 0.189 | 0.075 |
Note: Significant P-values are indicated in bold and italics. BMI, body mass index; FTND, Fagerstrom Test for Nicotine Dependence.
aIndependent factor design: Intercept + BMI + FTND score + Education + Gender + Group + Gender × group.
bExact statistic.
In the univariate analysis with simple contrasts, partial eta2 values were calculated to evaluate the effect sizes of the independent factors, especially the disease groups, on each of the score variables (supplementary table 2). To identify the score variables that can discriminate between subjects with SZ and MD or HC, we compared the partial eta2 in these tests for HC or MD group in contrast to SZ group. MD group was assigned the highest partial eta2 value (0.045) in the test on the score variable at 0.1 M AMN at 15 min, while HC group was assigned the highest partial eta2 value (0.115) in the test on the total score, indicating that these 2 score variables can best discriminate between SZ and MD or SZ and HC, respectively (table 3). In the test on the score variable at 0.1 M AMN at 15 min, both the HC and MD groups had significantly higher scores than the SZ group (MD vs SZ: P = .001; HC vs SZ: P = .001). Male subjects showed significantly higher scores than female subjects (P = .004). In the test on the total score, the HC group showed significantly higher score values than the SZ group (P < .0001), while gender (P = .037) and education (P = 0.004) had an effect on the score as well.
Table 3.
Parameter Estimates in Univariate Tests (Parta)
| Dependent Variable | Independent Variable | B | SE | t | Sig. | 95% Confidence Interval | Partial eta2 | |
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| Score at 0.1 M AMN at 15 min | Intercept | 2.630 | 0.304 | 8.648 | <0.001 | 2.031 | 3.229 | 0.226 |
| BMI | −0.009 | 0.010 | −0.882 | 0.378 | −0.030 | 0.011 | 0.003 | |
| FTND score | 0.032 | 0.042 | 0.770 | 0.442 | −0.051 | 0.115 | 0.002 | |
| Education | −0.080 | 0.043 | −1.888 | 0.060 | −0.164 | 0.003 | 0.014 | |
| Gender = male | 0.337 | 0.116 | 2.906 | 0.004 | 0.109 | 0.565 | 0.032 | |
| Gender = female | 0b | |||||||
| Group = HC | 0.466 | 0.137 | 3.411 | 0.001 | 0.197 | 0.735 | 0.043 | |
| Group = MD | 0.530 | 0.152 | 3.493 | 0.001 | 0.231 | 0.829 | 0.045c | |
| Group = SZ | 0b | |||||||
| Total score | Intercept | 18.627 | 3.335 | 5.585 | <0.001 | 12.059 | 25.195 | 0.109 |
| BMI | −0.060 | 0.115 | −0.520 | 0.603 | −0.286 | 0.167 | 0.001 | |
| FTND score | 0.151 | 0.462 | 0.327 | 0.744 | −0.759 | 1.062 | 0.000 | |
| Education | −1.343 | 0.467 | −2.877 | 0.004 | −2.262 | −0.424 | 0.031 | |
| Gender = male | 2.661 | 1.271 | 2.093 | 0.037 | 0.158 | 5.164 | 0.017 | |
| Gender = female | 0b | |||||||
| Group = HC | 8.641 | 1.498 | 5.767 | <0.001 | 5.690 | 11.592 | 0.115d | |
| Group = MD | 3.033 | 1.665 | 1.822 | 0.070 | −0.246 | 6.311 | 0.013 | |
| Group = SZ | 0b | |||||||
Note: Significant P-values are indicated in bold and italics. AMN, aqueous methyl nicotinate; BMI, body mass index; FTND, Fagerstrom Test for Nicotine Dependence; HC, healthy control ; MD, mood disorder; SZ, schizophrenia.
aSee supplementary table 1 for the full version of this table.
bThis parameter is set to zero because it is redundant.
cThe highest partial eta2 value for the MD group compared with the SZ group.
dThe highest partial eta2 value for the HC group compared with the SZ group.
