Abstract
Every day, humans make countless decisions that require the integration of information about potential benefits (i.e. rewards) with other decision features (i.e. effort required, probability of an outcome or time delays). Here, we examine the overlap and dissociation of behavioral preferences and neural representations of subjective value in the context of three different decision features (physical effort, probability and time delays) in a healthy adult life span sample. While undergoing functional neuroimaging, participants (N = 75) made incentive compatible choices between a smaller monetary reward with lower physical effort, higher probability, or a shorter time delay versus a larger monetary reward with higher physical effort, lower probability, or a longer time delay. Behavioral preferences were estimated from observed choices, and subjective values were computed using individual hyperbolic discount functions. We found that discount rates were uncorrelated across tasks. Despite this apparent behavioral dissociation between preferences, we found overlapping subjective value-related activity in the medial prefrontal cortex across all three tasks. We found no consistent evidence for age differences in either preferences or the neural representations of subjective value across adulthood. These results suggest that while the tolerance of decision features is behaviorally dissociable, subjective value signals share a common representation across adulthood.
Keywords: decision preferences, subjective value, hyperbolic discounting, medial prefrontal cortex, aging
Introduction
People face a myriad of decisions every day, ranging from the crucial to the trivial. Should I walk up the stairs or take the elevator? Should I use a financial windfall to buy bonds or invest in stocks? Should I purchase a new car now or wait until the end-of-the-year sales? Real-world decision making involves taking into account factors such as the amount of effort required to achieve an outcome, the probability that an outcome will be realized, or the amount of time until an outcome is realized. Each individual’s decision preferences vary based on how these features diminish the subjective value of a decision’s potential outcome. Thus, the choice to pursue financial well-being, social satisfaction, and physical health depend upon individual differences in the discounting of these features and their computation of subjective value. A better understanding of individual differences in discounting behavior and the representation of subjective value in the brain, and whether this behavior and these representations are consistent across adulthood, may provide insights into everyday decision making across adulthood.
Theoretically, it has been posited that probability and time discounting share similar cognitive mechanisms (Estle et al., 2006; Green & Myerson, 2004). However, the empirical literature examining this conjecture is mixed, with some studies finding behavioral correlations between probability and time discounting (Mitchell, 1999; Richards et al., 1999), while others only find correlations within a particular patient group or experimental condition (Myerson et al., 2003; Scheres et al., 2006), and still others find no evidence of correlated preferences across tasks (Holt et al., 2003; Ohmura et al., 2005; Olson et al., 2007; Peters & Büchel, 2009; Reynolds et al., 2004; Weber & Huettel, 2008). Fewer theoretical and empirical studies have compared effort discounting to probability or time discounting, with limited support for behavioral correlations between effort and probability discounting (Massar et al., 2015; Prévost et al., 2010). To our knowledge, only two studies have examined the relationships between discounting behavior across all three features: one finding correlations across all three features in a small sample (N = 11) of smokers (Mitchell, 2004) and our own study which only found a small correlation between effort and time discounting in a moderate sample (N= 92) of healthy adults (Seaman et al., 2016). Given that the literature examining behavioral consistency of preferences across decision features is inconclusive, one goal of this study was to examine behavioral correlations between preferences across all three decision features (effort, probability and time) in healthy adults.
From behavioral preferences, and how they vary as a function of decision features, one can make inferences about the subjective value (SV) of each option presented during decision-making. Variation in SV, or the value of an option after any discounting, across trials within a task can be used to identify brain regions where neural activity is correlated with SV (Kable & Glimcher, 2007). One strength of this approach is that SV signals can be localized independent of individual differences in decision preferences across subjects. In fact, previous research has shown similar SV representation in individuals with dramatically different discount rates (Kable & Glimcher, 2007, 2010). Comprehensive meta-analyses of human neuroimaging studies of decision making using these methods suggest a network of regions, where brain activity is highly correlated with SV that includes the medial prefrontal cortex, posterior cingulate cortex and ventral striatum (Acikalin, Gorgolewski, & Poldrack, 2017; Bartra, McGuire, & Kable, 2013; Clithero & Rangel, 2014). These studies, mostly in healthy young adults, suggest a common neural system representing SV across decision features.
In addition to the evidence for a unitary corticostriatal system for SV representation, there is also evidence for regional specialization depending on the particular decision feature being considered. For instance, studies have suggested that SV representations during effort discounting are significantly stronger in the anterior cingulate and insula cortices compared to time discounting (Massar et al., 2015; Prévost et al., 2010) and in the midcingulate and supplementary motor area during effort discounting compared to probability discounting (Burke et al., 2013). SV representation during probability discounting has been shown to be greater in the superior parietal cortex and middle occipital lobes compared to time discounting (Peters & Büchel, 2009) and anterior insula compared to effort discounting (Burke et al., 2013). Finally, SV representations during temporal discounting have been shown to be significantly greater in the posterior cingulate, frontopolar, and lateral–parietal cortices compared to probability discounting (Peters & Büchel, 2009) and in the ventral–medial prefrontal cortex, ventral striatum, and right supramarginal and superior temporal gyri compared to effort discounting (Prévost et al., 2010). In summary, there is less clarity in the existing studies regarding regional specialization for different decision features. The varying regions and effects across studies could be due to differences in tasks or the small samples sizes (N range = 18–23), creating a lack of power and contributing to a lack of reliability and reproducibility (Poldrack et al., 2017). It should also be noted that these studies only tested young adults. It remains unclear whether these patterns in the overlap and dissociation of SV representation between discounting tasks are reliable and generalizable across adulthood.
Emerging theories suggest that changes in cognition, emotion, motivation, and experience across adulthood influence age differences in decision making (Brown & Ridderinkhof, 2009; Hsu et al., 2008; Mather, 2006; Peters et al., 2007; Samanez-Larkin & Knutson, 2015). Behavioral work has begun to investigate the differential effects of various decision features (effort, probability and time) on decision making across adulthood. While there is only one published study of preferences for physical effort in older adults (Seaman et al., 2016) that showed no age differences in effort discounting on a hypothetical task, studies of cognitive effort using an incentive–compatible task suggest that older adults discount effort more than younger adults (Westbrook, Kester, & Braver, 2013). In the probability domain, a quantitative meta-analysis of studies of probabilistic decision making revealed that choice behavior does not consistently differ between younger and older adults; instead, age effects vary depending on cognitive task demands (Mata et al., 2011). The research is also inconclusive in the time domain, with some studies reporting a decrease in temporal discounting with age (Eppinger, Nystrom, & Cohen, 2012; Green, Fry, & Myerson, 1994), while other studies find no age differences (Roalf, Mitchell, Harbaugh, & Janowsky, 2012; Samanez-Larkin et al., 2011) or an increase in discounting with age (Read & Read, 2004). In summary, behavioral studies suggest potential differential effects of aging on decision making across different decision features, although there is no strong evidence for consistent age differences in preferences. If there are differences, it would be interesting to investigate how these decision features and SVs are differentially represented by younger, middle-aged, and older adults. Further, it is not clear whether the same or different neural systems are computing and representing SV across adulthood.
