Abstract
Background
Blockade of D3 receptor, a member of the dopamine D2-like receptor family, has been suggested as a possible medication for schizophrenia. Blonanserin has high affinity in vitro for D3 as well as D2 receptors. We investigated whether a single dose of 12 mg blonanserin, which was within the daily clinical dose range (i.e., 8–24 mg) for the treatment of schizophrenia, occupies D3 as well as D2 receptors in healthy subjects.
Methods
Six healthy males (mean 35.7±7.6 years) received 2 positron emission tomography scans, the first prior to taking blonanserin, and the second 2 hours after the administration of a single dose of 12 mg blonanserin. Dopamine receptor occupancies by blonanserin were evaluated by [11C]-(+)-PHNO.
Results
Occupancy of each region by 12 mg blonanserin was: caudate (range 64.3%–81.5%; mean±SD, 74.3±5.6%), putamen (range 60.4%–84.3%; mean±SD, 73.3%±8.2%), ventral striatum (range 40.1%–88.2%; mean±SD, 60.8%±17.1%), globus pallidus (range 65.8%–87.6%; mean±SD, 75.7%±8.6%), and substantia nigra (range 56.0%–88.7%; mean±SD, 72.4%±11.0%). Correlation analysis between plasma concentration of blonanserin and receptor occupancy in D2-rich (caudate and putamen) and D3-rich (globus pallidus and substantia nigra) regions showed that EC50 for D2-rich region was 0.39 ng/mL (r=0.43) and EC50 for D3-rich region was 0.40 ng/mL (r=0.79).
Conclusions
A single dose of 12 mg blonanserin occupied D3 receptor to the same degree as D2 receptor in vivo. Our results were consistent with previous studies that reported that some of the pharmacological effect of blonanserin is mediated via D3 receptor antagonism.
Keywords: D2 receptor, D3 receptor, blonanserin, positron emission tomography
Significance Statement
The focus of this study was on the occupancies of blonanserin for both dopamine D2 and D3 receptors in vivo. This study found that 12 mg blonanserin occupied D3-rich region (i.e., 75.7% in globus pallidus and 72.4% in substantia nigra) as much as D2-rich region (i.e., 74.3% in caudate and 73.3% in putamen) in healthy volunteers. Correlation analysis between the plasma concentration of blonanserin and receptor occupancy showed that the EC50 values of D2-rich regions and D3-rich regions are very similar (0.39 ng/mL, r=0.43 and 0.40 ng/mL, r=0.79). Our results indicated the possibility that some of the pharmacological effect of blonanserin is mediated via D3 receptor antagonism.
Introduction
The mechanism of the antipsychotic effect of neuroleptics mainly focuses on the antagonism of D2 receptor (Seeman, 2002). D2 receptor antagonism can also induce some adverse effects (e.g., Parkinsonism, hyperprolactinemia), and therefore many researchers have been studying the effects of antipsychotics on the dopaminergic system and other monoaminergic systems, and also the association between pharmacological treatment and the change of monoaminergic systems in vivo.
Dopamine D3 receptor, a member of the dopamine D2-like receptor family, localizes in the limbic area and coexists with D2 receptors in the substantia nigra as well as in many other areas in human brain. D3 receptor has similarities to other members of the D2-like receptor family, but D3 receptor has very high affinity for dopamine and modulates dopamine release as an autoreceptor (Gross and Drescher, 2012). Some previous studies have reported that selective D3 receptor antagonists might increase the extracellular dopamine concentrations in the medial prefrontal cortex (Gross and Drescher, 2012) and are also able to increase acetylcholine (Kuroki et al., 1999). These pharmacological studies and studies of rodent D3 receptors have suggested that blockade of D3 receptor might represent a new treatment mechanism for schizophrenia (Gross et al., 2013).
