Abstract
Chronic meningitis caused by Sporothrix sp. is occasionally described in immunosuppressed patients. We report the challenges in diagnosing and managing 2 nonimmunocompromised patients with hydrocephalus and chronic meningitis caused by Sporothrix brasiliensis. This more virulent species appears to contribute more atypical and severe cases than other related species.
Keywords: Sporotrichosis, Sporothrix brasiliensis, chronic meningitis, hydrocephalus, virulence, CNS
Chronic meningitis caused by Sporothrix spp. has occasionally been described in patients with immunosuppression from alcohol abuse, cirrhosis, transplantation, diabetes, and Hodgkin’s disease. It has recently been increasingly reported in AIDS patients as part of a cat-associated epidemic of sporotrichosis in Rio de Janeiro State, Brazil [1, 2]. Sporotrichosis has therefore been suggested as a differential diagnosis of chronic meningitis in immunosuppressed patients living in endemic or hyperendemic sporotrichosis areas [3]. The clinical outcomes of chronic meningitis due to Sporothrix sp. in immunosuppressed patients are poor, with an overall 50% mortality rate [1]. We present 2 cases of hydrocephalus due to unsuspected chronic meningoencephalitis caused by Sporothrix brasiliensis in nonimmunocompromised adults and highlight the challenges in diagnosing and managing these cases.
CASE 1
A previously healthy 46-year-old male presented in January 2016 with a 1-month history of headaches, retro-orbital pain, episodes of confusion, and gait impairment. His previous medical history was unremarkable, and he denied alcohol use. The patient was from the countryside of Paraná Sate, where he was a quarry worker. Initial investigation showed a cerebrospinal fluid (CSF) with very mild mononuclear pleocytosis (7 cells/mm3), low glucose (12 mg/dL), and elevated protein (148 mg/dL), but negative microbiological analyses. Magnetic resonance imaging (MRI) revealed basal meningitis (Figure 1A). Because tuberculosis is highly endemic in Brazil, he was empirically treated for tuberculous meningitis with the standard regimen for 4 months, without showing signs of improvement.
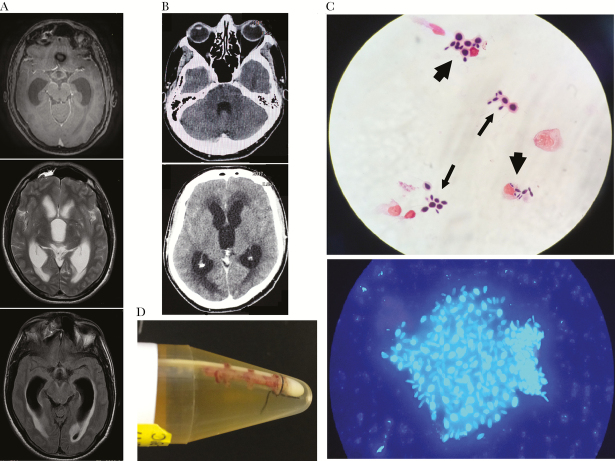
Figure 1.
Magnetic resonance imaging (MRI) and computed tomography (CT) scan of the brain and direct mycological exam of cerebrospinal fluid (CSF). A, Pretreatment (March 2016) brain T1-weighted MRI of patient 1 showing hydrocephalus and skull base meningeal contrast enhancement. B, Pretreatment (May 2017) CT scan of the brain of patient 2 showing communicating hydrocephalus and transependymal edema without anomalous intraparenchymal contrast enhancement or intraparenchymal lesions. C, Upper image: baseline CSF direct mycological exam (August 2017) of patient 2 showing yeast cells suggestive of Sporothrix sp. free (thin arrows) or engulfed by macrophages (thick arrows). Lower image: calcofluor white staining of patient 2’s CSF sample collected on August 2017, showing a yeast aggregate surrounded by extracellular matrix suggestive of a biofilm-like structure. D, fibrin sheath on the tip of the ventriculoperitoneal shunt removed (August 2017) from patient 2: culture of the material from the tip yielded Sporothrix sp., subsequently identified by molecular method as S. brasiliensis.