There is currently very little empirical evidence directly linking specific psychological and neural systems with adult age differences in SV representation. The vast majority of emerging studies on decision making across adulthood document age differences in behavior or choice without specifying the underlying psychological, computational and neural mechanisms. Due to marked age-related changes in the structure and function of the medial prefrontal cortex (Fjell et al., 2014; Raz et al., 2005), as well as age-related differences in connectivity between the medial prefrontal cortex and other regions (Bennett et al., 2010; Samanez-Larkin et al., 2012), it is plausible that SV representation may change in the aging brain. To our knowledge, only one study has examined SV representation in older adults. The study found that older adults with stronger representation of SV during intertemporal choice also had lower neural signal variability and performed better on a separate probabilistic decision-making task (Halfmann et al., 2016). This suggests weaker SV representations could be due to greater neural signal variability, and this nosier representation leads to worse decision performance. However, this study did not compare SV representations across adulthood, so the question remains whether or not SV representation is consistent from young adulthood through middle age and into older adulthood. If there is weaker and more variable SV-related neural signal in older age, this could make decision making more difficult or more inconsistent as people age (Tymula et al., 2013).
Here, we examined sensitivity to and tolerance of effort, probability and time in monetary decision-making tasks across adulthood. Young adults, middle-aged adults and older adults completed three different two alternative, forced-choice decision tasks assessing tolerance for effort requirements, probability and temporal delays while undergoing functional magnetic resonance imaging (fMRI). Given inconsistent results in the literature examining behavioral correlations across these three decision features, we predicted there would be little to no association between discounting behavior in this study. Based on the aging literature, we hypothesized that on incentive–compatible tasks, effort discounting would increase with age, while probability and temporal discounting would remain relatively stable. Given the age-related structural and functional declines in frontostriatal regions described above, we predicted an age-related decrease in representation of SV in the ventral striatum and medial prefrontal cortex. However, we also considered the possibility that there would be no age-related differences in SV representation, given the preservation of function in these regions for relatively simple decision tasks (Samanez-Larkin & Knutson, 2015).
Method
Participants and procedures
Seventy-five participants (age: M = 49.71, s.d. = 17.92, range = 22–83 years old) were included in all the analyses. They were a subset of 89 healthy volunteers who completed the fMRI tasks described below as part of a multiday multimodal neuroimaging study that included MRI and positrom emission tomography imaging. Fourteen of the 89 subjects were excluded due to poor data quality (see Supplementary Methods) leaving a final sample of 75. Participants were recruited from the Nashville community using the Vanderbilt School of Medicine subject database of healthy adults, Research Match (www.researchmatch.org), and a combination of newspaper, radio and local television advertisements (Seaman et al., 2016). The Vanderbilt University and Yale University Institutional Review Boards approved all experimental procedures. Participants gave informed consent and were compensated with at least $350 for the entire study, with a potential for additional money (up to $60) based on task performance.
Cognitive assessment
Participants completed a battery of cognitive assessments during the study. Table 1 displays the mean performance on this test battery and correlation of each measure with age. As in Seaman et al. (2016), this sample displayed normal performance on neuropsychological tests, with the expected significant age differences in measures of fluid intelligence (e.g. Digit Span) and lack of age differences in crystallized intelligence (e.g. Vocabulary) across adulthood.
Table 1.
Participant characteristics
| Variable | R [95% CI] with age | M (s.d.) |
|---|---|---|
| Age | 49.71 (17.92) | |
| Gender | 42F/33M | |
| Digit span | –0.44 [–0.61, –0.23] | 16.24 (4.04) |
| Letter–Number Sequencing | –0.58 [–0.71, –0.4] | 11.31 (2.98) |
| Numeracya | –0.26 [–0.46, –0.04] | 11.85 (3.16) |
| Paired associates delayed recallb | –0.62 [–0.74, –0.45] | 5.82 (2.42) |
| Shipley Vocabulary Subscale | 0.13 [–0.11, 0.34] | 33.37 (5.4) |
| Trails testc | 0.39 [0.18, 0.57] | 38.17 (30.22) |
Notes: Digit Span and Paired Associates Delayed Recall from the WMS-III, Wechsler Memory Scale, Third Edition, (Wechsler, 1997 b); WAIS-III, Letter-Number Sequencing, (Wechsler, 1997a); Numeracy, (Peters et al., 2007); Shipley Vocabulary Subscale, (Shipley, 1940); Trails Test, (Corrigan & Hinkeldey, 1987). Significant correlations denoted in bold.
Numeracy score for one participant was not recorded.
Delayed Recall not recorded for four participants.
Trails test score is the difference in time to complete Trail A and Trail B.
Tasks
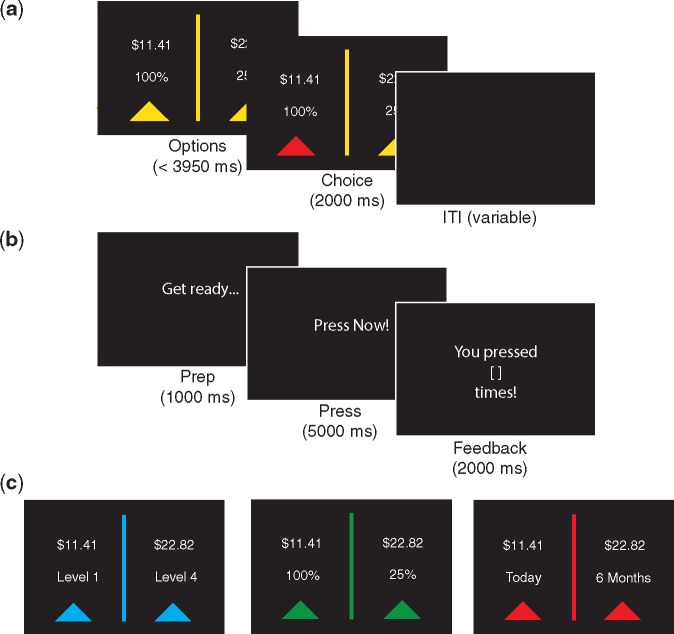
We sought to examine individual differences in neural SV representation across three decision features (effort, probability and time) in a within-subjects design. Participants completed the same 82 unique trials in the same order on each of the three two-alternative forced-choice tasks. E-Prime code and trial-level stimulus information for all three tasks are available on Open Science Framework (OSF; https://osf.io/bths8/). Each task was completed in its own block, and task blocks were performed in the order that they are described below (Figure 1) while undergoing fMRI. Participants were compensated with the payout from one trial from each task using Amazon gift cards that were scheduled for electronic delivery at the chosen time. On an earlier study visit, participants also completed effort, probability and time discounting tasks for monetary (as well as social and health related) rewards, but the outcomes were hypothetical (results reported in Seaman et al., 2016). For all three tasks, on each trial, the smaller reward was randomly chosen from a uniform distribution between $5 and $15, and the larger reward was 25%, 50%, 100%, or 150% greater. There were also two control trials per task, one where both options had the same reward (but different decision features) and one where the options had the minimum difference possible in decision features (but vastly different reward).