Many atypical antipsychotics and some typical antipsychotics have high affinities for both D2 and D3 receptors (Girgis et al., 2011). Recent technological progress has allowed us to visualize the distribution of D3 receptors in vivo. [11C]-(+)-PHNO is a D3/D2 agonist radioligand for positron emission tomography (PET) with preferential in vivo selectivity for dopamine D3 over D2 receptor (Willeit et al., 2006; Ginovart et al., 2007). Almost all of the signal of [11C]-(+)-PHNO in both caudate and putamen represented D2 receptor sites, while up to 100% of the signal in substantia nigra, 67% in globus pallidus, and 26% in ventral striatum represented D3 receptor sites (Searle et al., 2010). An in vitro affinity study suggested that antipsychotics would occupy D3 receptors as much as D2 receptors, although antipsychotics reportedly occupied D3 receptors moderately less than D2 receptors (Girgis et al., 2011), and a PET study with [11C]-(+)-PHNO reported that antipsychotics (i.e., clozapine, risperidone, olanzapine) did not decrease or even increased the in vivo nondisplaceable binding potential (BPND) of D3 receptors in human brain (Graff-Guerrero et al., 2009). Another study also reported that chronically administered antipsychotics (i.e., clozapine, olanzapine, and haloperidol) showed lower selectivity for D3 compared with D2 receptors ex vivo than in vitro in rat brain (McCormick et al., 2010).
Blonanserin is an atypical antipsychotic, but unlike other atypical antipsychotics, the binding affinity for D2 receptors (Ki=0.284 nM) is slightly higher than that for 5-HT2A receptors (Ki=0.64 nM) in vitro (Murasaki et al., 2008). Blonanserin has high in vitro affinity for dopamine D3 receptor (Ki=0.277 nM), similar to that for D2 receptor (Ki=0.284 nM) (Baba et al., 2008). Blonanserin occupied a D3-rich region (i.e., cerebellum lobe 9–10) similarly to a D2-rich region (i.e., striatum) in rat brain, while risperidone, olanzapine, and aripiprazole did not (Baba et al., 2008). Blonanserin demonstrated efficacy against cognitive impairment induced by phencyclidine by inhibiting both dopamine D3 and serotonin 5-HT2A receptors in rat (Hida et al., 2015). Thus, blonanserin has been thought to have D3 antagonism identical to D2 and clinical efficacy by D3 receptors. However, there has been no study of the evaluation of D3 occupancy by blonanserin.
In this study, we investigated whether a clinical dose of blonanserin occupies D3 receptors as well as D2 receptors in healthy subjects.
Methods
Subjects
Six healthy male volunteers (range 27–46 years; mean±SD, 35.7±7.6) were enrolled. None had a history of present or past psychiatric, neurological, or somatic disorders, or alcohol or substance-related problems. After thorough explanation of the study, written informed consent was obtained from all participants. This study was approved by the review board of Nippon Medical School Hospital, Tokyo, Japan.
Study Design
This study was designed as a single administration, open-label protocol. Each subject underwent 2 PET scans, the first prior to taking blonanserin, and the second 2 hours after being administered of 12 mg blonanserin, which targeted the time-to-maximum blood concentration of blonanserin (Saruwatari et al., 2010).
PET Procedures
PET scans were carried out with Eminence SET-3000GCT-X (Shimadzu Corp) to measure regional brain radioactivity. This scanner provides 99 sections with an axial field of view of 26.0 cm. Spatial resolution was 3.45 mm in-plane and 3.72 mm axially full-width at half-maximum. A head fixation device was used during the scans. A 15-min transmission scan was done to correct for attenuation using a 137Cs source. Dynamic PET scan was performed for 90 min (1 min×15, 5 min×15) after i.v. bolus injection of [11C]-(+)-PHNO. Injected radioactivity was 196.4 to 385.0 MBq (348.8±64.2 (mean±SD MBq) for drug-free condition; 339.8±70.2 MBq for blonanserin condition). The injected mass of [11C]-(+)-PHNO was 1.9 – 2.5 μg (2.2±0.3 μg for drug-free condition; 2.4±0.1 μg for blonanserin condition). Specific radioactivity was 41.7 – 95.4 GBq/µmol (77.7±7.5 GBq/µmol for drug-free condition; 77.9±7.5 GBq/µmol for blonanserin condition) at the time of injection.
MRI Procedures
Magnetic resonance (MR) images of the brain were acquired with 1.5T MR imaging, Intera 1.5T Achieve Nova (Philips Medical Systems) as proton density image (echo time=17 msec; repetition time=6000 msec; field of view=22 cm, 2-dimensional, 256×256; slice thickness=2 mm; number of excitations=2). These images were used for analysis of the PET scans.