Quasi-Meta-analysis of the 2 Discriminative Scores in SZ Vs HC and MD Vs SZ
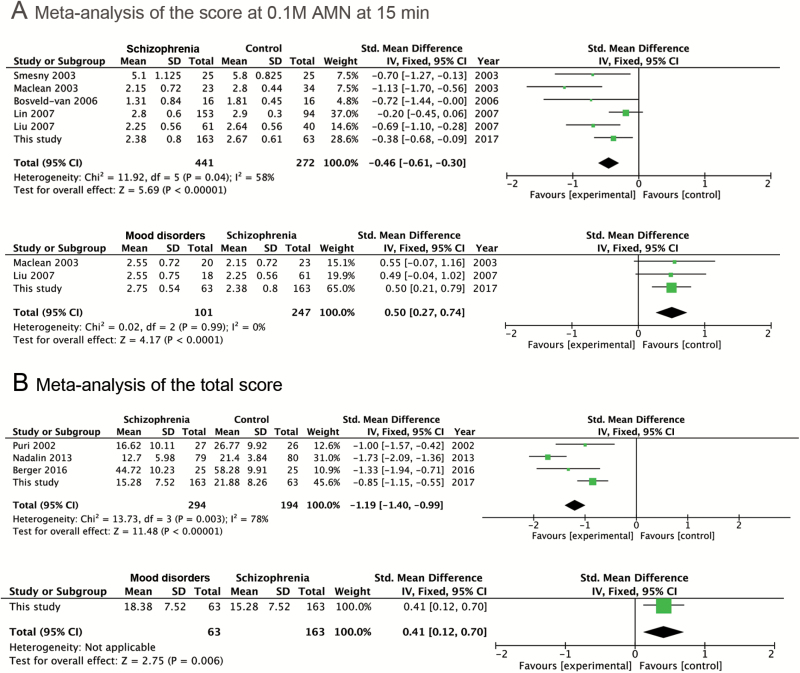
The score variable at 0.1 M AMN at 15 min is identified as the most discriminant variable for SZ vs MD in this study. We investigated its effect in SZ vs HC as well for reference. Five studies were found with the means and standard deviations (SDs) of this score in SZ vs HC group (figure 2A above), while only 2 studies had the score data for SZ vs MD group (figure 2A below). The patients belong to MD group in the 2 studies were all diagnosed as bipolar disease. We add our own data to the meta-analysis to achieve overall effects. The overall effect of the score at 0.1 M AMN at 15 min in SZ vs MD comparison was 4.17 (P-value < 0.0001), indicating that SZ group had got significantly smaller value of this score compared to MD group, which was in line with our result. In SZ vs HC comparison, the overall effect was statistically significant as well (z = 5.69, P-value < .00001).
Fig. 2.
Tables and forest plots for the meta-analysis of the score at 0.1 M AMN at 15 min and the total score. Study references: Smesny et al21; Maclean et al17; Bosveld-van et al19; Liu et al7; Lin et al8; Puri et al22; Nadalin et al23; Berger et al.20
For the total score that was identified as the most discriminant variable for SZ vs HC comparison, much fewer studies were found to have calculated this data. Only 3 studies reported this data for SZ vs HC comparison and no studies for SZ vs MD. Nevertheless, as shown in figure 2B, the overall effect of the total score for SZ vs HC comparison is very prominent (z = 11.48, P-value < .00001). As no other studies calculated total score for MD group, we only list our own data (z = 2.75) in figure 2B for reference.
Identification of Niacin-Blunted SZ Subgroup by Discriminative Scores
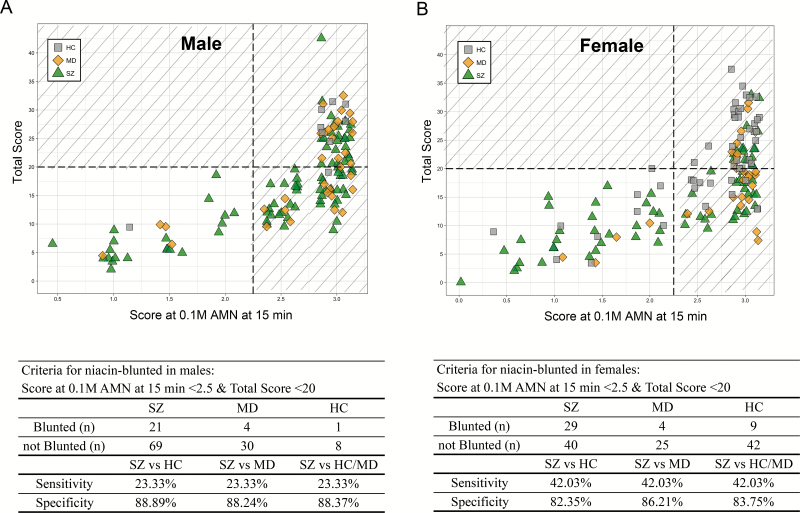
We plotted the distribution of our subjects by the 2 discriminative scores identified in previous analysis (the score at 0.1 M AMN at 15 min and the total score) in figure 3. As gender was proved to be a confounder for the overall flush response scores and particularly for the 2 discriminative scores, we identified the niacin-blunted SZ subgroup in males and females separately. In pursuit of good specificity and proper sensitivity, we set the criteria for the niacin-blunted subgroup of SZ patients as “the score at 0.1 M AMN at 15 min <2.5” and “the total score<20.” As shown in figure 3A and 3B, all subjects in the lower left part of the divided regions were niacin-blunted subjects. By these criteria, we can diagnose SZ with the sensitivity of 23.33% in males and 42.03% in females. The niacin-blunted SZ subgroup we identified accounted for 30.67% of the total SZ patients. As for disease specificity of the subgroup, we got as high as 88.89% in males and 82.35% in females in subjects with SZ or HC; 88.24% in males and 86.21% in females in patients with SZ or MD; 88.37% in males and 83.75% in females among all 3 groups. Thus, though with relatively low diagnostic sensitivity, we were able to screen out a subgroup of SZ patients with high specificity by the criteria we established.
