Abstract
Background
The observed link between positive psychotic experiences (PE) and psychosis spectrum disorder (PSD) may be stronger depending on concomitant presence of PE with other dimensions of psychopathology. We examined whether the effect of common risk factors for PSD on PE is additive and whether the impact of risk factors on the occurrence of PE depends on the co-occurrence of other symptom dimensions (affective dysregulation, negative symptoms, and cognitive alteration).
Method
Data from the Netherlands Mental Health Survey and Incidence Study 2 were used. Risk factors included childhood adversity, cannabis use, urbanicity, foreign born, hearing impairment, and family history of affective disorders. Logistic regression models were applied to test (1) the additive effect of risk factors (4 levels) on PE and (2) the moderating effects of symptom dimensions on the association between risk factors (present/absent) and PE, using additive interaction, expressed as the interaction contrast ratio.
Results
Risk factors were additive: the greater the number of risk factors, the greater the odds of PE. Furthermore, concomitant presence of the other symptom dimensions all increased the impact of risk factors on PE. After controlling for age, sex, and education, only affective dysregulation and negative symptoms remained significant moderators; only affective dysregulation remained a significant moderator if all dimensions were adjusted for each other.
Conclusions
Risk factors may not be directly associated with PE but additively give rise to a multidimensional subthreshold state anticipating the multidimensional clinical syndrome. Early motivational and cognitive impairments in the context of PE may be reducible to affective dysregulation.
Keywords: risk factors, symptom dimensions, psychotic experiences
Introduction
The attenuated form of positive psychosis expression, commonly referred to as psychotic experiences (PE), is prevalent in the general population, with rates between 5% and 8%.1,2 It frequently co-occurs with affective dysregulation,3,4 and predicts both PSD5–7 and mental disorders at large, including mood and anxiety disorders.4,8 The WHO World Mental Health Surveys demonstrated a bidirectional temporal relation between PE and most mental disorders—the preceding condition increasing the risk of the other over time.9 Similarly, increased levels of psychosis admixture in nonpsychotic disorders have been observed to impact illness severity,10 comorbidity,3 poor outcome,11 functional impairment,12,13 and suicidality.14,15 Furthermore, in a recent study on the distribution of ultra-high risk criteria in the general population, however without applying the help-seeking criterion, the presence of nonpsychotic mental disorders and functional deficits was more likely when attenuated psychotic symptoms co-occurred with cognitive deficits (ie, cognitive–perceptive basic symptoms and cognitive disturbances).16
The initial manifestation of a mental condition typically represents a mixture of signs and symptoms accompanied by impairment in various emotional and neurocognitive processes that may include aberrant salience, motivational alterations, affective dysregulation, and anxiety states.17 The degree of impairment in these dynamically interacting processes, mediated by underlying biological vulnerabilities, predict the degree of progression from a subtle mental state (subthreshold PE) toward clinical disorder (schizophrenia).17 Taken together, these findings suggest that various symptom dimensions and underlying neurocognitive processes co-occur in both clinical and general populations, and interact with each other between traditional diagnostic categories (eg, depressed mood interact with delusions of reference)11,18 and within traditional diagnostic categories (eg, auditory hallucinations interact with paranoid ideation).19,20
There is evidence that affective dysregulation and psychosis expression co-occur and share a considerable amount of pathoetiological background.21 The Bipolar-Schizophrenia Network on Intermediate Phenotypes study yielded neurobiological commonalities cutting across the classical Kraepelinian dichotomy,22,23 while genome-wide association studies consistently showed significant overlap between affective disorders and PSD.24 In agreement with molecular genetic data, we demonstrated that polygenic risk score (PRS) for schizophrenia in healthy participants and nonill relatives of patients with PSD was expressed not only as positive schizotypy but also in the domains of affective regulation, neurocognition, and attribution of salience.25 Furthermore, PRS for schizophrenia was associated with lifetime mood episodes (both depressive and manic) in relatives and healthy controls.25 Confirming the shared vulnerability theory, environmental exposure (like cannabis use, childhood adversity, urbanicity, and hearing impairment) likewise is associated with psychosis and with affective and stress-related phenotypes.26–29
It is therefore reasonable to hypothesize that environmental exposure, along with genetic vulnerability, may synergistically alter the degree of blending of affective dysregulation and psychosis expression, thus triggering progression toward a more serious clinical condition. Converging evidence indicates that the degree of psychotic admixture in affective disorders is contingent on the level of environmental exposure that is linked to PSD, such as cannabis use30 and childhood trauma.31,32 Previously, our group demonstrated that both childhood adversity and cannabis exposure additively increased the likelihood of admixture of psychosis expression in affective disorders in a dose–response fashion,33 and that the level of connectivity between different psychopathological dimensions increased as a function of the environmental risk load.34
In line with our multidimensional approach to explore the impact of various risk factors on psychopathology in the general population, this study aimed to investigate to what degree the association between common risk factors for PSD (childhood adversity, cannabis use, urbanicity, foreign born, hearing impairment, and family history of affective disorders) and PE is contingent on other components of the multidimensional psychosis spectrum (affective dysregulation, negative symptoms, and cognitive alteration) in the general population. The study also aimed to clarify to what degree risk factors are additive (linearly increasing) or redundant (not adding to each other).