Measurement of Plasma Concentration of Blonanserin
Venous blood samples were taken just before second PET scans (2 hours after taking 12 mg blonanserin), collected in tubes containing EDTA-2Na, and centrifuged at 3000 rpm for 10 min at 4ºC. Separated plasma samples were stored at -80ºC until analysis. Plasma concentration was measured by validated method using high-performance liquid chromatography-tandem mass spectrometry with a target lower quantification limit of 0.001 ng/mL (Sekisui Medical Co., Ltd.).
Data Analysis
MR images were co-registered to summated PET images with the mutual information algorithm using PMOD (version 3.4; PMOD Technologies Ltd). Regions of interest (ROIs) were defined for the caudate, putamen, ventral striatum, globus pallidus, and substantia nigra, and were drawn manually in accordance with Tziortzi’s study (Tziortzi et al., 2011). We defined the caudate and putamen as D2-rich regions and the substantia nigra and globus pallidus as D3-rich regions, according to Searle’s study (Searle et al., 2010). ROIs were drawn manually on overlaid summated PET and co-registered MR images of each subject. By matching the targeted frame to the average of the first 10 frames (i.e., 0–10 minutes), motion corrections were conducted in 3 scans of 2 subjects because of head movements.
Quantitative estimate of binding of [11C]-(+)-PHNO was performed using a simplified reference tissue model (Lammertsma and Hume, 1996), with the cerebellar cortex as reference region. This model has been validated to reliably estimate BPND, which compares the concentration of radioligand in the receptor-rich region with the receptor-free region (Innis et al., 2007), for [11C]-(+)-PHNO (Ginovart et al., 2007).
Receptor occupancy by drugs was calculated by the following equation:
where occupancy is the receptor occupancy, BPNDbase is BPND under drug-free condition, and BPNDdrug is BPND under blonanserin condition.
We used a 1-site binding model, the same as a previous study (Graff-Guerrero et al., 2010). The relationship between plasma concentration and receptor occupancy was shown by the following equation:
where C is the plasma concentration of drug, Emax is the maximum occupancy, and EC50 is the plasma concentration required to achieve 50% occupancy (Takano et al., 2006; Graff-Guerrero et al., 2010). Emax was fixed at 1 and EC50 >0, the same as in previous occupancy studies (Takano et al., 2006; Graff-Guerrero et al., 2010). Since discrepancy of D3 receptor occupancy between in vitro and in vivo studies has been reported (Graff-Guerrero et al., 2010; McCormick et al., 2010), correlations between plasma concentration and receptor occupancy were examined.
Results
Five subjects each completed two 90-min PET scans with [11C]-(+)-PHNO. One subject provided partial data: the first PET scan (drug-free condition) was stopped at 70 min because of the subject’s anxiety. We then included both conditions of 0 to 70 min PET data of this subject.
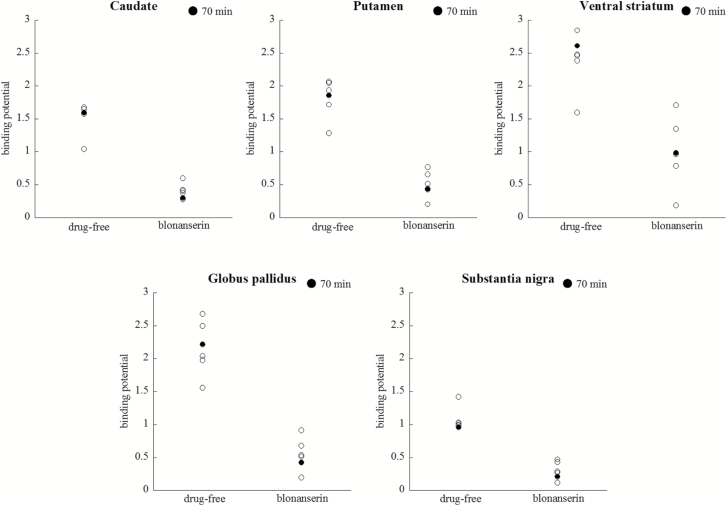
Figure 1 shows BPND of each ROI in each condition. Average BPND in drug-free condition was as follows: caudate (BPND range 1.04–1.68; mean±SD, 1.53±0.24), putamen (BPND range 1.28–2.06; 1.82±0.29), ventral striatum (BPND range 1.59–2.85; 2.40±0.43), globus pallidus (BPND range 1.56–2.68; 2.16±0.40), and substantia nigra (BPND range 0.96–1.42; 1.06±0.17). Average BPND in blonanserin condition was as follows: caudate (BPND range 0.27–0.60; 0.40±0.12), putamen (BPND range 0.20–0.77; 0.50±0.19), ventral striatum (BPND range 0.19–1.70; 0.99±0.52), globus pallidus (BPND range 0.19–0.91; 0.54±0.24), and substantia nigra (BPND range 0.11–0.47; 0.30±0.13). The average level of receptor occupancy by a single dose of blonanserin 12 mg was as follows: caudate (range 64.3–81.5; 74.3±5.6%), putamen (range 60.4–84.3; 73.3±8.2%), ventral striatum (range 40.1–88.2; 60.8±17.1%), globus pallidus (range 65.8–87.6; 75.7±8.6%), and substantia nigra (range 56.0–88.7; 72.4±11.0%) (Table 1).