Fig. 1.
Discounting tasks. (a) Trial structure for discounting tasks. Participants had up to 3950 ms to make a choice. (b) Task sequences for button-pressing task. On half of the effort trials, participants completed the button-pressing task between the choice phase and the intertrial interval (ITI). (c) Sample options for discounting tasks. Left: effort; Middle: probability; Right: time.
Effort expenditure for rewards task
The effort expenditure for rewards task (EEfRT) was adapted from an existing paradigm that used finger pressing as the physical effort required for earning a reward (Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009). On each trial, participants chose between a smaller reward available for a lower amount of physical effort (button presses) and a larger reward available for a higher amount of effort. The effort required for the smaller reward was set as 20%, 40% or 60% (of each participant’s maximum press rate), while the effort required for the larger reward was set as 20% or 40% higher than the smaller reward with no effort >80% required. The number of button presses required for each level of effort was individually determined based on an initial calibration procedure in which participants pressed a button with their pinky finger as many times and as rapidly as possible in a few short intervals. On half of the trials, after making a choice, participants were shown a 1-second “Ready” screen and then completed the button-pressing task.
Probabilistic discounting task
The probabilistic decision-making paradigm is similar to a number of recent two-alternative forced choice mixed gamble tasks (Levy & Glimcher, 2011). On each trial, participants chose between a smaller reward with a higher probability (100%, 75% and 50%), while the probability of the larger reward was set to 25% or 50% lower than the smaller reward with no probability lower than 25% included.
Temporal discounting task
The temporal discounting tasks were adapted from a previously used paradigm (McClure, Laibson, Loewenstein, & Cohen, 2004). On each trial, participants chose between an early reward and a late reward. The delay of the early reward was set to today, 2 or 4 weeks, while the delay of the late reward was set to 2 or 4 weeks after the early reward with no delays >6 weeks included.
Computational modeling
Computational models were used to estimate behavioral preferences and compute subject-specific SVs for use in brain imaging analyses. For each participant and each task, SVs were modeled with a hyperbolic discounting function,
where R represents the monetary reward, k represents the discount rate, and C represents either: (a) proportion of maximum finger press rate from the practice run for effort, (b) odds against winning ((1-P(win))/P(win)) for probability or (c) delay in days for time. Data were fit with a softmax decision function (see Supplementary Methods). This model provided a good fit the data (Supplementary Table S1). The estimated k values per participant were used to calculate the SV of the chosen option (SV) on each trial within each participant, which were used in the fMRI analyses described below.
fMRI acquisition and preprocessing
Brain images were collected using a 3-T Phillips Intera Achieva MRI scanner using a 32-channel head coil. High-resolution structural scans were acquired using T1-weighted, high-resolution anatomical scans (repetition time = 8.9 ms, echo time = 4.6 ms, field of view = 256 × 256, voxel dimensions = 1 × 1 × 1 mm), facilitating localization and coregistration of the functional data. Functional scans of the whole brain were acquired with 38 oblique 3.2-mm-thick slices (in-plane resolution 3 × 3 mm, gap = 0.35 mm) at a repetition time of 2 s, with a T2*-weighted (echo time = 28 ms, flip angle = 79 degrees).
Analysis of functional neuroimaging data was conducted using analysis of functional neuroimages (AFNI) software (Cox, 1996). Preprocessing of functional time series data included slice-time correction to account for nonsimultaneous acquisition, motion correction in six directions to account for motion between volumes, Gaussian spatial smoothing to minimize anatomical differences (FWHM = 4 mm), normalization to convert to percent signal change relative to the mean activation for the entire experiment and high-pass filtering (.011 Hz) to remove slow trends. Visual inspection of the motion correction estimates confirmed that no included subject’s head moved more than 4 mm in any dimension from one volume acquisition to the next. As noted above, 10 participants were excluded for head motion that exceeded this.
Whole-brain analyses
Following the procedures recommended by the AFNI group (Coxet al., 2016), voxel-wise statistical thresholds were set to P < 0.001, uncorrected, at the whole-brain level. The minimum cluster size of 48 contiguous, face-to-face voxels for a cluster-level correction of P < 0.05 was estimated using the updated autocorrelation function in AFNI’s 3dFWHMx and 3dClustSim. For each participant, the preprocessed time series data were analyzed with multiple regression models in AFNI. At the subject level, models included a regressor modeling the choice period of each trial and a regressor modeling the SV of the chosen option (SVcho). Before inclusion in the regression models, these covariates were convolved with a single gamma function to model the hemodynamic response. Subject-level regression models also included six covariates for residual motion. For each contrast of interest, T-statistic maps were transformed in Z-scores and spatially normalized by warping into MNI-152 stereotactic space. Anatomical images were warped into MNI template space (see Supplementary Methods). The spatial normalization parameters resulting from each subject’s anatomical image warping were applied to the statistical maps produced in the first-level fMRI analyses before group analyses. The residual error time series from these subject-level models were used to estimate the noise smoothness values for cluster estimation.
Statistical maps were then generated using a single general linear model to examine the mean effect of SVcho across subjects (i.e. regression intercept) and the linear effect of age on SVcho. We then performed conjunction analyses (Nichols et al., 2005) to identify regions where the correlation of brain activity with SV exceeded a threshold of Z = 3.291. We examined this conjunction for all three tasks as well as this conjunction for each pair of tasks (effort & probability, effort & time and probability & time). Finally, we used paired t-tests to identify regions where there were significant differences in SV representation between each pair of tasks.
Data: Code and data used in the manuscript can be viewed 60 and downloaded from https://osf.io/26mqt/. Unthresholded statistical maps for neuroimaging results available at: https://neurovault.org/collections/3184/.