On readmission, the patient was wheelchair bound due to motor weakness and lack of balance and was confused and disoriented. MRI showed hydrocephalus, and a ventriculoperitoneal shunt (VPS) placement was indicated. This procedure resulted in partial neurological improvement. The patient was discharged from the hospital but kept on antituberculosis therapy. HIV serology was negative. The patient presented relapses of the central nervous system (CNS) manifestations that were caused by obstruction of the CSF pathway secondary to formation of a pseudocyst around the peritoneal tip of the shunt, which accumulated large volumes of CSF. The CSF pathway was reestablished each time via a surgical procedure; 3 consecutive CSF samples were collected and showed mild mononuclear pleocytosis, low glucose, and elevated protein; all were negative on direct mycological exam (DME), but 2 yielded Sporothrix sp.
The isolate was subsequently identified as S. brasiliensis by DNA sequencing of the calmodulin gene (GenBank MG869808). The patient was treated with amphotericin deoxycholate (accumulated dose 2 g), with good clinical response. As neither itraconazole nor serum level monitoring was available [4], he was treated with fluconazole (800 mg/d), scheduled for 1 year; no relapses were recorded at the time of writing. However, the patient presented neurological sequelae (ataxia and extrinsic ocular motor paresis related to basal meningitis), which are slowly improving.
CASE 2
Case 2 was a previously healthy 40-year-old male with progressive weight loss and lethargy dating from October 2016. He was a farmer in the countryside of Bahia State. He had been treated for “depression,” but his symptoms evolved, with headaches, vomiting, and confusion. He was HIV-negative and denied alcohol abuse. In April 2017, a cranial computed tomography scan showed hydrocephalus and signs suggestive of mild meningeal enhancement in some areas (posterior fossa) (Figure 1B). CSF analysis revealed glucose 40 mg/dL, protein 48 mg/dL, and mild pleocytosis (8 mononuclear cells/mm3); microbiology tests were negative. The patient received a provisional diagnosis of idiopathic hydrocephalus and received a VPS. Hydrocephalus improved, and the patient was discharged but required a subsequent neurosurgical procedure to reestablish the CSF pathway. He was referred to our service in August 2017 because of obstruction of the VPS and abdominal discomfort and distension.
As with patient 1, a peritoneal pseudocyst with 1500 mL of CSF was found, and a clinical hypothesis of peritonitis due to secondary bacterial infection (although cultures for bacteria were negative) was made. This led to empirical treatment with vancomycin and ceftazidime; abdominal symptoms improved significantly. CSF analysis at this time showed 12 mononuclear cells, glucose 53 mg/dL, and protein 43 mg/dL; however, DME yielded yeast forms, and culture revealed Sporothrix sp. (Figure 1C). The peritoneal tip of the VPS showed a fibrin sheath (Figure 1D), which was also positive for Sporothrix sp. on culture. Molecular identification revealed the species S. brasiliensis (GenBank MG867724), as in case 1. The catheter was removed, and a new VPS was positioned. A qualitative immunolectrophoresis test for anti-Sporothrix antibodies yielded positive results in serum and CSF.
However, the patient’s follow-up was complicated. Subsequent episodes of obstruction accompanied by recrudescence of hydrocephalus required successive neurosurgical procedures, including a neuro-endoscopic ventricular septostomy and, ultimately, a ventriculoatrial shunt with 2 proximal catheters with a Y-connector. The patient was started on amphotericin deoxycholate, but attempts to move to itraconazole failed because of the development of severe pharmacodermy. The patient remains hospitalized, receiving liposomal amphotericin and monitoring of hydrocephalus. All subsequent CSF analyses were negative for Sporothrix but still presented mild biochemical and cellular abnormalities, with the exception of the most recent (December 2017), which was normal.