Fig. 3.
Bivariate sample distribution plots of the flush score at 0.1 M AMN at 15 min and the total score for males (A) and females (B), respectively. The points in the plots were properly jittered on the x-axis direction for better demonstration. The grey squares in the plots represent healthy controls; the yellow diamonds represent MD patients; the green triangles represent SZ patients. With a vertical dotted line drawn between 2 and 2.5 of the score at 0.1 M AMN at 15 min, and a horizontal line at 20 of the total score, subjects in the lower left part (the unshaded area) of the plot were identified as niacin-blunted. Related statistics are listed in the tables below the plots.
Discussion
A Niacin-Blunted Subgroup of Patients With SZ Can Be Identified Using the Semi-Quantitative Skin Flush Scores
Most of the relevant studies have observed diminished niacin flush responses in patients with SZ in the last century, regardless of whether the patients received oral or topical niacin treatments.6 In this study, we employed the modified niacin skin test described by Ward et al3 to observe the epidermal flush response in a comparatively large sample of Chinese patients with SZ compared to healthy controls and patients with MD. We confirmed that the SZ group showed delayed and attenuated skin flush reactions after niacin stimulation, while the MD group exhibited delayed but not attenuated or even accentuated reactions in response to the niacin treatment. This property of MD group was more evident when AMN concentration is high (such as 0.1 M AMN).
We identified the total score variable as the most effective indicator for differentiating SZ patients from HC group and the score variable at 0.1 M at 15 min as the most discriminant score for SZ versus MD comparison. This result was successfully validated in a quasi-meta-analysis. From the forest plot for the total score in SZ vs HC comparison (figure 2B), we can see that this score were significantly different in all of the studies no matter using 4-point scale or 7-point scale,20 indicating the efficiency of this indicator for differentiating SZ from healthy controls. For the score at 0.1 M at 15 min in SZ vs MD comparison (figure 2A), studies with small sample size7,17 could only identify a trend of difference, but together with our study, the distinctiveness of this score was finally established, which is highly valuable in clinical practice, since MD group (especially the bipolar disorder subgroup) has been frequently misdiagnosed with SZ.24
It’s interesting to compare the niacin responses in the 3 MD subgroups (mania, bipolar disorder (BP), and depressive disorder). It has been reported that a much lower proportion of individuals who did not respond to niacin were observed in the BP group than in the SZ group.7,16 Skin flush reaction is delayed but not attenuated in the BP group17 and unipolar depression group.25 Hudson et al16 even found the bipolar affective disorder subjects exhibited marked response to the niacin challenge. Bipolar disorder subjects in this study didn’t exhibit exceeding flushing compared with healthy controls, but it’s the mania subgroup that showed delayed but marked responses (even more remarkable than healthy controls at higher AMN concentration). In contrast, subjects with depressive disorder showed the most delayed responses among the 3 subgroups of MD. It seems that the more the negative symptom or mood a subgroup is featured by, the more the flushing responses delayed in niacin skin tests. In SZ, reduced niacin sensitivity has been reported to be associated with greater functional impairment26 and more negative symptoms.21 Similar association may exist in MDs between the negative symptoms and the delay of responses. However, not like SZ, as shown in figure 1D, even the subgroup of MD with the severest negative symptoms, ie, depressive disorder, could achieve a flushing response comparable with healthy controls at 0.1 M AMN at 20 min. Therefore, our study revealed that the niacin-bluntness (both delayed and attenuated) phenomenon was quite specific for SZ.
Using the 2 indicators, we identified a niacin-blunted subgroup of SZ with 88%–89% specificity and 23.33% sensitivity in males and 82%–86% specificity and 42.03% sensitivity in females. To compare our results with previous studies, we listed the studies that have reported the prevalence (sensitivity) and specificities of their niacin-blunted SZ subgroups in table 4. In the studies that used the semi-quantitative measurement for flushing responses (including our study), the niacin-blunted SZ subgroups with more than 80% specificity all had prevalence below 50% (from 13.7% to 49.2%), indicating a stable but minority presence of this patient subgroup despite the heterogeneity in study designs. In addition, Lin and Liu’s study investigated Chinese subjects as well but their specificities were a bit higher than ours. Such discrepancies might originate from different diagnostic systems we used (ICD-10 vs DSM-IV), which however need further investigations. As for studies that used quantitative measurement of flush responses, it seems that they have identified SZ niacin-blunted subgroups with relatively higher sensitivities/prevalence (from 31% to 92%), while their specificities were all above 80%. In addition, it’s worth to note that the most recent work by Yao et al18 also utilized bivariate cut-offs to differentiate SZ subgroup from healthy controls and bipolar or depressive patients, which achieved stable prevalence (31%–32%) and pretty high specificities (87%–97%) in 2 independent cohorts, indicating the effectiveness of such bivariate strategy in niacin skin test.