Results
Decision preferences and discount rates
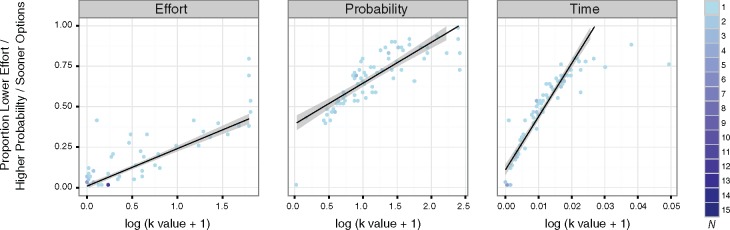
Decision preferences were quantified in two ways. First, we computed a score indicating the proportion of lower effort/higher probability/sooner options selected. This score has been commonly used in the literature. It does not make specific assumptions about the curvature of the discount function but also likely lacks sensitivity. We also used computational modeling with hyperbolic discount functions to estimate each subject’s discount rate (k) for effort, probability and time. As expected, for each task there was a strongly significant relationship between the simple proportion choice score and discount rate (Figure 2), and these relationships remained significant controlling for age (Table 2).
Fig. 2.
Decision preferences and discount rates. Robust correlations between decision preferences (proportion of lower effort/higher probability/sooner options chosen) and log-transformed discount rates (k values). Left: effort; Middle: probability; Right: time. The color bar on the far right represents the number of individual participants represented by that data point.
Table 2.
Decision preferences and discount rates: partial correlations [95% BCa CI] controlling for age
| Decision feature | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1) Preferences for effort | |||||
| 2) Preferences for probability | 0.007 [–0.208, 0.232] | ||||
| 3) Preferences for time | 0.317 [0.15, 0.462] | –0.032 [–0.23, 0.188] | |||
| 4) Effort log (k + 1) | 0.865 [0.721, 0.915] | –0.032 [–0.232, 0.207] | 0.254 [0.091, 0.415] | ||
| 5) Probability log (k + 1) | –0.038 [–0.24, 0.154] | 0.838 [0.734, 0.889] | –0.04 [–0.253, 0.171] | –0.088 [–0.282, 0.124] | |
| 6) Time log (k + 1) | 0.216 [–0.005, 0.424] | –0.044 [–0.219, 0.148] | 0.862 [0.76, 0.926] | 0.103 [–0.095, 0.299] | –0.037 [–0.211, 0.126] |
Notes: Decision preferences quantified as the proportion of lower effort/higher probability/sooner choices. Discount rates are k parameter values from the softmax hyperbolic discounting function. Because of their skewed distribution, discount rates are log-transformed. Significant correlations are denoted in bold typeface. Bias-corrected and accelerated bootstrapped confidence intervals (DiCiccio et al., 1996) were computed using 2000 replications.
There was no significant relationship between age and effort discounting (Supplementary Figure S1). Like our prior study (Seaman et al., 2016), there was a pronounced floor effect in the effort data, making it difficult to detect an age effect if it exists. As predicted, there was no relationship between age and probability discounting. However, contrary to our predictions, there was a small, significant relationship between age and temporal discounting, with older age having higher discount rates. Because this relationship did not extend to the full behavioral sample (R = 0.12, 95% CI [–0.1, 0.32], N = 89), nor did it replicate in a hypothetical version of this task (Seaman et al., 2016), the age difference does not appear to be reliable.
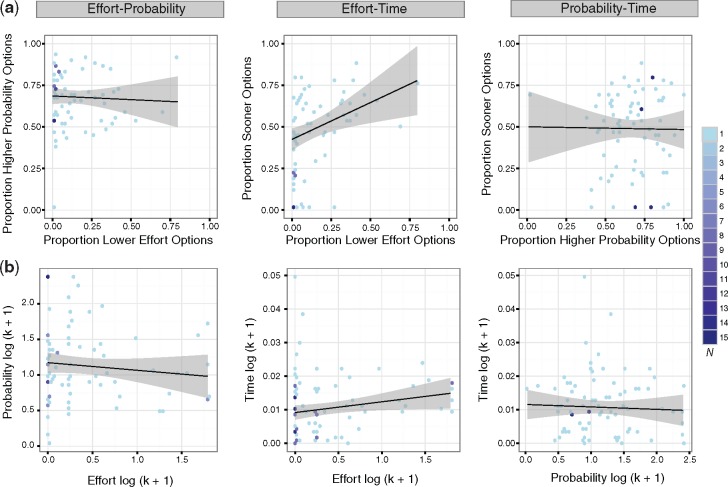
To determine whether there was consistency in behavioral preferences across tasks, we examined the correlation between preferences (using both the simple proportion score and discount rates) across each pair of features. For the effort and time tasks, there was a significant relationship between the proportion scores (Figure 3a, Table 2) but not between the discount rates (k; Figure 3b;Table 2). There was no evidence suggesting proportion scores or discount rates were correlated between any other pair of tasks. Thus, there is very limited evidence for consistency in behavioral preferences across tasks.
Fig. 3.
Behavioral consistency across decision preferences and discount rates. (a) Robust correlations between decision preferences for effort, probability, and time and (b) between effort, probability, and temporal discount rates (log-transformed). Left: probability over effort; Middle: time over effort; Right: time over probability. Because of their skewed distribution, the discount rates are log-transformed. The color bar on the far right represents the number of individual participants represented by that data point.
Neural representation of subjective value
Using the discount rates (k) described above, the subjective value of the chosen option (SVcho) was calculated for each trial. First-level regression models in individual subjects examined the neural representation of SVcho across the brain. Second-level regression analyses examined averages across subjects (intercept) and linear effects of age on SV representation. Whole-brain analyses found several regions where brain activity correlated with SVcho within each task (Table 3; Figure 4), controlling for age. During the effort task, SVcho correlated with activity in a large bilateral medial prefrontal region, including the anterior (pregenual) cingulate, neighboring medial frontal gyrus/medial frontal pole, and subcortical regions, including the bilateral thalamus and left caudate. During the probability task, SVcho correlated with activity in a set of large bilateral regions, including the anterior (pregenual) cingulate, medial frontal gyrus/pole, and middle/posterior cingulate. SVcho during the probability task also correlated with activity in subcortical regions, including the thalamus, and insula, as well as the cerebellum. During the time task, SVcho correlated with bilateral activity in the pregenual cingulate cortex, adjacent medial wall, and the right precuneus.
Table 3.