Figure 1.
Binding potential (BPND) of [11C]-(+)-PHNO for every region of interest before and after taking 12 mg blonanserin. Filled circles represent the subject with 70-min PET scans.
Table 1.
Average Binding Potential and Occupancy of Dopamine Receptors in Each Region
| Region of Interest | Drug-free | Blonanserin | Occupancy (%) |
|---|---|---|---|
| Caudate | 1.53±0.24 | 0.40±0.12 | 74.3±5.6 |
| Putamen | 1.82±0.29 | 0.50±0.20 | 73.3±8.2 |
| Globus pallidus | 2.16±0.40 | 0.54±0.24 | 75.7±8.6 |
| Ventral striatum | 2.40±0.43 | 0.99±0.52 | 60.8±17.1 |
| Substantia nigra | 1.06±0.17 | 0.30±0.13 | 72.4±11.0 |
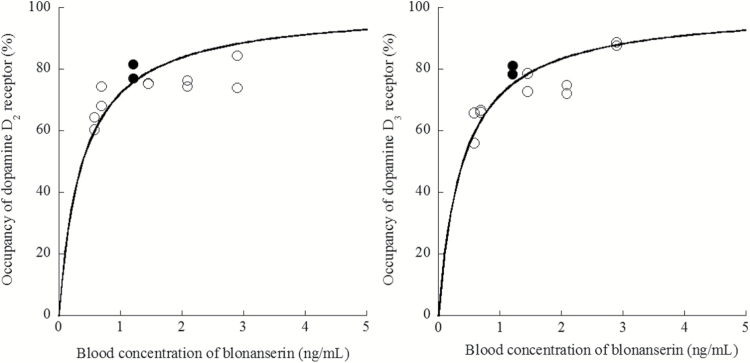
The average drug concentration of blonanserin was 1.49±0.88 (mean±SD) ng/mL (range 0.58–2.90). Correlations between plasma concentration of blonanserin and receptor occupancy in D2-rich and D3-rich ROI are shown in Figure 2. EC50 was 0.39 ng/mL (df=11, r=0.43) for D2-rich region and 0.40 ng/mL (df=11, r=0.79) for D3-rich region.
Figure 2.
Correlation between receptor occupancy in D2-rich (caudate and putamen) and D3-rich (globus pallidus and substantia nigra) regions with [11C]-(+)-PHNO and plasma concentration of 12 mg blonanserin. Filled circles represent the subject with 70-min PET scans.
Discussion
In this study, we examined the receptor occupancies in both D2-rich and D3-rich regions by a single dose of 12 mg blonanserin using [11C]-(+)-PHNO PET. A single dose of 12 mg blonanserin occupied dopamine receptors in D3-rich regions (i.e., substantia nigra, globus pallidus) as much as in D2-rich regions (i.e., caudate, putamen). Receptor occupancy in the striatum by 12 mg blonanserin (74.3% for caudate and 73.3% for putamen) was almost the same as by [11C]raclopride (68.5% for the striatum as calculated based on our previous data; Tateno et al., 2013). Thus, a single dose of 12 mg blonanserin, within the clinical daily dose range for the treatment of schizophrenia, occupied D3 receptors to approximately the same extent as D2 receptors. Thus, this is the first study to show that this dopamine antagonist occupied both D2 and D3 receptors at about the same levels.