Table 4.
Summary of Niacin Skin-Flush Studies in SZ and Other Psychiatry Diseases
| Study | Subjects | Area | Subgrouping Indicator | Cutoff Indicators | Sensitivity (Subgroup Prevalence) | Specificity |
|---|---|---|---|---|---|---|
| Qualitative measurement | ||||||
| Horrobin1 | Not available | UK | Flush status | No visible occurrence of skin flushing | 80.0% | Not available |
| Rybakowski and Weterle2 | 33 SZ and 18 DE | Poland | Flush status | No visible occurrence of skin flushing | 24.0% | 100% in SZ and DE |
| Glen et al27 | 126 SZ with predominantly negative symptoms | UK | Flush status | No visible occurrence of skin flushing | 52.0% | Not available |
| Semi-quantitative measurement | ||||||
| Ward et al3 | 35 SZ and 22 HC | UK | Skin flush score at 0.01 M at 5 min | Score = 0 or 1 | 83.0% | 77% in SZ and HC |
| Puri et al28 | 21 SZ and 20 HC | UK | Skin flush score at 0.001 M at 15 min | Score = 0 | 90.0% | 75% in SZ and HC |
| Puri et al22 | 27 SZ and 26 HC | UK | Over-all sum score of skin flush | Score = ≤21 | 77.8% | 65.38% in SZ and HC |
| Tavares et al29 | 38 SZ and 28 HC | Brazil | Skin flush score at 0.01 M at 10 min | Score = 0 or 1 | 23.7% | 85.8% in SZ and HC |
| Smesny et al21 | 25 SZ and 25 HC | Australia | Skin flush score at 11 min at 0.001 M and 0.1 M | Not available | 84.0% | 76% in SZ and HC |
| Lin et al8 | 153 SZ and 94 HC | Taiwan | Skin flush score at 0.01 M at 10 min | Score = 0 | 13.7% | 96.8% in SZ and HC |
| Score = 0 or 1 | 24.2% | 90.4% in SZ and HC | ||||
| Liu et al7 | 61 SZ and 18 BP and 40 HC | Taiwan | Skin flush score at 0.01 M at 10 min | Score = 0 or 1 | 49.2% | 92.5% in SZ and HC; 88.2% in SZ and BP |
| This study | 163 SZ and 63 MD and 63 HC | Mainland China | Skin flush score at 0.1 M at 15 min and total score | Score at 0.1 M at 15 min < 2.5 and total score < 20 | 23.3%–42.0%a | 82.4%–88.9%a |
| Quantitative measurement | ||||||
| Hudson et al16 | 33 SZ and 18 BP and 28 HC | Canada | Thermal index | Index ≤ 1.5 | 42.9% | 94.4% in SZ and BP; 100% in SZ and HC; 97.8% in SZ, BP, and HC |
| Hudson et al30 | 23 SZ and 30 HC | Canada | Thermal index | Index ≤ 1.5 | 43.0% | 97% in SZ and HC |
| Smesny et al21 | 25 SZ and 25 HC | Australia | Combination of spectroscopic data with the calculated min_step value | Not available | 92.0% | 84% in SZ and HC |
| Messamore et al4 | 27 SZ and 21 HC | Poland | log10(EC50), Fmin, Fmax | Not available | 74.0% | 81% in SZ and HC |
| Ross et al31 | 27 SZ and 26 BP and 31 HC | Canada | Delta F | Delta F ≤ 30 | 70.0% | 86% in SZ and HC; 81% in SZ and BP |
| Yao et al18 | 70 SZ and 59 BP and 87 HC | America | log10(EC50), Fmax | Beyond 90th percentile of log10 (EC50) and within 60th percentile of Fmax | 31.0% | 95% in SZ and HC; 97% in SZ and BP |
| 90 SZ and 30 BP-MDD and 93 HC | 32.0% | 95% in SZ and HC; 87% in SZ and BP-MDD | ||||
Note: SZ, schizophrenia; DE, depression; BP, bipolar disease; MD, mood disorders; HC, healthy control; BP-MDD, bipolar and major depressive disorder.
aPlease refer to the main text for details.