Neural representation of subjective value of the chosen option controlling for age
| Region | R | A | S | Z | # Voxels |
|---|---|---|---|---|---|
| Effort | |||||
| L medial frontal pole/pregenual cingulate | –8 | 50 | 4 | 5.50 | 789 |
| L precentral gyrus | –22 | –14 | 66 | 4.75 | 479 |
| L inferior frontal gyrus | –32 | 20 | –20 | 4.62 | 268 |
| L ventral striatum | –8 | 10 | –2 | 5.61 | 237 |
| L cerebellum | –20 | –50 | –20 | 4.16 | 88 |
| R thalamus | 8 | –16 | 4 | 4.90 | 55 |
| Probability | |||||
| L medial frontal pole/pregenual cingulate | –8 | 50 | 10 | 5.50 | 869 |
| L cerebellum | –28 | –74 | –30 | 5.15 | 461 |
| R posterior cingulate cortex | 2 | –44 | 34 | 5.90 | 354 |
| L inferior parietal lobule | –44 | –62 | 48 | 5.33 | 182 |
| L inferior temporal gyrus | –64 | –16 | –18 | 4.33 | 87 |
| L thalamus | –14 | –26 | 0 | 4.33 | 60 |
| L insula | –34 | –8 | 10 | 4.23 | 55 |
| Time | |||||
| L medial frontal pole/pregenual cingulate | –8 | 56 | 0 | 4.72 | 286 |
| R precuneus | 2 | –80 | 48 | 4.52 | 146 |
| L superior frontal gyrus | –22 | 34 | 52 | 4.60 | 119 |
| R inferior parietal lobule | 52 | –58 | 52 | 4.46 | 54 |
Note. RAS are coordinates for peak voxels within a cluster in MNI space. R, right; A, anterior; S, superior. N =75.
Fig. 4.
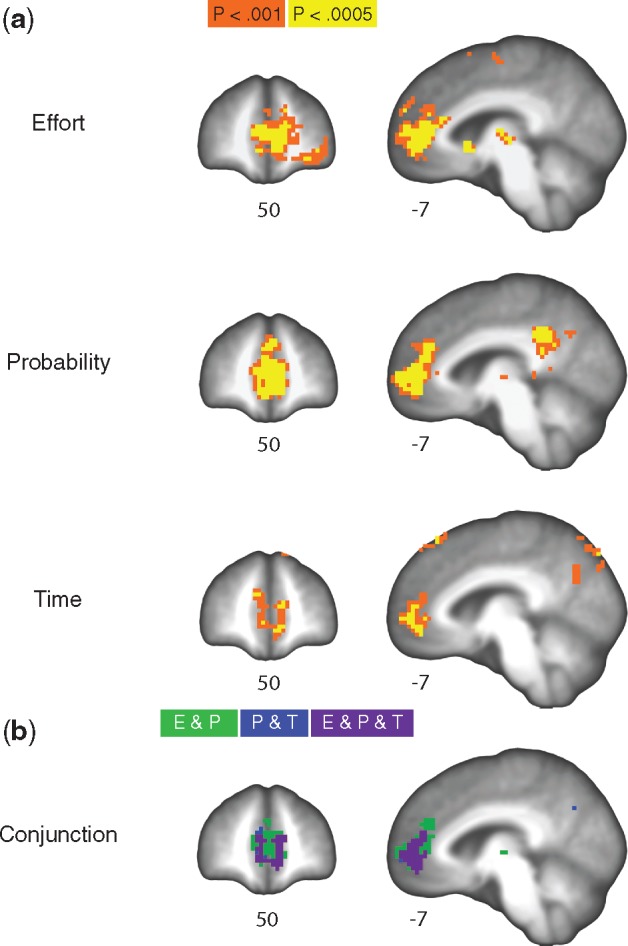
Neural representation of subjective value. (a) Subjective value representation during effort, probability, and time tasks. Unthresholded statistical maps available at: https://neurovault.org/collections/3184/. (b) Conjunction of subjective value representation during effort and probability tasks (green), probability and time tasks (blue), and effort, probability, and time tasks (purple). The conjunction of subjective value representation during effort and time tasks is obscured by the other conjunctions in the figure. Results are displayed overlaid onto the mean structural scan of all participants in the sample and plotted at an uncorrected threshold of P < 0.001 (corrected to P <0.05).
We used a conjunction analysis to identify areas of overlapping SVcho representations across the three tasks (Table 4), controlling for age. As predicted, a bilateral region of the medial frontal cortex, centered on the pregenual cingulate, was significantly correlated with SVcho for all three tasks (Figure 4, bottom panel, purple). We also separately examined pairwise conjunctions across each pair of tasks. These analyses revealed a large bilateral region of overlap for the conjunction of SVcho for effort and probability (Figure 3, bottom panel, green and purple). They also revealed a smaller region of overlap within the medial prefrontal cortex for the conjunction of probability and time (Figure 4, bottom panel, blue and purple) and a nearly identical region for the conjunction of effort and time (not shown in Figure 4).
Table 4.
Conjunctions of neural representation of the subjective value of the chosen option
| Region | R | A | S | # Voxels |
|---|---|---|---|---|
| Effort & Probability & Time | ||||
| L pregenual cingulate/medial fontal pole | –7 | 52 | 1 | 158 |
| Effort & Probability | ||||
| L pregenual cingulate/medial frontal gyrus | –2 | 48 | 9 | 424 |
| L thalamus | –12 | –21 | 5 | 11 |
| L superior frontal gyrus | –24 | 56 | 8 | 9 |
| L claustrum | –34 | –13 | –2 | 5 |
| Effort & Time | ||||
| L pregenual cingulate | –3 | 48 | 4 | 172 |
| R anterior cingulate | 8 | 38 | 14 | 7 |
| Probability & Time | ||||
| L medial frontal gyrus/pregenual cingulate | –2 | 48 | 5 | 221 |
| R anterior cingulate | 11 | 38 | 14 | 2 |
| L precuneus | –6 | –71 | 42 | 2 |
Note. RAS are coordinates for center of mass of each cluster in MNI space. R, right; A, anterior; S, superior. N = 75.
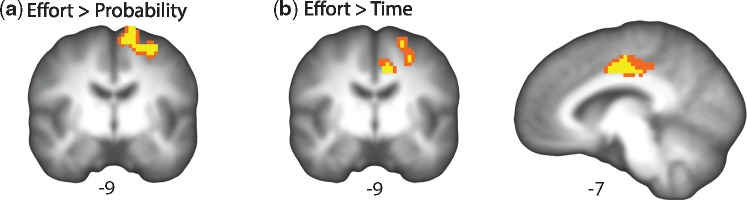
Finally, we used paired t-tests to examine significant differences in SVcho representations between each pair of tasks (Table 5), controlling for age. As displayed in Figure 5a, there was a left lateralized cortical region extending from the precentral gyrus to the supplementary motor area where the SVcho representation was significantly greater for the effort compared to the probability task. There was also a bilateral region of the middle cingulate gyrus, where the SVcho representation was significantly greater for effort compared to time (Figure 5b). There were no significant differences in SVcho for probability and time.