Since dopaminergic hypofunction in the prefrontal cortex has been implicated in the pathogenesis of negative symptoms (Davis et al., 1991) and cognitive dysfunctions of schizophrenia (Sawaguchi, 2000), D3 receptor antagonism might improve the negative symptoms and cognitive deficits of schizophrenia. Animal studies have reported that blonanserin showed cognitive efficacy via D3 receptor antagonism and that it had a beneficial effect on prefrontal dopamine transmission (Gross et al., 2013; Nakajima et al., 2013; Hida et al., 2015). Other clinical studies showed that blonanserin induced improvements in verbal fluency and executive function (Tenjin et al., 2012; Hori et al., 2014), which might be related to the effect of this antipsychotic on dopamine transmission prefrontal cortex function. It has also been reported that functional connectivity between the prefrontal cortex and salience/executive control networks negatively associated with midbrain D3 receptor availability (Cole et al., 2011). Many PET studies have suggested that around 70% to 80% D2 receptor occupancy in the striatum is required for an antipsychotic effect with a lower risk of extrapyramidal adverse effects (Farde et al., 1992; Nordström et al., 1993; Kapur et al., 2000). Previous studies showed that 150 mg of ABT-925, a selective D3 receptor antagonist, possibly improves positive and negative symptoms of schizophrenia, and that it occupied about 30% of D3 receptors in substantia nigra and globus pallidus (Graff-Guerrero et al., 2010; Bhathena et al., 2013). A recent study showed that a clinical dose of cariprazine, a D3-preferring dual D3/D2 receptor partial agonist for the treatment of schizophrenia, occupied at least 76% of D3 receptors, along with 45% of D2 receptors (Girgis et al., 2016). However, the adequate degree of D3 receptor occupancy for an antipsychotic effect or improvement of motivation and/or cognition has been unclear. Our result provided evidence that a single dose of 12 mg blonanserin antagonizes D3 receptor as much as D2 receptor, as has been indicated by previous studies (Baba et al., 2015), and therefore further study regarding what percentage of D3 occupancy by antipsychotics might improve cognition could be beneficial for treatment strategies of schizophrenia.
The mechanism underlying the discrepancies between in vitro and in vivo bindings of several antipsychotics to dopamine D3 receptors (Graff-Guerrero et al., 2010; McCormick et al., 2010) is still unclear. However, several explanations have been proposed. Girgis presented the upregulation scenario (Girgis et al., 2011). The study in baboons demonstrated binding of both D2 and D3 receptors by an acute dose of haloperidol (haloperidol: 70% of putamen and 61% of globus pallidus, clozapine: 43% of putamen and 21% of globus pallidus). Girgis further suggested that upregulation of D3 receptor induced by chronic use of antipsychotics might affect the discrepancy between the results of in vitro affinity study and in vivo PET study (Girgis et al., 2011). Our study participants were healthy volunteers with a single administration, and our result was consistent with this scenario. Another explanation could be the influence of endogenous dopamine. Schotte reported that the D2/D3 potency ratios of antipsychotics (i.e., clozapine, olanzapine, risperidone, haloperidol) in vivo were 2 to 10 times higher than those of in vitro competitive binding experiments (Schotte et al., 1996), and they suggested that this effect was due to the in vivo inhibitory influence of endogenous dopamine. It has been suggested that antipsychotics inhibit D2 and D3 receptors in a dose-dependent manner, but the relationship between drug concentration and inhibitory effect differed according to the respective antipsychotics. Tadori reported that most antipsychotics at clinical dose sufficiently antagonize D2 receptor signals but not D3 receptor signals, while blonanserin, haloperidol, and fluphenazine inhibit D3 receptor at a similar level to D2 receptor (Tadori et al., 2011). Their study suggested that a clinical dose of antipsychotics, except for blonanserin, haloperidol, and fluphenazine, preferentially inhibits D2 receptor compared with D3 receptor in vivo (Tadori et al., 2011).
There are several limitations to this study. First, we performed PET scan after only a single administration of an antipsychotic in 6 healthy subjects. It has been reported that chronic use of antipsychotics increases D3 receptors among patients with schizophrenia (Graff-Guerrero et al., 2009). Another study of drug-naïve patients with schizophrenia showed that 2.5 weeks of antipsychotic treatment doubled BPND of D3 receptors (Mizrahi et al., 2011). These studies indicated that the density of D3 receptors in patients treated with antipsychotics might be different from that in healthy controls. Thus, to evaluate the precise relationship between drug concentration and both D2 and D3 receptor occupancies, further study with larger sample size, including patients, will be needed. Second, 1 of our 6 subjects provided only 70-min data from the PET scan. It was considered that this might affect the evaluation of BPND. However, the relationship between occupancy of receptors and drug concentration was almost unchanged when excluding this subject (in caudate: r = 1.00, EC50 =0.38; in putamen: r=0.77, EC50=0.42; in ventral striatum: r=0.62, EC50=0.82; in globus pallidus: r=0.79, EC50=0.38; in substantia nigra: r=0.85, EC50=0.46).