The niacin skin test has confounders. Substance use, such as smoking and alcohol intake, were not identified as confounders in this study, which is consistent with other studies.7,31,32 Age and gender did not influence the skin test results in the study by Ward et al,3 but Smesny et al33 observed faster and more intense erythema in response to niacin in females. They then published another study that further emphasized the importance of considering age and gender in clinical niacin studies.5 In this study, age was not a confounder because we balanced the ages of the subjects in the SZ, MD, or HC groups and no differences in age were observed between the niacin-blunted subgroup and non-niacin-blunted individuals of the same disease. Gender appeared to be a confounding factor in this study. It affected niacin flush responses, particularly the score at 0.1 M AMN at 15 min (P-value = .005). We observed that males were more sensitive to niacin than females in our study. Smesny et al5 also found an effect of gender on flushing responses, though males displayed a significantly weaker flush response than females in their study. It has been reported that male hormone testosterone possesses vasodilatory properties as well.34 More efforts should be made to clarify the gender effect on niacin skin flushing responses. Moreover, we found that although in SZ group females were enriched in the niacin-blunted subgroup (21 out of 90 males vs 29 out of 69 females, chi-square P-value = .02), no such enrichment were observed in the MD group or the HC group, suggesting there may be gender-related heterogeneity of the physiological pathways underlying the niacin-blunted phenotype in patients of different diseases.35
Impaired niacin sensitivity had once only been observed in patients with first-episode SZ but not chronic SZ patients in a small cohort study.36 Chronic patients are featured by long medication history and multiple episodes. It has been repeatedly reported that neither medication status nor doses or classes of antipsychotic medication was associated with niacin flush responses in patients with SZ,8,16,31 but whether different disease status influences the niacin responses is still questionable. However, along with this study, many peer studies21,31 had found a subgroup of niacin-blunted patients in chronic SZ patients as well, supporting that niacin skin test could be generally utilized in all SZ patients.
Pathological Implications for the Niacin-Blunted Subgroups of Patients With SZ
The skin flush response to niacin is caused by prostaglandins synthesized from AA (arachidonic acid) molecules on the membranes of dermal cells. A depletion of AA and other essential fatty acids from the phospholipids on the membrane of the red blood cells (RBC) and brain tissues of patients with SZ has been observed by several studies,23,37 thus the blunted skin flush response to niacin might be caused by a reduced level of membrane AA.22,38 Though direct correlation between niacin sensitivity and RBC membrane AA level has not been established yet,17,23,39 a well designed study in 1996 had found the association of unsaturated essential fatty acids (especially AA) with niacin flushing in SZ patients’ RBC membrane samples,27 and the nonflushing phenotype in SZ patients along with their reduced levels of membrane essential fatty acids could even be reversed by clozapine treatment. Fatty acid levels on RBC membranes could reflect their levels in the brain,40 therefore this result is in consistent with the membrane phospholipid hypothesis of SZ, which focuses on abnormal fatty acid spectrum in neural cell membranes that hampers neuron activity and signal transduction, such as dopamine pathway, in brains of SZ.41,42
Reduced levels of membrane essential fatty acids could be caused by multiple reasons. Phospholipase A2 (PLA2) is a key esterase that cleaves the glycerophospholipids at the sn-2 ester bond to release the fatty acid (mainly AA) from the membrane. Increased activity of PLA2 has been observed in patients with SZ who do not respond to niacin,29 which would induce an excess release of essential fatty acids from the membranes and then lead to niacin bluntness. In addition, a genetic variant near the promoter region of the PLA2 gene has also been associated with SZ.43 Such variant may influence the transcription and then the amount of PLA2 enzymes in cells. We have measured PLA2 levels in the plasma of SZ patients and found a statistically significant increase of this enzyme in the patients (data have not been published), which is the same as several other studies found.44,45 Such an increase of the amount of PLA2 enzyme may also cleaves excess membrane fatty acid and cause niacin bluntness in SZ patients.
Shortage of the fatty acids on the membrane could be caused by oxidative stress as well. Converging lines of evidence, especially from proteomic and metabolomics studies in recent years, points to elevated oxidative stress in SZ,46 which is featured by disturbed mitochondria function and increased reactive oxygen species (ROS). ROS in the brain would not only affect neuronal function such as neurotransmitter synthesis and synaptic plasticity but also oxidize membrane fatty acids, especially those unsaturated essential fatty acids, causing reduced level of membrane AA.
Drop of the fatty acids from membranes no matter by PLA2 enzyme or ROS attack would probably lead to an increase of free fatty acids in blood. In fact, our team have revealed a phenomenon of elevated free fatty acids and ketone bodies in SZ patients’ serum in a metabolomic study47 and later a validating lipidomic study.48 We proposed for increased lipolysis in SZ patients and a transfer of energy substance to the brain for energy supply. Such an increased consumption of free fatty acids in SZ might additionally contribute to the reduced level of membrane fatty acids. For example, there might be not enough free fatty acids left for readily repair of the membranes damaged by enzymes or oxidative stress.