Table 5.
Dissociations in neural representation of the subjective value of the chosen option
| Region | R | A | S | Z | # Voxels |
|---|---|---|---|---|---|
| Effort > Probability | |||||
| L precentral gyrus | –32 | –4 | 54 | 4.72 | 225 |
| Effort > Time | |||||
| R supplemental motor area | 4 | –20 | 48 | 4.74 | 153 |
| R cerebellum/fusiform gyrus | 20 | –98 | –19 | –4.98 | 69 |
| L precentral gyrus | –28 | –8 | 52 | 4.21 | 59 |
Note. RAS are coordinates for peak voxels within a cluster in MNI space. R, right; A, anterior; S, superior. N = 75.
Fig. 5.
Dissociations in neural representation of subjective value. (a) Region where the subjective value representation is significantly greater during the effort task than during the probability task. (b) Regions where the subjective value representation is significantly greater during the effort task than during the time task. Results are displayed overlaid onto the mean structural scan of all participants in the sample and plotted at an uncorrected threshold of P < 0.001 (corrected to P < 0.05). Unthresholded statistical maps available at: https://neurovault.org/collections/3184.
We examined linear effects of age on SV representations in whole-brain analyses for each task. During the effort task, there were significant age effects in the representation of SVcho in the right middle temporal gyrus and the left superior temporal gyrus as well as the right lingual and calcarine gyrus (Supplementary Table S1 and Figure S3), with less SV representation in these regions with age. There were no significant age differences in the neural representation of SV during the probability and time tasks.
Discussion
Here, we examined preferences for effort, probability, and time in monetary decisions across adulthood. With one unreliable exception, we found that preferences for lower physical effort, higher probability, or shorter time delays were uncorrelated. Despite this behavioral dissociation, we found overlapping SV-related activity in the medial prefrontal cortex across all three tasks. In other words, even if subjects have different discount rates across tasks, once these differences are accounted for in calculating SV, SV scales with medial frontal activity regardless of the objectively-stated value. These results suggest that while the tolerance of these decision features is behaviorally dissociable, the decision feature-discounted value signals share a common neural representation.
Both qualitative (Kable & Glimcher, 2009; Peters & Büchel, 2010) and quantitative (Acikalin et al., 2017; Bartra et al., 2013; Clithero & Rangel, 2014; Levy & Glimcher, 2012) reviews have highlighted the role of the medial prefrontal cortex in subjective valuation. Our results are generally consistent with meta-analyses of SV signals along the frontal midline (Acikalin et al., 2017; Bartra et al., 2013; Clithero & Rangel, 2014). Although researchers have assumed SV is localized in more ventromedial portions of PFC, meta-analyses consistently show that value signals are represented in a broad portion of the medial prefrontal cortex, including the pregenual cingulate gyrus and medial portions of superior frontal gyrus. The regions reported here, particularly for effort and probability, are located within the superior extent of the clusters identified in these meta-analyses.
Many studies have reported SV signals in both the MPFC and the nucleus accumbens/ventral striatum (Acikalin et al., 2017; Bartra et al., 2013; Clithero & Rangel, 2014; Kable & Glimcher, 2010; Levy & Glimcher, 2012; Peters & Büchel, 2010). However, one of these meta-analyses, which (similar to this study) examined parametric modulation of activity with SV, showed that the signal is stronger in the medial prefrontal cortex (MPFC) than the ventral striatum (Clithero & Rangel, 2014). Somewhat consistent with these findings, we found a strong representation in the MPFC, but did not find reliable representation of SV in the ventral striatum. One possible explanation for the lack of striatal activation across the sample is a subthreshold effect of age on activity in this region. However, preservation of function in the striatum with age has been well documented by our lab (Samanez-Larkin et al., 2007; Samanez-Larkin & Knutson, 2015) and others (Spaniol, Bowen, Wegier, & Grady, 2015), and we did not observe a significant effect of age on SV representation in the striatum in any of the three tasks. Thus, it is possible that the lack of a ventral striatal effect across the sample is due to the broad age range, but future studies are needed to fully examine this explanation.
Although differences in SV across tasks were minimal in our study, prior studies have identified differences in SV representation across tasks (Burke et al., 2013; Massar et al., 2015; Peters & Büchel, 2009). Given that the existing literature examining neural representation of SV across decision features is very small (three studies), has small sample sizes (N = 18–23) and uses very different experimental tasks, these inconsistent effects are not very surprising. More research using larger sample sizes may be needed to provide enough power to detect potential differences and reduce false-positive rates (Poldrack et al., 2017). It is also possible that more consistent differences in SV representations across tasks could be identified with multivariate analysis methods (Kahnt, in press).
In the present study, we only found differences in SV representation comparing the effort task to the other two tasks. One potential explanation for this difference is that the effort task was the only task where participants actually experienced the consequences of their decision; on half of the trials, participants actually completed a round of button pressing before progressing on to the next trial. Thus, this difference in activity could be due to subjects preparing to make a motor response and not due to differences in subjective valuation. This interpretation is supported by the fact that the regions differentially activated by the effort task were regions associated with motor planning and preparation. Also, as in the previous behavioral study of effort discounting with hypothetical rewards (Seaman et al., 2016), there is a strong floor effect in the behavioral data and this restricted range may have reduced the reliability of our discounting parameter estimates. Future studies should explore other physical effort-based discounting tasks (Kurniawan et al., 2011; Kurniawan et al., 2010), cognitive effort-based discounting (Schmidt, Lebreton, Cléry-Melin, Daunizeau, & Pessiglione, 2012; Westbrook et al., 2013), and different computational models of behavior (Klein-Flügge, Kennerley, Saraiva, Penny, & Bestmann, 2015), to see whether these regions are truly specific to SV representations involving effort.
Contrary to our predictions, we found no evidence for adult age differences in behavior, suggesting that decision preferences may be relatively stable across adulthood. Further, the absence of age differences in neural representations of SV suggests that neural mechanisms supporting SV are preserved with age. This may be viewed as somewhat surprising, given prior work suggesting integration deficits during decision-making in older age (Samanez-Larkin & Knutson, 2015). However, these integration deficits were hypothesized to be most pronounced during more cognitively demanding tasks (Mata et al., 2011), and the simple choice tasks used here required relatively minimal integration of information over time. Our results are also contrary to some prior studies, which have reported age differences in discounting behavior (Eppinger et al., 2012), although as noted above this literature is mixed (Read & Read, 2004; Samanez-Larkin et al., 2011). Studies that use smaller samples and extreme-group designs (Eppinger et al., 2012; Green et al., 1994) have been more likely to identify age differences. Studies with larger samples, comparable to the one here or larger, have not consistently identified adult age differences in decision preferences, especially in the time domain (Löckenhoff et al., 2016, 2017). However, it is important to acknowledge that our older subjects may be healthier than average subjects who complete behavioral studies. Due to the extensive screening for PET imaging that was also part of this protocol, the older adults in our sample may not be representative of the aging population as a whole. To the extent that we can generalize to the larger population, the findings suggest that discounted value signals are represented similarly from young adulthood through middle age into older age. Finally, the null effect of age could be due to the reward used in this study (money), which may be less motivating for older adults (Seaman et al., 2016). Our prior study suggested that older adults may be more motivated than younger adults to obtain health and social rewards. One challenge for futures studies will be to design ways to examine discounting for social and health rewards with incentive–compatible tasks.