Method
Study Population
Data were obtained from the Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2) designed to investigate the prevalence, incidence, course, and consequences of mental disorders in the Dutch general population. The baseline data of NEMESIS-2 were collected from 2007 to 2009. The study was approved by the Medical Ethics Review Committee for Institutions on Mental Health Care and written informed consent was collected from participants. To ensure representativeness of the sample in terms of age (between the ages of 18 and 65 at baseline), region, and population density, a multistage random sampling procedure was applied.35 Dutch literacy was an inclusion criterion. Nonclinician, trained interviewers applied the Composite International Diagnostic Interview (CIDI) version 3.0 and additional questionnaires during home visits. Details of NEMESIS-2 were provided elsewhere.35,36 The first wave (T0) enrolled 6646 participants (response rate 65.1%; average duration: 95 minutes), who were followed up in 2 visits within 6 years: successive response rates at year 3 (T1) and year 6 (T2) were 80.4% (n = 5303; excluding those who deceased; duration: 84 minutes) and 87.8% (n = 4618; duration: 83 minutes), respectively. Data from all waves were utilized. Rates at baseline reflect lifetime occurrence; rates at T1 and T2 reflect interval (baseline T1 and T1–T2) occurrence. Attrition between T0 and T137 and between T1 and T238 was not significantly associated with any of the 12-month mental disorders at T0 controlled for sociodemographics, except for alcohol and drug dependence at T1 which were significantly related with attrition at T2.38
Dimensions of Psychopathology
Psychotic Experiences.
To assess PE, a questionnaire constructed based on CIDI 1.1 was used. Participants were asked at baseline (T0; life time symptoms) and at follow-ups (T1 and T2; 3-year symptoms) whether they had experienced any of a list of 20 positive psychotic symptoms (with a binary response: 0 = “no” and 1 = “yes”; items are listed in supplementary table S1).32,39 For the purpose of this analysis, a dichotomous PE variable was defined as positive if any of the positive symptom items were rated positively.
Affective Dysregulation.
The CIDI 3.0 was used to assess depressive, manic, and anxiety symptoms. Affective dysregulation was considered present when participants endorsed at least one of the CIDI 3.0 core symptoms of Depressive Episode, Panic Disorder, Social Phobia, Generalized Anxiety Disorder, and Manic Episode. Affective dysregulation was assessed at each time-point (T0, T1, and T2).
Negative Symptoms.
The negative symptom dimension was constructed using 4 items based on interviewer observation: poor personal hygiene and inadequate independent living skills (ie, neatness and cleanness of the participant’s residence; were assessed at T1 and T2); lack of emotional expression and poverty of speech (were assessed at T2). Presence of negative symptoms was defined as a rating of “present” on any of these items, which were dichotomized as present = “0” and absent = “1.” Presence of negative symptoms at T1 and/or T2 was used as a person-level indicator of “trait” negative symptom at T0.
Cognitive Alteration.
The forward and backward digit span tasks from the Wechsler Adult Intelligence Scale—III40 was used to assess short-term attention and working memory performance, respectively, at T1. A binary cognitive alteration variable was constructed using a cut-off point to define the group of participants with the lowest 20% raw scores of combined forward and backward tasks of digit span. Presence of cognitive alteration at T1 was used as a person-level trait indicator of cognitive alteration at T0 and T2.
Risk Factors
Family History of Affective Disorders.
Family history includes depressive, manic, and anxiety items (panic disorder, specific phobia, social phobia, agoraphobia, generalized anxiety disorder) as well as items on drugs and alcohol use. Assessment of family history was limited to the participants who screened positive for affective disorders: depression, mania, and anxiety disorders (panic disorder, specific phobia, and generalized anxiety disorder).
Childhood Adversity.
Childhood adversity (CA) was assessed at baseline (T0) across 5 domains (emotional neglect, peer victimization, physical abuse, psychological abuse, and sexual abuse; before age 16) using a questionnaire based on the NEMESIS-1 trauma questionnaire.35 Subjects were asked whether they experienced emotional neglect, psychological abuse, peer victimization or physical abuse on ≥2 occasions, or sexual abuse on ≥1 occasion. A person-level continuous CA variable was constructed using the sum score of the 5 domains. In accordance with previous research,41 CA was dichotomized at the 80th percentile.
Cannabis Exposure.
Cannabis use was assessed with the section Illegal Substance Use of the CIDI 3.0 at baseline (T0; lifetime) and at follow-ups (T1 and T2; 3-year). If subjects reported cannabis use, they were rated on frequency of use in the period of most frequent use on a scale of 1 (never) to 7 (every day). Consistent with previous work,42 a binary variable was constructed by using the cut-off value of once per week or more in the period most frequent use.
Urban Environment.
Exposure to an urban environment until age 16 years was assessed at baseline (T0) and analyzed as a person-level variable across the 3 waves at 5 levels: (1) countryside (distances to amenities is bigger), (2) village (<25000 inhabitants), (3) small city (25000–50000 inhabitants), (4) medium city (50000–100000 inhabitants), 5) large city (>100000 inhabitants). Conforming to previous work using the NEMESIS-I dataset,34 the cut-off of more than 50000 inhabitants was used to define the binary variable of urban area.
Foreign Born.
Country of birth was assessed at baseline (T0) and analyzed as a person-level variable across the 3 waves. It was used as a proxy for minority status (born in the Netherlands = “0” and born in other countries = “1”).
Hearing Impairment.
Hearing impairment was assessed at each time-point (T0, T1, and T3), based on self-reported hearing impairment in the last 12 months (absent = “0” and present = “1”).