Therefore, given our observations, we support the notion that niacin skin test may serve as an indirect but convenient measurement of membrane fatty acid composition that might reflect PLA2 enzyme activity, in vivo oxidative stress or lipid metabolism imbalance in SZ, which coincides with the “phospholipid spectrum disorders” concept proposed by Peet et al.49 More studies are encouraged to consolidate this conclusion. On the other hand, we still could not exclude other pathogenic abnormalities in the AA-prostaglandin pathway that may underlie niacin bluntness in SZ. Genes such as prostaglandin synthase-2 (PTGS2) and long-chain fatty acid-CoA ligase 4 (FACL4),35 have also been found associated with flush responses in patients with SZ.23 Possible deficiency of in vivo niacin could increase the threshold of skin niacin concentration for flushing response, which is worth investigating as well.
The Utility of the Niacin Skin Test in SZ Research and Clinical Practice
This subgroup of SZ patients may share common abnormalities in the membrane phospholipid-AA-prostaglandin pathway, in fatty acid metabolism or even in the endogenous niacin concentration.50 Such abnormalities could have etiological implications for SZ, providing new angles to explore the disease. More importantly, it can also facilitate the design of efficient drugs for targeted treatment of these niacin-blunted SZ patients in the future. A recent study showed that the niacin skin test could also be used as a disease predictor to identify adolescents with higher risks of developing into psychosis.20 Therefore, niacin skin test can be potentially used as a new tool not only for enlightening SZ researches but also for predicting, diagnosing, and treating of the disease.6
Conclusion
In this study, we used niacin skin test in a relatively large sample set and successfully identify a subgroup of niacin-blunted SZ patients out from MD patients and healthy controls with impressive specificity. These niacin-blunted SZ patients account for about one-third of all SZ patients and were more prevalent in females than in males. Our result provides extra clues for etiological research of SZ and helps to optimize clinical strategies and facilitate precise diagnosis and personalized medicine for patients with SZ.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by the Natural Science Foundation of China (81771440 and 81361120389), Ministry of Science and Technology of China (2016YFC1306802 and 2016YFC1306900), Grants of Shanghai Brain-Intelligence Project from STCSM (16JC1420501), Science and Technology Commission of Shanghai Municipality (13dz2260500), and Science and Technology Plan Project of Wuhu, Anhui Province (2016ZD17).
Supplementary Material
Acknowledgement
We thank M. S. Pengkun Wang, M. S. Shujiao Du, M. S. Jiayi Sun, and B. S. Chuangye Xu for their kind assistance for the data processing. The authors declare no conflict of interest.
References
- 1. Horrobin DF. Schizophrenia: a biochemical disorder?Biomedicine. 1980;32:54–55. [PubMed] [Google Scholar]
- 2. Rybakowski J, Weterle R. Niacin test in schizophrenia and affective illness. Biol Psychiatry. 1991;29:834–836. [DOI] [PubMed] [Google Scholar]
- 3. Ward PE, Sutherland J, Glen EM, Glen AI. Niacin skin flush in schizophrenia: a preliminary report. Schizophr Res. 1998;29:269–274. [DOI] [PubMed] [Google Scholar]
- 4. Messamore E, Hoffman WF, Janowsky A. The niacin skin flush abnormality in schizophrenia: a quantitative dose-response study. Schizophr Res. 2003;62:251–258. [DOI] [PubMed] [Google Scholar]
- 5. Smesny S, Rosburg T, Klemm S, et al. The influence of age and gender on niacin skin test results—implications for the use as a biochemical marker in schizophrenia. J Psychiatr Res. 2004;38:537–543. [DOI] [PubMed] [Google Scholar]
- 6. Nadalin S, Buretić-Tomljanović A, Rubesa G, Tomljanović D, Gudelj L. Niacin skin flush test: a research tool for studying schizophrenia. Psychiatr Danub. 2010;22:14–27. [PubMed] [Google Scholar]
- 7. Liu CM, Chang SS, Liao SC, et al. Absent response to niacin skin patch is specific to schizophrenia and independent of smoking. Psychiatry Res. 2007;152:181–187. [DOI] [PubMed] [Google Scholar]