Prior studies of SV representation have focused almost exclusively on young adults (Acikalin et al., 2017; Bartra et al., 2013; Clithero & Rangel, 2014; Kable & Glimcher, 2007), which limits the generalizability of those results across adulthood. By using an adult life span sample, we show that there is consistency in the representation of SV across adulthood in the medial prefrontal cortex. Together, the results suggest that value signals are intact and decision preferences may be stable well into older age, but much variance in brain signals and behavior between subjects remains unexplained by age. Given this variance, individual differences within age-groups may facilitate the identification of individuals who are at risk of making poor decisions in everyday life (Halfmann, Hedgcock, & Denburg, 2013; Halfmann et al., 2016).
Supplementary Material
Acknowledgements
This research was supported by National Institute on Aging Pathway to Independence Award R00-AG042596, National Institute on Aging grant R01-AG044838, and National Institute on Aging Training Grant T32-AG000029. Some of the results reported in this manuscript were presented in posters at the annual meetings of the Society for Neuroeconomics (2014, 2015, and 2016) and the Cognitive Neuroscience Society (2017).
References
- Acikalin M.Y., Gorgolewski K.J., Poldrack R.A. (2017). A coordinate-based meta-analysis of overlaps in regional specialization and functional connectivity across subjective value and default mode networks. Frontiers in Neuroscience, 11, 1–11. doi: 10.3389/fnins.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage, 76, 412–27. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett I.J., Madden D.J., Vaidya C.J., Howard D.V., Howard J.H. (2010). Age‐related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Human Brain Mapping, 31(3), 378–90. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.B., Ridderinkhof K.R. (2009). Aging and the neuroeconomics of decision making: a review. Cognitive, Affective, & Behavioral Neuroscience, 9(4), 365–79. doi: 10.3758/cabn.9.4.365. [DOI] [PubMed] [Google Scholar]
- Burke C.J., Brünger C., Kahnt T., Park S.Q., Tobler P.N. (2013). Neural integration of risk and effort costs by the frontal pole: only upon request. Journal of Neuroscience, 33(4), 1706–13. doi: 10.1523/JNEUROSCI.3662-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero J.A., Rangel A. (2014). Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience, 9(9), 1289–302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan J.D., Hinkeldey N.S. (1987). Relationships between parts A and B of the Trail Making Test. Journal of Clinical Psychology, 43(4), 402–9. doi: 10.1002/1097-4679(198707)43: 4<402:: aid-jclp2270430411>3.0.co; 2-e. [DOI] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Reynolds R.C., Taylor P.A. (2016). AFNI and clustering: false positive rates redux. BioRxiv, 065862. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCiccio T.J., Efron B., Hall P., et al. (1996). Bootstrap confidence intervals. Statistical Science, 11(3), 189–212. doi: 10.1214/ss/1032280214. [Google Scholar]
- Eppinger B., Nystrom L.E., Cohen J.D. (2012). Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS One, 7(5), e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estle S.J., Green L., Myerson J., Holt D.D. (2006). Differential effects of amount on temporal and probability discounting of gains and losses. Memory & Cognition, 34(4), 914–28. doi: 10.3758/bf03193437. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Grydeland H., et al. (2014). Accelerating cortical thinning: unique to dementia or universal in aging? Cerebral Cortex, 24(4), 919–34. doi: 10.1093/cercor/bhs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L., Fry A.F., Myerson J. (1994). Discounting of delayed rewards: a life-span comparison. Psychological Science, 5(1), 33–6. doi: 10.1111/j.1467-9280.1994.tb00610.x. [Google Scholar]
- Green L., Myerson J. (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin, 130(5), 769. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann K., Hedgcock W., Denburg N.L. (2013). Age-related differences in discounting future gains and losses. Journal of Neuroscience, Psychology, and Economics, 6(1), 42–54. doi: 10.1037/npe0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann K., Hedgcock W., Kable J., Denburg N.L. (2016). Individual differences in the neural signature of subjective value among older adults. Social Cognitive and Affective Neuroscience, 11(7), 1111–20. doi: 10.1093/scan/nsv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt D.D., Green L., Myerson J. (2003). Is discounting impulsive?: evidence from temporal and probability discounting in gambling and non-gambling college students. Behavioural Processes, 64(3), 355–67. doi: 10.1016/S0376-6357(03)00141-4. [DOI] [PubMed] [Google Scholar]
- Hsu M., Lin H.-T., McNamara P.E. (2008). Neuroeconomics of decision-making in the aging brain: the example of long-term care. Advances in Health Economics and Health Services Research, 20, 203–25. [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience, 10(12), 1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2009). The neurobiology of decision: consensus and controversy. Neuron, 63(6), 733–45. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2010). An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. Journal of Neurophysiology, 103(5), 2513–31. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T. (in press). A decade of decoding reward-related fMRI signals and where we go from here. Neuroimage, doi: 10.1016/j.neuroimage.2017.03.067. [DOI] [PubMed] [Google Scholar]
- Klein-Flügge M.C., Kennerley S.W., Saraiva A.C., Penny W.D., Bestmann S. (2015). Behavioral modeling of human choices reveals dissociable effects of physical effort and temporal delay on reward devaluation. PLoS Computational Biology, 11(3), e1004116. doi: 10.1371/journal.pcbi.1004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan I.T., Guitart-Masip M., Dolan R.J. (2011). Dopamine and effort-based decision making. Frontiers in Neuroscience, 5, 1–10. doi: 10.3389/fnins.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan I.T., Seymour B., Talmi D., Yoshida W., Chater N., Dolan R.J. (2010). Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. Journal of Neurophysiology, 104(1), 313–21. doi: 10.1152/jn.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.J., Glimcher P.W. (2011). Comparing apples and oranges: using reward-specific and reward-general subjective value representation in the brain. Journal of Neuroscience, 31(41), 14693–707. doi: 10.1523/JNEUROSCI.2218-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.J., Glimcher P.W. (2012). The root of all value: a neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–38. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff C.E., Rutt J.L., Samanez-Larkin G.R., O’Donoghue T., Reyna V.F. (2017). Preferences for temporal sequences of real outcomes differ across domains but do not vary by age. The Journals of Gerontology. Series B, Psycological Sciences and Social Sciences. doi: 10.1093/geronb/gbx094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff C.E., Rutt J.L., Samanez-Larkin G.R., O'Donoghue T., Reyna V.F., Ganzel B. (2016). Dread sensitivity in decisions about real and imagined electrical shocks does not vary by age. Psychology and Aging, 31(8), 890–901. doi: 10.1037/pag0000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massar S.A., Libedinsky C., Weiyan C., Huettel S.A., Chee M.W. (2015). Separate and overlapping brain areas encode subjective value during delay and effort discounting. Neuroimage, 120, 104–13. doi: 10.1016/j.neuroimage.2015.06.080. [DOI] [PubMed] [Google Scholar]