DISCUSSION
The presented cases highlight the challenges in diagnosing and managing chronic meningitis caused by Sporothrix brasiliensis. Although currently unusual, this issue will likely be of growing importance due to the continuous expansion of the sporotrichosis epidemic in Brazil, which is causing atypical and more severe cases [5]. The diagnosis was delayed in both patients; they neither presented clinico-laboratorial evidence of immunosuppression nor were from municipalities where cases of human or feline sporotrichosis had been previously reported, according to the local health authorities. However, a case of cutaneous sporotrichosis was documented in patient 1’s municipality several months after the presented case, likely transmitted by a cat with an illness typical of sporotrichosis. Unfortunately, the animal could not be located for confirmation. In neither case was CNS involvement preceded by manifestations of cutaneous-lymphatic sportrichosis or associated disseminated disease. In immunosuppressed patients, CNS involvement is commonly part of a constellation of manifestations suggestive of hematogenous dissemination [2, 3]. Therefore, case 1 was empirically treated for tuberculosis for more than 4 months, whereas case 2 was diagnosed as idiopathic hydrocephalus 8 months before Sporothrix brasiliensis was isolated from CSF. These 2 cases are similar to those previously described in the literature with regards to both the difficulty in recovering the agent from the CSF [1, 2, 6, 7] and the delay in starting appropriate treatment, likely worsening the prognosis. Published cases refer to several weeks to many months from initial symptom presentation to identification of the fungus [1]. The usual scenario is that of a patient presenting the clinical syndrome of chronic meningitis, manifested initially by headache that progressed to lethargy, confusion, or other less frequent manifestations such as vomiting, seizures, gait disturbances, and other neurological deficits. It is noteworthy, like in the cases reported here, that fever was not always present. Preceding or associated cutaneous-lymphatic sporotrichosis was also not always present, particularly in nonimmunocompromised patients. Moreover, most patients, like these, did not report a history of unusual environmental exposure to Sporothrix sp. CSF analysis is also indistinguishable from that of chronic meningitis caused by other agents: elevated protein and low glucose levels were generally described, as well as low to moderate pleocytosis, although cases with 0 cells have already been described [1, 6]. The patients in this report followed this pattern, with case 1 presenting more prominent CSF alterations than case 2. Brain imaging, when reported, was either normal or showed meningeal enhancement; in a few patients there were signs of vasculitis and infarcts. Parenchymal lesions were occasionally described, mostly in immunosuppressed, HIV-infected patients [1, 7].
In light of the lack of awareness of Sporothrix sp. as a cause of chronic meningitis and the difficulties in obtaining positive fungal cultures from CSF in meningeal sporotrichosis, Scott et al. [6] recommended, 30 years ago, systematic serological testing of the CSF for antibodies to Sporothrix sp. when investigating chronic meningitis cases with difficult etiological diagnoses. In Brazil, serological testing is restricted to a few research laboratories. Our laboratory (LIM-53) developed a qualitative immunoelectropheresis test for detecting anti-Sporothrix sp. serum antibodies in the 1980s, which will now be included in the screening panel for deep mycoses (histoplasmosis, aspergillosis paracoccidioidomycosis). This test was performed in patient 2, yielding positive results in serum and CSF. However, despite its high specificity, it still has low sensitivity (≤40%) and needs improvement. The importance of serological diagnosis was recently demonstrated by a case report, which could only be diagnosed through CSF antibody detection [7].
The conditions of both patients were and still are difficult to manage. Although they presented very mild CSF pleocytosis, they evolved with hydrocephalus and required ventricular shunts. Moderate hydrocephalus was also present in several of the patients described in the literature [1], but placement of VPS was not frequently reported, except in Scott et al.’s case series, where 5 of the 7 patients underwent VPS; only 1 of these patients died, a patient with a 6-month-delayed diagnosis who could only be treated with a small amount of amphotericin B [6]. More recently, Freitas et al. [2] reported that 2 of the 4 HIV-infected patients from the hyperendemic area of Rio de Janeiro died of hydrocephalus complications; however, it was not mentioned whether these patients underwent VPS. In the patients in this study, the ventricular shunts were very difficult to manage due to frequent obstructions, requiring successive surgical interventions to restore the CSF pathway, indicating the presence of a significant and persistent local inflammatory reaction. Both patients responded to treatment; however, patient 1 developed ataxia and extrinsic ocular motor paresis secondary to basal meningitis, from which he has yet to recover. Patient 2 remains hospitalized on antifungal therapy and in the process of neurological rehabilitation.