- 8. Lin SH, Liu CM, Chang SS, et al. Familial aggregation in skin flush response to niacin patch among schizophrenic patients and their nonpsychotic relatives. Schizophr Bull. 2007;33:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demily C, Jacquet P, Marie-Cardine M. How to differentiate schizophrenia from bipolar disorder using cognitive assessment?Encephale. 2009;35:139–145. [DOI] [PubMed] [Google Scholar]
- 10. Ray MT, Shannon Weickert C, Webster MJ. Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl Psychiatry. 2014;4:e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ou J, Li M, Xiao X. The schizophrenia susceptibility gene ZNF804A confers risk of major mood disorders. World J Biol Psychiatry. 2016;18:1–6. [DOI] [PubMed] [Google Scholar]
- 12. Thomas EA, Dean B, Scarr E, Copolov D, Sutcliffe JG. Differences in neuroanatomical sites of apoD elevation discriminate between schizophrenia and bipolar disorder. Mol Psychiatry. 2003;8:167–175. [DOI] [PubMed] [Google Scholar]
- 13. Whaley AL. Symptom clusters in the diagnosis of affective disorder, schizoaffective disorder, and schizophrenia in African Americans. J Natl Med Assoc. 2002;94:313–319. [PMC free article] [PubMed] [Google Scholar]
- 14. Knobler HY, Hanin B, Itzchaky S, Maizel S, Shalom D, Edelman S, Lerner Y. Overlap between schizophrenia and affective disorders—a family study. Harefuah 1994;126:241–244, 304. [PubMed] [Google Scholar]
- 15. Kempf L, Hussain N, Potash JB. Mood disorder with psychotic features, schizoaffective disorder, and schizophrenia with mood features: trouble at the borders. Int Rev Psychiatry. 2005;17:9–19. [DOI] [PubMed] [Google Scholar]
- 16. Hudson CJ, Lin A, Cogan S, Cashman F, Warsh JJ. The niacin challenge test: clinical manifestation of altered transmembrane signal transduction in schizophrenia?Biol Psychiatry. 1997;41:507–513. [DOI] [PubMed] [Google Scholar]
- 17. Maclean R, Ward Pe Fau - Glen I, Glen I Fau - Roberts SJ, Roberts Sj Fau - Ross BM, Ross BM. On the relationship between methylnicotinate-induced skin flush and fatty acids levels in acute psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:927–933. [DOI] [PubMed] [Google Scholar]
- 18. Yao JK, Dougherty GG Jr, Gautier CH, et al. Prevalence and specificity of the abnormal niacin response: a potential endophenotype marker in schizophrenia. Schizophr Bull. 2016;42:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bosveld-van Haandel L, Knegtering R, Kluiter H, van den Bosch RJ. Niacin skin flushing in schizophrenic and depressed patients and healthy controls. Psychiatry Res. 2006;143:303–306. [DOI] [PubMed] [Google Scholar]
- 20. Berger GE, Smesny S, Schäfer MR, et al. Niacin skin sensitivity is increased in adolescents at ultra-high risk for psychosis. PLoS One. 2016;11:e0148429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smesny S, Berger G, Rosburg T, et al. Potential use of the topical niacin skin test in early psychosis—a combined approach using optical reflection spectroscopy and a descriptive rating scale. J Psychiatr Res. 2003;37:237–247. [DOI] [PubMed] [Google Scholar]
- 22. Puri BK, Hirsch SR, Easton T, Richardson AJ. A volumetric biochemical niacin flush-based index that noninvasively detects fatty acid deficiency in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:49–52. [DOI] [PubMed] [Google Scholar]
- 23. Nadalin S, Giacometti J, Jonovska S, Tomljanović D, Buretić-Tomljanović A. The impact of PLA2G4A and PTGS2 gene polymorphisms, and red blood cell PUFAs deficit on niacin skin flush response in schizophrenia patients. Prostaglandins Leukot Essent Fatty Acids. 2013;88:185–190. [DOI] [PubMed] [Google Scholar]
- 24. Joyce PR. Age of onset in bipolar affective disorder and misdiagnosis as schizophrenia. Psychol Med. 1984;14:145–149. [DOI] [PubMed] [Google Scholar]
- 25. Ross BM, Ward P, Glen I. Delayed vasodilatory response to methylnicotinate in patients with unipolar depressive disorder. J Affect Disord. 2004;82:285–290. [DOI] [PubMed] [Google Scholar]
- 26. Messamore E. Niacin subsensitivity is associated with functional impairment in schizophrenia. Schizophr Res. 2012;137:180–184. [DOI] [PubMed] [Google Scholar]
- 27. Glen AI, Cooper SJ, Rybakowski J, Vaddadi K, Brayshaw N, Horrobin DF. Membrane fatty acids, niacin flushing and clinical parameters. Prostaglandins Leukot Essent Fatty Acids. 1996;55:9–15. [DOI] [PubMed] [Google Scholar]
- 28. Puri BK, Easton T, Das I, Kidane L, Richardson AJ. The niacin skin flush test in schizophrenia: a replication study. Int J Clin Pract. 2001;55:368–370. [PubMed] [Google Scholar]