Statistical Analysis
Analyses were performed using Stata 14.2.43 Consistent with previous work,33 data from all waves were analyzed cross-sectionally in the “long format” (each participant contributing 3 observations: T0, T1, and T2). This analytical strategy serves the purposes of increasing overall reliability and achieving consistency across a limited number of variables of interest that in some cases were assessed differently at different time points. Using the CLUSTER option, all analyses were corrected for clustering of multiple observations within subjects, and cluster-robust standard errors were computed. To evaluate the effect of the risk-loading on PE, logistic regression analysis, using the LOGISTIC command, was modeled with PE as dependent variable and the 4-level risk score (no = 0, low = 1, medium = 2, and high > 2 risk factors) as independent variable. The model was adjusted for sex, age, and education (1 = primary school, 2 = lower secondary education, 3 = higher secondary education, 4 = higher professional education). The LINCOM command was applied to test OR differences between groups with low and medium, medium and high, and low and high risk.
Logistic regression models were used to analyze whether the association between the presence of any risk factors (absent = 0; presence of one or more risk factors = 1) and PE was dependent on symptom dimensions (affective dysregulation, negative symptoms, and cognitive alteration). The interaction contrast ratio (ICR) method was applied to explore the interaction between risk factors and symptom dimensions in the model of PE. To test additive interaction, 4 exposure states were produced by the combination of each dimension and risk. In logistic models, the combinations served as the independent variables (3 dummy variables with nonexposed state as the reference category) and PE served as dependent variable. Using the ORs from these models, ICRs for each model were calculated using the NLCOM command in Stata: eg, ICR = OR (risk) + OR (affective dysregulation) − OR (risk) − OR (affective dysregulation) + 1. These models were further adjusted for age (continuous), sex, and education level (4 levels). In the final adjusted models, psychopathology dimensions were additionally controlled for each other.
Results
The total sample for the analyses included 16567 observations from subjects who participated at the 3 time-points (T0, n = 6646; T1, n = 5303; T2, n = 4618). The baseline demographics of the NEMESIS-2 sample have been described previously32 and an overview of the 3 time-points is presented in table 1. Supplementary table S1 reports the frequencies of individual PE items at each time-point.
Table 1.
Summary of Descriptive Data
| Baseline, n = 6646 | 3-Year Follow-Up, n = 5303 | 6-Year Follow-Up, n = 4618 | |
|---|---|---|---|
| Sex, female | 3672 (55.3) | 2922 (55.1) | 2558 (55.4) |
| Mean age, years (SD) | 44.3 (12.5) | 47.6 (12.4) | 50.9 (12.3) |
| Education | |||
| Primary education | 332 (5) | 226 (4.3) | 186 (4) |
| Lower secondary education | 1826 (27.5) | 1388 (26.2) | 1193 (25.8) |
| Higher secondary education | 2145 (32.3) | 1728 (32.6) | 1479 (32) |
| Higher professional education, university degree | 2343 (35.3) | 1961 (37) | 1760 (38.1) |
| Foreign born | 920 (13.8) | 650 (12.3) | 529 (11.5) |
Note: Data are given as number (percentage) unless otherwise indicated.
Dose–Response Relationship Between the Risk-Loading and PE
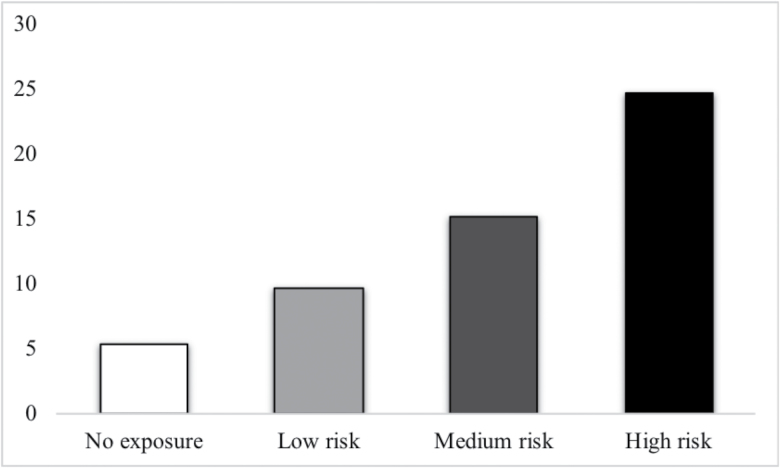
Analyzing environmental and familial risk load at 4 levels (no = 0, low = 1, medium = 2, and high > 2 risk factors), irrespective of PSD dimensions, revealed a dose–response relationship between risk and PE: With “no exposure” as the reference group, risk categories displayed progressively higher odds ratios: OR = 1.84, 95% CI = 1.58–2.13, P < .001 for the low risk group; OR = 3.11, 95% CI = 2.64–3.66, P < .001 for the medium risk group; OR = 5.78, 95% CI = 4.77–7, P < .001 for the high risk group. Furthermore, comparison with the LINCOM command indicated significant differences between the groups with low and medium risk (OR = 1.7, 95% CI = 1.47–1.94, P < .001), medium and high risk (OR = 1.86, 95% CI = 1.56–2.22, P < .001), and low and high risk (OR = 3.15 95% CI = 2.64–3.74, P < .001). Figure 1 shows the frequencies of individual risk factors within risk strata (no = 0, low = 1, medium = 2, and high > 2 risk factors) at baseline level. Figure 2 shows the prevalence of PE across the risk strata, including data from participants with information from all time-points.