It is not known how Sporothrix sp. reaches the CNS. In immunosuppressed patients, it is speculated that it is caused by hematogenic spread in the setting of a disseminated disease [1–3]. However, this would not apply to nonimmunocompromised patients without disseminated disease. An attractive yet speculative hypothesis relies on the recent discovery that the CNS has a functional lymphatic system connected to the body’s lymphatic system through deep cervical lymph nodes [8]. It is thus possible that our patients acquired the infection through either the respiratory route or an unnoticed traumatic skin inoculation, and cells from the initial local inflammatory response (eg, macrophages) that phagocytosed yeast cells would have migrated to the lymphatic circulation and eventually reached the CNS through this pathway. Indeed, Figure 1C shows some CSF macrophages engulfing yeast cells in patient 2. In addition, it has been suggested that, at least in experimental models, S. brasiliensis is more virulent in vivo than the other species [9]. In vitro, it has also been shown that Sporothrix brasiliensis has a greater ability to disarm murine macrophages, promoting its survival within macrophages (L. Rossato, PhD, S. A. Almeida, PhD, 2017, unpublished data), and to form biofilms [10]. In fact, biofilm-like structures were observed in patient 2’s CSF (Figure 1C), which may help to explain the recurrent hydrocephalus shunt catheter obstructions.
These cases carry some potentially important messages: that the Brazilian epidemic, due to the more virulent Sporothrix brasiliensis, contributes more to atypical and unexpectedly aggressive cases, even in nonimmunocompromised individuals without a clear-cut epidemiological link, compared with previous outbreaks due to Sporothrix schenckii [11–14]; that sporotrichosis should come to mind in cases of chronic meningitis with complex etiological diagnosis; and that serological tests should urgently be made widely available to enable earlier diagnosis, prevent severe CNS damage, and reduce mortality rates.
Acknowledgments
We thank Gilda M. B. Del Negro for the assistance in fungal gene sequencing, Mônica S. M. Vidal for the immunolectrophoresis assay, Daniel Ciampi for critical advices, and Justin H. Axel-Berg for English review.
Potential conflicts of interest. G.B. is a senior researcher from CNPq. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Galhardo MC, Silva MT, Lima MA et al. . Sporothrix schenckii meningitis in AIDS during immune reconstitution syndrome. J Neurol Neurosurg Psychiatry 2010; 81:696–9. [DOI] [PubMed] [Google Scholar]
- 2. Freitas DF, Lima MA, de Almeida-Paes R et al. . Sporotrichosis in the central nervous system caused by Sporothrix brasiliensis. Clin Infect Dis 2015; 61:663–4. [DOI] [PubMed] [Google Scholar]
- 3. Moreira JAS, Freitas DFS, Lamas CC. The impact of sporotrichosis in HIV‐infected patients: a systematic review. Infection 2015; 43:267–76. [DOI] [PubMed] [Google Scholar]
- 4. Kauffman CA, Bustamante B, Chapman SW, Pappas PG; Infectious Diseases Society of America Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:1255–65. [DOI] [PubMed] [Google Scholar]
- 5. Almeida-Paes R, de Oliveira MM, Freitas DF et al. . Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis 2014; 8:e3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scott EN, Kaufman L, Brown AC, Muchmore HG. Serologic studies in the diagnosis and management of meningitis due to Sporothrix schenckii. N Engl J Med 1987; 317:935–40. [DOI] [PubMed] [Google Scholar]
- 7. Hessler C, Kauffman CA, Chow FC. The upside of bias: a case of chronic meningitis due to Sporothrix schenckii in an immunocompetent host. Neurohospitalist 2017; 7:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louveau A, Smirnov I, Keyes TJ et al. . Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arrillaga-Moncrieff I, Capilla J, Mayayo E et al. . Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect 2009; 15:651–5. [DOI] [PubMed] [Google Scholar]
- 10. Brilhante RSN, de Aguiar FRM, da Silva MLQ et al. . Antifungal susceptibility of Sporothrix schenckii complex biofilms. Med Mycol. 2018; 56:297–306. [DOI] [PubMed] [Google Scholar]
- 11. Govender NP, Maphanga TG, Zulu TG et al. . An outbreak of lymphocutaneous Sporotrichosis among mine-workers in South Africa. PLoS Negl Trop Dis 2015; 9:e0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGuinness SL, Boyd R, Kidd S et al. . Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis 2016; 16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leads from the MMWR. Multistate outbreak of sporotrichosis in seedling handlers, 1988. JAMA 1988; 260:2806, 2811. [PubMed] [Google Scholar]
- 14. Pappas PG, Tellez I, Deep AE et al. . Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis 2000; 30:65–70. [DOI] [PubMed] [Google Scholar]