- 29. Tavares H, Yacubian J, Talib LL, Barbosa NR, Gattaz WF. Increased phospholipase A2 activity in schizophrenia with absent response to niacin. Schizophr Res. 2003;61:1–6. [DOI] [PubMed] [Google Scholar]
- 30. Hudson C, Gotowiec A, Seeman M, Warsh J, Ross BM. Clinical subtyping reveals significant differences in calcium-dependent phospholipase A2 activity in schizophrenia. Biol Psychiatry. 1999;46:401–405. [DOI] [PubMed] [Google Scholar]
- 31. Ross BM, Hughes B, Turenne S, Seeman M, Warsh JJ. Reduced vasodilatory response to methylnicotinate in schizophrenia as assessed by laser Doppler flowmetry. Eur Neuropsychopharmacol. 2004;14:191–197. [DOI] [PubMed] [Google Scholar]
- 32. Shah SH, Vankar GK, Peet M, Ramchand CN. Unmedicated schizophrenic patients have a reduced skin flush in response to topical niacin. Schizophr Res. 2000;43:163–164. [PubMed] [Google Scholar]
- 33. Smesny S, Riemann S, Riehemann S, Bellemann ME, Sauer H. Quantitative measurement of induced skin reddening using optical reflection spectroscopy—methodology and clinical application. Biomed Tech (Berl). 2001;46:280–286. [DOI] [PubMed] [Google Scholar]
- 34. English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res. 2001;33:645–652. [DOI] [PubMed] [Google Scholar]
- 35. Covault J, Pettinati H, Moak D, Mueller T, Kranzler HR. Association of a long-chain fatty acid-CoA ligase 4 gene polymorphism with depression and with enhanced niacin-induced dermal erythema. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:42–47. [DOI] [PubMed] [Google Scholar]
- 36. Smesny S, Rosburg T, Riemann S, et al. Impaired niacin sensitivity in acute first-episode but not in multi-episode schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2005;72:393–402. [DOI] [PubMed] [Google Scholar]
- 37. Fenton WS, Hibbeln J, Knable M. Essential fatty acids, lipid membrane abnormalities, and the diagnosis and treatment of schizophrenia. Biol Psychiatry. 2000;47:8–21. [DOI] [PubMed] [Google Scholar]
- 38. Messamore E. Relationship between the niacin skin flush response and essential fatty acids in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:413–419. [DOI] [PubMed] [Google Scholar]
- 39. Messamore E, Hoffman WF, Yao JK. Niacin sensitivity and the arachidonic acid pathway in schizophrenia. Schizophr Res. 2010;122:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yao Jk, Stanley JA, Reddy RD, Keshavan MS, Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol Psychiatry. 2002;52:823–830. [DOI] [PubMed] [Google Scholar]
- 41. Brunner J, Gattaz WF. Intracerebral injection of phospholipase A2 inhibits dopamine-mediated behavior in rats: possible implications for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1995;246:13–16. [DOI] [PubMed] [Google Scholar]
- 42. Schaeffer EL, Gattaz WF, Eckert GP. Alterations of brain membranes in schizophrenia: impact of phospholipase A(2). Curr Top Med Chem. 2012;12:2314–2323. [DOI] [PubMed] [Google Scholar]
- 43. Hudson CJ, Kennedy JL, Gotowiec A, et al. Genetic variant near cytosolic phospholipase A2 associated with schizophrenia. Schizophr Res. 1996;21:111–116. [DOI] [PubMed] [Google Scholar]
- 44. Ross BM, Hudson C, Erlich J, Warsh JJ, Kish SJ. Increased phospholipid breakdown in schizophrenia. Evidence for the involvement of a calcium-independent phospholipase A2. Arch Gen Psychiatry. 1997;54:487–494. [DOI] [PubMed] [Google Scholar]
- 45. Berger GE, Smesny S, Amminger GP. Bioactive lipids in schizophrenia. Int Rev Psychiatry. 2006;18:85–98. [DOI] [PubMed] [Google Scholar]
- 46. Koga M, Serritella AV, Sawa A, Sedlak TW. Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr Res. 2016;176:52–71. [DOI] [PubMed] [Google Scholar]
- 47. Yang J, Chen T, Sun L, et al. Potential metabolite markers of schizophrenia. Mol Psychiatry. 2013;18:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang X, Sun L, Zhao A, et al. Serum fatty acid patterns in patients with schizophrenia: a targeted metabonomics study. Transl Psychiatry. 2017;7:e1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peet M, Glen I, Horrobin DF.. Phospholipid Spectrum Disorders in Psychiatry and Neurology. 2nd ed Carnforth, UK: Marius Press; 2003. [Google Scholar]
- 50. Xu XJ, Jiang GS. Niacin-respondent subset of schizophrenia—a therapeutic review. Eur Rev Med Pharmacol Sci. 2015;19:988–997. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.