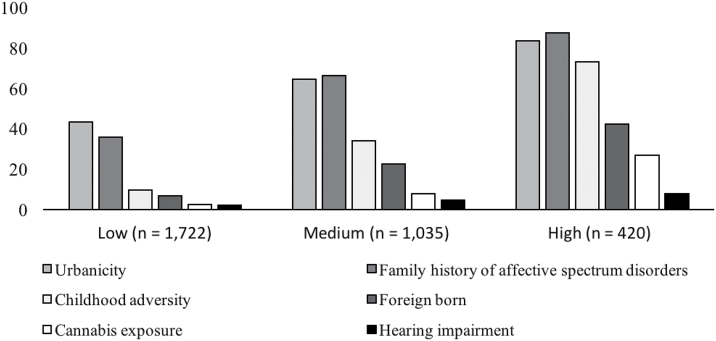
Fig. 1.
The frequencies of risk factors within risk factor strata at baseline assessment. This figure shows the frequencies of individual risk factors in each risk stratum at baseline: No risk factor = 0, low risk = 1 risk factor, medium risk = 2 risk factors, and high risk > 2 risk factors. Data were given in percentages based on the individual sample sizes of the low, medium, and high risk groups, respectively.
Fig. 2.
The prevalence of psychotic experience across risk strata. This figure reports the percentage of people with at least one PE within each risk stratum: No risk factors = 0, low risk = 1 risk factor, medium risk = 2 risk factors, and high risk > 2 risk factors.
Testing the Moderating Effects of Affective Dysregulation
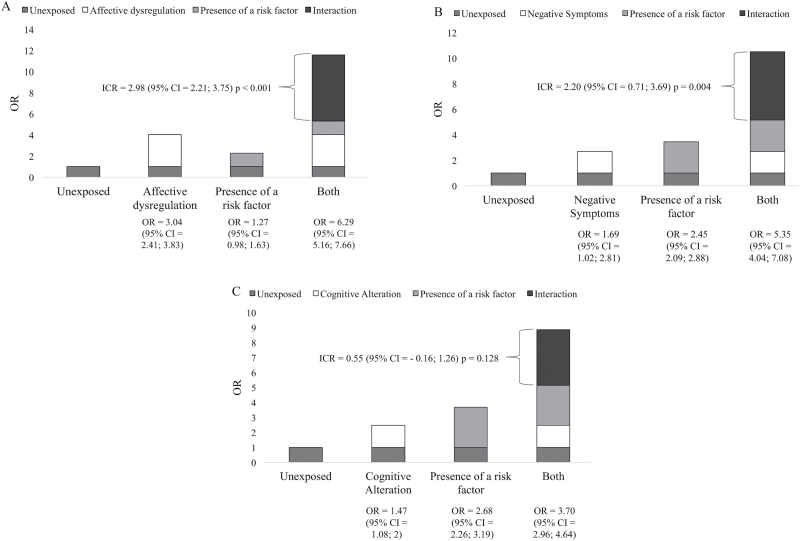
The association between risk and PE was greater if there was also evidence for affective dysregulation (table 2). Figure 3A shows that the adjusted OR for those with affective dysregulation and risk factors was 6.29, in comparison with ORs of 1.27 for those with risk factors only, and 3.04 for those with affective dysregulation only, yielding an ICR of 2.98 (P < .001). The additive effect of affective dysregulation remained significant after the other dimensions of psychopathology (negative symptoms, cognitive alteration) were controlled for, with an ICR of 2.74 (95% CI = 1.92–3.55, P < .001).
Table 2.
Additive Effect of Dimensional Components on the Association Between Risk Factors and Psychosis Expression
| Unadjusted Model | Adjusted Modela | Adjusted Modela (Corrected for the Other Dimensions) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ICR | 95% CI | P | ICR | 95% CI | P | ICR | 95% CI | P | |
| Affective dysregulation | 3.18 | 2.37; 3.98 | <.001 | 2.98 | 2.21; 3.75 | <.001 | 2.74 | 1.92; 3.55 | <.001 |
| Negative symptoms | 2.68 | 1.13; 4.23 | .001 | 2.20 | 0.71; 3.69 | .004 | −0.35 | −1.09; 0.39 | .355 |
| Cognitive alteration | 0.74 | 0.025; 1.45 | .042 | 0.55 | −0.16; 1.26 | .128 | −0.28 | −0.71; 0.15 | .199 |
Note: ICR, interaction contrast ratio; CI, confidence interval.
aAdjusted for age, sex, and education; controlled.
Fig. 3.
Figure shows the additive effects of symptom dimensions (A: affective dysregulation, B: negative symptoms, C: cognitive alteration) on the association between risk factors and PE adjusted for sex, age, and education.
Testing the Moderating Effects of Negative Symptoms
The association between risk and PE was greater if there was also evidence for negative symptoms (table 2). Figure 3B shows that the adjusted OR for those with negative symptoms and risk factors was 5.35, in comparison with ORs of 2.45 for those with risk factors only, and 1.69 for those with negative symptoms only, yielding an ICR of 2.20 (P = .004). After controlling for the other dimensions of psychopathology (affective dysregulation, cognitive alteration), there was no significant additive effect (ICR = −0.35, 95% CI = −1.09 to 0.39, P = .36).
Testing the Moderating Effects of Cognitive Alteration
The association between risk factors and PE was greater if there was also evidence for cognitive alteration (table 2). However, the additive effect was no longer significant in either the adjusted model (Figure 3C) nor in the model controlled for the other symptom dimensions (negative symptoms, affective dysregulation; ICR = −0.28, 95% CI = −0.71 to 0.15, P = .20).