- Mata R., Josef A.K., Samanez‐Larkin G.R., Hertwig R. (2011). Age differences in risky choice: a meta‐analysis. Annals of the New York Academy of Sciences, 1235, 18–29. (doi: 10.1111/j.1749-6632.2011.06200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. (2006). A review of decision-making processes: weighing the risks and benefits of aging In Carstensen L.L., Hartel C.R., editors. When I’m 64 (pp. 145–173). Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 306(5695), 503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mitchell S.H. (1999). Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology, 146(4), 455–64. doi: 10.1007/PL00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell S.H. (2004). Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine & Tobacco Research, 6(5), 819–28. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Myerson J., Green L., Hanson J.S., Holt D.D., Estle S.J. (2003). Discounting delayed and probabilistic rewards: processes and traits. Journal of Economic Psychology, 24(5), 619–35. doi: 10.1016/S0167-4870(03)00005-9. [Google Scholar]
- Nichols T., Brett M., Andersson J., Wager T., Poline J.-B. (2005). Valid conjunction inference with the minimum statistic. Neuroimage, 25(3), 653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ohmura Y., Takahashi T., Kitamura N. (2005). Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology, 182(4), 508–15. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Olson E.A., Hooper C.J., Collins P., Luciana M. (2007). Adolescents’ performance on delay and probability discounting tasks: contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Personality and Individual Differences, 43(7), 1886–97. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E., Dieckmann N., Dixon A., Hibbard J.H., Mertz C. (2007). Less is more in presenting quality information to consumers. Medical Care Research and Review, 64(2), 169–90. doi: 10.1177/10775587070640020301. [DOI] [PubMed] [Google Scholar]
- Peters J., Büchel C. (2009). Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. Journal of Neuroscience, 29(50), 15727–734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Büchel C. (2010). Neural representations of subjective reward value. Behavioural Brain Research, 213(2), 135–41. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Baker C.I., Durnez J., et al. (2017). Scanning the horizon: towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience, 18(2), 115–26. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost C., Pessiglione M., Météreau E., Cléry-Melin M.-L., Dreher J.-C. (2010). Separate valuation subsystems for delay and effort decision costs. Journal of Neuroscience, 30(42), 14080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Lindenberger U., Rodrigue K.M., et al. (2005). Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex, 15(11), 1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Read D., Read N.L. (2004). Time discounting over the lifespan. Organizational Behavior and Human Decision Processes, 94(1), 22–32. doi: 10.1016/j.obhdp.2004.01.002. [Google Scholar]
- Reynolds B., Richards J.B., Horn K., Karraker K. (2004). Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes, 65(1), 35–42. doi: 10.1016/S0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Richards J.B., Zhang L., Mitchell S.H., Wit H. (1999). Delay or probability discounting in a model of impulsive behavior: effect of alcohol. Journal of the Experimental Analysis of Behavior, 71(2), 121–43. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf D.R., Mitchell S.H., Harbaugh W.T., Janowsky J.S. (2012). Risk, reward, and economic decision making in aging. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 67B(3), 289–98. doi: 10.1093/geronb/gbr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Gibbs S.E., Khanna K., Nielsen L., Carstensen L.L., Knutson B. (2007). Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience, 10(6), 787–91. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Knutson B. (2015). Decision making in the ageing brain: changes in affective and motivational circuits. Nature Reviews Neuroscience, 16(5), 278–89. doi: 10.1038/nrn3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Levens S.M., Perry L.M., Dougherty R.F., Knutson B. (2012). Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. Journal of Neuroscience, 32(15), 5333–7. doi: 10.1523/JNEUROSCI.5756-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Mata R., Radu P.T., Ballard I.C., Carstensen L.L., McClure S.M. (2011). Age differences in striatal delay sensitivity during intertemporal choice in healthy adults. Frontiers in Neuroscience, 5, 1–12. doi: 10.3389/fnins.2011.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A., Dijkstra M., Ainslie E., et al. (2006). Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia, 44(11), 2092–103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Schmidt L., Lebreton M., Cléry-Melin M.-L., Daunizeau J., Pessiglione M. (2012). Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biology, 10(2), e1001266. doi: 10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman K.L., Gorlick M.A., Vekaria K.M., Hsu M., Zald D.H., Samanez-Larkin G.R. (2016). Adult age differences in decision making across domains: increased discounting of social and health-related rewards. Psychology and Aging, 31(7), 737–46. doi: 10.1037/pag0000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley W.C. (1940). A self-administering scale for measuring intellectual impairment and deterioration. The Journal of Psychology, 9(2), 371–77. doi: 10.1080/00223980.1940.9917704. [Google Scholar]
- Spaniol J., Bowen H.J., Wegier P., Grady C. (2015). Neural responses to monetary incentives in younger and older adults. Brain Research, 1612, 70–82. doi: 10.1016/j.brainres.2014.09.063. [DOI] [PubMed] [Google Scholar]
- Treadway M.T., Buckholtz J.W., Schwartzman A.N., Lambert W.E., Zald D.H. (2009). Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One, 4(8), e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymula A., Belmaker L.A.R., Ruderman L., Glimcher P.W., Levy I. (2013). Like cognitive function, decision making across the life span shows profound age-related changes. Proceedings of the National Academy of Sciences, 110(42), 17143–8. doi: 10.1073/pnas.1309909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B.J., Huettel S.A. (2008). The neural substrates of probabilistic and intertemporal decision making. Brain Research, 1234, 104–15. doi: 10.1016/j.brainres.2008.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1997a). WAIS-III: Wechsler Adult Intelligence Scale. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D. (1997b). Wechsler Memory Scale (WMS-III). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Westbrook A., Kester D., Braver T.S. (2013). What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PLoS One, 8(7), e68210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.