Discussion
We explored the possible existence of a risk-loading effect on PE and investigated whether the association between the risk for PSD and PE was contingent on multidimensional psychopathology (affective dysregulation, negative symptoms, and cognitive alteration). The findings were that: (1) risk factors additively increased the likelihood of PE in a dose–response fashion; (2) affective dysregulation, negative symptoms, and cognitive alteration additively increased the association between risk factors and PE; (3) the association of risk factors with affective dysregulation and negative symptoms remained significant in the model adjusted for age, sex, and education; (4) the association of risk factors with affective dysregulation remained significant in the final model, when all PSD dimensions were controlled for each other.
Multidimensional Psychopathology
There is growing evidence that psychosis expression represents a severity indicator for multidimensional psychopathology cutting across traditional diagnostic boundaries,8,14,44 and that nonpsychotic symptoms, such as affective dysregulation,12,45 negative symptoms,13,46 and neurocognitive alteration13,46,47 precede the early stages of PSD and predict progression to more severe states. The current population-based study provides additional support for this concept by showing that epidemiological risk factors for PSD are not exclusively associated with PE, but rather with the degree of amalgamated multidimensional psychopathology.48–50
Our findings are in agreement with the literature showing symptom dimensions interrelate within19,20 and between11,18 different diagnoses, and that the severity of clinical outcomes of diverse clinical representations may depend on the degree of interconnection between those dimensions.7,13,20,51 For instance, the presence of PE in anxiety or depression disorders was shown to predict severity of clinical outcomes and treatment response7,52,53; and by investigating fluctuations of momentary mental states (eg, paranoia, positive, and negative affect) in daily life, studies using the experience sampling methodology found that an increased connectivity between momentary states was associated with symptom severity and need for care.54–56
Consistent with previous research, current findings suggest that risk factors operate by intensifying the multidimensional blending of affective and cognitive processes,51,57–60 as well as the negative symptom domain13,61 in the development of PSD. This finding fits well with our previous findings showing that the risk-loading (childhood trauma, urbanicity, cannabis use, and discrimination) amplifies connectivity between different symptom dimensions.34 Similarly, exposure to cannabis use and childhood trauma was found to increase associations between hallucination and delusion in healthy and in genetically at risk populations18,19; while another study reported that exposure to childhood trauma had a stronger correlation with a combined symptom network rather than the individual symptoms.32 Our finding that only the effect of affective dysregulation remained significant after the adjustment for the other dimensions is furthermore compatible with the theory of an “affective path to psychosis.”57,62
The Risk-Loading in the Context of Liability Threshold Model
The results echo findings from previous research showing a dose–response relationship between risk factors and PE,33,63 emphasizing the effects of risk-loading for PSD. Recently, researchers attempted to construct a “polyenviromic risk score” (PERS) for psychosis (the sum of weighted scores of known environmental risk factors based on their association with psychosis reported in meta-analyses). Despite several methodological issues, this proof-of-concept study showed that higher PERS predicted greater risk of developing psychosis in genetically at-risk individuals.64 Additionally, recent studies of individualized risk calculators for psychosis focusing on demographic, clinical, and some environmental predictors may provide insight into estimating psychosis risk in clinical settings.65,66
It is plausible to argue that the mechanisms underlying the development of PSD may be best understood in the context of the liability-threshold model67 that posits the combination of various genetic and environmental factors—with each factor adding to the risk load—adding to the manifestation of a phenotypic outcome. The distribution of liability may not be continuous, as the apparent phenomenological and temporal continuity of psychotic experiences with PSD may in fact reflect an underlying discontinuous population distribution consisting of vulnerable and nonvulnerable individuals.68,69
To further investigate environmental and genetic risk loading, future studies should focus on constructing reproducible total scores of environmental exposures, along with a single metric of aggregated molecular genetic variation (PRS), to disentangle the additive effects of gene–environment interplay on the development and course of PSD.
Given the complexity of multi-dimensional psychopathology, the network approach to symptoms (“symptomics”), a rapidly evolving analytical strategy, may also provide an alternative platform to gain insight into the role of gene–environment interplay in the development and progression of PSD, with initial findings showing (at least) some promise but requiring replication.70,71
Limitations
The primary strength of this study was the multi-domain clinical phenotyping and the use of a large and representative population cohort collected at 3 time-points over 6 years. However, various methodological limitations should be considered when interpreting the findings.
First, the cross-sectional analysis of the dataset merging data from each time-point in the “long-format,” while decreasing threats to external validity, cannot be taken as absolutely confirmatory of causality.72 Ideally, a time series analysis of a birth cohort—followed up with regular in-depth assessments at short-enough intervals to capture emerging psychopathology stretching over the period at risk for emerging mental disorders—is required to yield essential information to understand the impact of psychosis liability on the emotional, behavioral, and cognitive components of PSD. With no such data available now or in the near future, our practical strategy, despite its shortcomings, expands our knowledge-base, implying that the association between psychosis risk and psychosis expression is moderated by existing multidimensional psychopathology.
Second, although the dataset includes a fine-grained assessment of positive PE and affective symptomatology, there were only 4 proxy items appraising the negative symptom dimension. A more thorough assessment, using a validated rating scale with focus on measuring the negative symptom dimension as well as PE in the general population (such as the Structured Interview for Schizotypy—Revised (SIS-R) and the Community Assessment of Psychic Experiences (CAPE)) could have been beneficial in capturing the negative symptom dimension across the full range.
Third, our approach to risk stratification through aggregating vulnerability factors assumes a linear increase in the risk for psychosis as a function of the number of risk factors and weighs each risk factors equally by overlooking any specific feature pertaining to individual risk factors and their synergistic effects.
Although current findings are in line with those of previous studies in different population-based datasets, replications are necessary. We aim for reproducing the findings in the European network of national schizophrenia networks studying gene–environment interaction dataset, which includes heterogeneous, international, multi-ethnic samples of patients, relatives, and healthy controls.73,74
Conclusion
Consistent with previous findings, this study demonstrates that the association between psychosis expression and risk-loading (environmental and familial) is contingent on the dimensions of PSD, lending further support to the framework of an affective path to psychosis. Also, as predicted by the liability-threshold model, in which vulnerability for a phenotypic outcome can be modeled as a continuous metric of quantifiable risk, the aggregated risk-loading increased the odds of psychosis expression in a dose–response fashion.
Overall, our recent findings, combined with strong evidence from unbiased population-based cohorts, demonstrate the need for reconstructing the framework of psychosis by integrating multidimensional measurement of psychopathology to advance our understanding of the complex network of biopsychosocial mechanisms underlying the early progression of psychopathology and to dissect diverse developmental paths to psychosis.75
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
Financial support has been received from the Ministry of Health, Welfare and Sport, with supplementary support from the Netherlands Organization for Health Research and Development (ZonMw) and the Genetic Risk and Outcome of Psychosis (GROUP) investigators. These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Grants were received to write this paper on data from NEMESIS: Supported by the European Community’s Seventh Framework Program under Grant agreement No. HEALTH-F2-2009–241909 (Project EU-GEI).
Acknowledgment
NEMESIS-2 is conducted by the Netherlands Institute of Mental Health and Addiction (Trimbos Institute) in Utrecht.
References
- 1. van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. [DOI] [PubMed] [Google Scholar]
- 2. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 3. DeVylder JE, Burnette D, Yang LH. Co-occurrence of psychotic experiences and common mental health conditions across four racially and ethnically diverse population samples. Psychol Med. 2014;44:3503–3513. [DOI] [PubMed] [Google Scholar]
- 4. Kaymaz N, van Os J, de Graaf R, Ten Have M, Nolen W, Krabbendam L. The impact of subclinical psychosis on the transition from subclinicial mania to bipolar disorder. J Affect Disord. 2007;98:55–64. [DOI] [PubMed] [Google Scholar]
- 5. Dominguez MD, Wichers M, Lieb R, Wittchen HU, van Os J. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull. 2011;37:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaymaz N, Drukker M, Lieb R et al. Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42:2239–2253. [DOI] [PubMed] [Google Scholar]
- 7. Wigman JT, van Nierop M, Vollebergh WA et al. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity—implications for diagnosis and ultra-high risk research. Schizophr Bull. 2012;38:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rössler W, Hengartner MP, Ajdacic-Gross V, Haker H, Gamma A, Angst J. Sub-clinical psychosis symptoms in young adults are risk factors for subsequent common mental disorders. Schizophr Res. 2011;131:18–23. [DOI] [PubMed] [Google Scholar]
- 9. McGrath JJ, Saha S, Al-Hamzawi A et al. The bidirectional associations between psychotic experiences and DSM-IV mental disorders. Am J Psychiatry. 2016;173:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armando M, Lin A, Girardi P et al. Prevalence of psychotic-like experiences in young adults with social anxiety disorder and correlation with affective dysregulation. J Nerv Ment Dis. 2013;201:1053–1059. [DOI] [PubMed] [Google Scholar]
- 11. Wigman JT, van Os J, Abidi L et al. Subclinical psychotic experiences and bipolar spectrum features in depression: association with outcome of psychotherapy. Psychol Med. 2014;44:325–336. [DOI] [PubMed] [Google Scholar]
- 12. Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dominguez MD, Saka MC, can Saka M, Lieb R, Wittchen HU, van Os J. Early expression of negative/disorganized symptoms predicting psychotic experiences and subsequent clinical psychosis: a 10-year study. Am J Psychiatry. 2010;167:1075–1082. [DOI] [PubMed] [Google Scholar]
- 14. Kelleher I, Devlin N, Wigman JT et al. Psychotic experiences in a mental health clinic sample: implications for suicidality, multimorbidity and functioning. Psychol Med. 2014;44:1615–1624. [DOI] [PubMed] [Google Scholar]
- 15. Honings S, Drukker M, van Nierop M et al. Psychotic experiences and incident suicidal ideation and behaviour: disentangling the longitudinal associations from connected psychopathology. Psychiatry Res. 2016;245:267–275. [DOI] [PubMed] [Google Scholar]
- 16. Schultze-Lutter F, Michel C, Ruhrmann S, Schimmelmann BG. Prevalence and clinical relevance of interview-assessed psychosis-risk symptoms in the young adult community. Psychol Med. 2018;48:1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Os J. The dynamics of subthreshold psychopathology: implications for diagnosis and treatment. Am J Psychiatry. 2013;170:695–698. [DOI] [PubMed] [Google Scholar]
- 18. Smeets F, Lataster T, Dominguez MD et al. Evidence that onset of psychosis in the population reflects early hallucinatory experiences that through environmental risks and affective dysregulation become complicated by delusions. Schizophr Bull. 2012;38:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smeets F, Lataster T, Viechtbauer W, Delespaul P; G.R.O.U.P Evidence that environmental and genetic risks for psychotic disorder may operate by impacting on connections between core symptoms of perceptual alteration and delusional ideation. Schizophr Bull. 2015;41:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smeets F, Lataster T, van Winkel R, de Graaf R, Ten Have M, van Os J. Testing the hypothesis that psychotic illness begins when subthreshold hallucinations combine with delusional ideation. Acta Psychiatr Scand. 2013;127:34–47. [DOI] [PubMed] [Google Scholar]
- 21. Upthegrove R, Marwaha S, Birchwood M. Depression and schizophrenia: cause, consequence, or trans-diagnostic issue?Schizophr Bull. 2017;43:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheffield JM, Kandala S, Tamminga CA et al. Transdiagnostic associations between functional brain network integrity and cognition. JAMA Psychiatry. 2017;74:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ivleva EI, Bidesi AS, Keshavan MS et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cross-Disorder Group of the Psychiatric Genomics Consortium , Lee SH, Ripke S et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Os J, van der Steen Y, Islam MA, Gülöksüz S, Rutten BP, Simons CJ; GROUP Investigators Evidence that polygenic risk for psychotic disorder is expressed in the domain of neurodevelopment, emotion regulation and attribution of salience. Psychol Med. 2017;47:2421–2437. [DOI] [PubMed] [Google Scholar]
- 26. Misiak B, Krefft M, Bielawski T, Moustafa AA, Sąsiadek MM, Frydecka D. Toward a unified theory of childhood trauma and psychosis: a comprehensive review of epidemiological, clinical, neuropsychological and biological findings. Neurosci Biobehav Rev. 2017;75:393–406. [DOI] [PubMed] [Google Scholar]
- 27. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot—a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heinz A, Deserno L, Reininghaus U. Urbanicity, social adversity and psychosis. World Psychiatry. 2013;12:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gevonden MJ, Myin-Germeys I, van den Brink W, van Os J, Selten JP, Booij J. Psychotic reactions to daily life stress and dopamine function in people with severe hearing impairment. Psychol Med. 2015;45:1665–1674. [DOI] [PubMed] [Google Scholar]
- 30. Reeves LE, Anglin DM, Heimberg RG et al. Anxiety mediates the association between cannabis use and attenuated positive psychotic symptoms. Psychiatry Res. 2014;218:180–186. [DOI] [PubMed] [Google Scholar]
- 31. Catalan A, Angosto V, Díaz A et al. Relation between psychotic symptoms, parental care and childhood trauma in severe mental disorders. Psychiatry Res. 2017;251:78–84. [DOI] [PubMed] [Google Scholar]
- 32. van Nierop M, Viechtbauer W, Gunther N et al. ; Genetic Risk and Outcome of Psychosis investigators. Childhood trauma is associated with a specific admixture of affective, anxiety, and psychosis symptoms cutting across traditional diagnostic boundaries. Psychol Med. 2015;45:1277–1288. [DOI] [PubMed] [Google Scholar]
- 33. Guloksuz S, van Nierop M, Lieb R, van Winkel R, Wittchen HU, van Os J. Evidence that the presence of psychosis in non-psychotic disorder is environment-dependent and mediated by severity of non-psychotic psychopathology. Psychol Med. 2015;45:2389–2401. [DOI] [PubMed] [Google Scholar]
- 34. Guloksuz S, van Nierop M, Bak M et al. Exposure to environmental factors increases connectivity between symptom domains in the psychopathology network. BMC Psychiatry. 2016;16:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Graaf R, Ten Have M, van Dorsselaer S. The Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2): design and methods. Int J Methods Psychiatr Res. 2010;19:125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Graaf R, ten Have M, van Gool C, van Dorsselaer S. Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study-2. Soc Psychiatry Psychiatr Epidemiol. 2012;47:203–213. [DOI] [PubMed] [Google Scholar]
- 37. de Graaf R, van Dorsselaer S, Tuithof M, ten Have M. Sociodemographic and psychiatric predictors of attrition in a prospective psychiatric epidemiological study among the general population. Result of the Netherlands Mental Health Survey and Incidence Study-2. Compr Psychiatry. 2013;54:1131–1139. [DOI] [PubMed] [Google Scholar]
- 38. De Graaf R, Van Dorsselaer S, Tuithof M, ten Have M.. Sociodemographic and Psychiatric Predictors of Attrition in the Third Wave of the Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2). Utrecht: Trimbos-instituut; 2015. [Google Scholar]
- 39. Andrews G, Peters L. The psychometric properties of the Composite International Diagnostic Interview. Soc Psychiatry Psychiatr Epidemiol. 1998;33:80–88. [DOI] [PubMed] [Google Scholar]
- 40. Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 41. van Dam DS, van Nierop M, Viechtbauer W et al. ; Genetic Risk and Outcome of Psychosis (GROUP) Investigators. Childhood abuse and neglect in relation to the presence and persistence of psychotic and depressive symptomatology. Psychol Med. 2015;45:1363–1377. [DOI] [PubMed] [Google Scholar]
- 42. van Winkel R, van Beveren NJ, Simons C; Genetic Risk and Outcome of Psychosis (GROUP) Investigators AKT1 moderation of cannabis-induced cognitive alterations in psychotic disorder. Neuropsychopharmacology. 2011;36:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. StataCorp. Stata Statistical Software [computer program]. Version 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 44. Navarro‐Mateu F, Alonso J, Lim C et al. The association between psychotic experiences and disability: results from the WHO World Mental Health Surveys. Acta Psychiatr Scand. 2017;136:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Häfner H, Maurer K, Trendler G, an der Heiden W, Schmidt M, Könnecke R. Schizophrenia and depression: challenging the paradigm of two separate diseases—a controlled study of schizophrenia, depression and healthy controls. Schizophr Res. 2005;77:11–24. [DOI] [PubMed] [Google Scholar]
- 46. Häfner H, Löffler W, Maurer K, Hambrecht M, an der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100:105–118. [DOI] [PubMed] [Google Scholar]
- 47. Reichenberg A, Caspi A, Harrington H et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2009;167:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Os J, Guloksuz S. A critique of the ‘ultra‐high risk’ and ‘transition’ paradigm. World Psychiatry. 2017;16:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Os J, Reininghaus U. Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry. 2016;15:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reininghaus U, Böhnke JR, Hosang G et al. Evaluation of the validity and utility of a transdiagnostic psychosis dimension encompassing schizophrenia and bipolar disorder. Br J Psychiatry. 2016;209:107–113. [DOI] [PubMed] [Google Scholar]
- 51. Freeman D, Dunn G, Fowler D et al. Current paranoid thinking in patients with delusions: the presence of cognitive-affective biases. Schizophr Bull. 2013;39:1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kelleher I, Keeley H, Corcoran P et al. Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry. 2012;201:26–32. [DOI] [PubMed] [Google Scholar]
- 53. Jeppesen P, Clemmensen L, Munkholm A et al. Psychotic experiences co-occur with sleep problems, negative affect and mental disorders in preadolescence. J Child Psychol Psychiatry. 2015;56:558–565. [DOI] [PubMed] [Google Scholar]
- 54. Wigman JT, van Os J, Thiery E et al. Psychiatric diagnosis revisited: towards a system of staging and profiling combining nomothetic and idiographic parameters of momentary mental states. PLoS One. 2013;8:e59559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Os J, Lataster T, Delespaul P, Wichers M, Myin-Germeys I. Evidence that a psychopathology interactome has diagnostic value, predicting clinical needs: an experience sampling study. PLoS One. 2014;9:e86652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kramer I, Simons CJ, Wigman JT et al. Time-lagged moment-to-moment interplay between negative affect and paranoia: new insights in the affective pathway to psychosis. Schizophr Bull. 2014;40:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bird JC, Waite F, Rowsell E, Fergusson EC, Freeman D. Cognitive, affective, and social factors maintaining paranoia in adolescents with mental health problems: a longitudinal study. Psychiatry Res. 2017;257:34–39. [DOI] [PubMed] [Google Scholar]
- 58. Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44:181–191. [DOI] [PubMed] [Google Scholar]
- 59. Freeman D, Startup H, Dunn G et al. The interaction of affective with psychotic processes: a test of the effects of worrying on working memory, jumping to conclusions, and anomalies of experience in patients with persecutory delusions. J Psychiatr Res. 2013;47:1837–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Rossum I, Dominguez MD, Lieb R, Wittchen HU, van Os J. Affective dysregulation and reality distortion: a 10-year prospective study of their association and clinical relevance. Schizophr Bull. 2011;37:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Binbay T, Drukker M, Elbi H et al. Testing the psychosis continuum: differential impact of genetic and nongenetic risk factors and comorbid psychopathology across the entire spectrum of psychosis. Schizophr Bull. 2012;38:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. [DOI] [PubMed] [Google Scholar]
- 63. Morgan C, Reininghaus U, Reichenberg A, Frissa S, Hotopf M, Hatch SL; SELCoH Study Team Adversity, cannabis use and psychotic experiences: evidence of cumulative and synergistic effects. Br J Psychiatry. 2014;204:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Padmanabhan JL, Shah JL, Tandon N, Keshavan MS. The “polyenviromic risk score”: aggregating environmental risk factors predicts conversion to psychosis in familial high-risk subjects. Schizophr Res. 2017;181:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cannon TD, Yu C, Addington J et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fusar-Poli P, Rutigliano G, Stahl D et al. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry. 2017;74:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gottesman II, Shields J. A polygenic theory of schizophrenia. Proc Natl Acad Sci USA. 1967;58:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kaymaz N, Van Os J. Extended psychosis phenotype–yes: single continuum–unlikely: a commentary on ‘Why we need more debate on whether psychotic symptoms lie on a continuum with normality’ by David (2010). Psychol Med. 2010;40:1963–1966. [DOI] [PubMed] [Google Scholar]
- 69. Linscott RJ, van Os J. Systematic reviews of categorical versus continuum models in psychosis: evidence for discontinuous subpopulations underlying a psychometric continuum. Implications for DSM-V, DSM-VI, and DSM-VII. Annu Rev Clin Psychol. 2010;6:391–419. [DOI] [PubMed] [Google Scholar]
- 70. Isvoranu AM, Borsboom D, van Os J, Guloksuz S. A network approach to environmental impact in psychotic disorder: brief theoretical framework. Schizophr Bull. 2016;42:870–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Isvoranu AM, van Borkulo CD, Boyette LL, Wigman JT, Vinkers CH, Borsboom D; Group Investigators A network approach to psychosis: pathways between childhood trauma and psychotic symptoms. Schizophr Bull. 2017;43:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moffa G, Catone G, Kuipers J et al. Using directed acyclic graphs in epidemiological research in psychosis: an analysis of the role of bullying in psychosis. Schizophr Bull. 2017;43:1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schizophrenia ENoNNsG-EIi. Identifying gene–environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull. 2014;40:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van Os J, Rutten BP, Poulton R. Gene–environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48:229–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.