In conclusion, our study using [11C]-(+)-PHNO demonstrated that a single dose of 12 mg blonanserin could occupy D3 receptors to the same degree as D2 receptors in vivo. Our results indicated the possibility that some of the pharmacological effect of blonanserin was mediated via D3 receptor antagonism.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japanese government.
Statement of Interest
Y.O. has received grants or speaker’s honoraria from Dainippon Sumitomo Pharma, GlaxoSmithKline, Janssen Pharmaceutical, Otsuka, Pfizer, Eli Lilly, Astellas, Yoshitomi, and Meiji within the past 3 years. The remaining authors declare no interest.
Acknowledgments
We are grateful to Dr. Alan A. Wilson for advice on the synthesis of [11C]-(+)-PHNO. We thank Koji Nagaya, Koji Kanaya, Megumi Hongo, and Minoru Sakurai for their assistance in performing the PET experiments and MRI scanning, and Michiyo Tamura for her help as clinical research coordinator (Clinical Imaging Center for Healthcare, Nippon Medical School, Tokyo, Japan).
References
- Baba S, Enomoto T, Horisawa T, Hashimoto T, Ono M(2015)Blonanserin extensively occupies rat dopamine D3 receptors at antipsychotic dose range. J Pharmacol Sci 127:326–331. [DOI] [PubMed] [Google Scholar]
- Bhathena A, Wang Y, Kraft JB, Idler KB, Abel SJ, Holley-Shanks RR, Robieson WZ, Spear B, Reden L, Katz DA(2013). Association of dopamine-related genetic loci to dopamine D3 receptor antagonist ABT-925 clinical response. Transl Psychiatry 3:e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Searle GE, Plisson C, Tziortzi AC, Nichols TE, Gunn RN, Matthews PM, Rabiner EA, Beaver JD(2012)Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex 22:2784–2793. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M(1991)Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148:1474–1486. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G(1992)Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 49:538–544. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, Kapur S, Wilson AA(2007)Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab 27:857–871. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Slifstein M, D’Souza D, Lee Y, Periclou A, Ghahramani P, Laszlovszky I, Durgam S, Adham N, Nabulsi N, Huang Y, Carson RE, Kiss B, Kapás M, Abi-Dargham A, Rakhit A(2016)Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [(11)C]-(+)-PHNO. Psychopharmacology (Berl) 233:3503–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Xu X, Miyake N, Easwaramoorthy B, Gunn RN, Rabiner EA, Abi-Dargham A, Slifstein M(2011)In vivo binding of antipsychotics to D3 and D2 receptors: a PET study in baboons with [11C]-(+)-PHNO. Neuropsychopharmacology 36:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mamo D, Shammi CM, Mizrahi R, Marcon H, Barsoum P, Rusjan P, Houle S, Wilson AA, Kapur S(2009)The effect of antipsychotics on the high-affinity state of D2 and D3 receptors: a positron emission tomography study with [11C]-(+)-PHNO. Arch Gen Psychiatry 66:606–615. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Redden L, Abi-Saab W, Katz DA, Houle S, Barsoum P, Bhathena A, Palaparthy R, Saltarelli MD, Kapur S(2010)Blockade of [11C](+)-PHNO binding in human subjects by the dopamine D3 receptor antagonist ABT-925. Int J Neuropsychopharmacol 13:273–287. [DOI] [PubMed] [Google Scholar]
- Gross G, Drescher K(2012). The role of dopamine D(3) receptors in antipsychotic activity and cognitive functions. Hndb Exp Pharmacol 213:167–210. [DOI] [PubMed] [Google Scholar]
- Gross G, Wicke K, Drescher KU(2013)Dopamine D₃ receptor antagonism–still a therapeutic option for the treatment of schizophrenia. Naunyn Schmiedebergs Arch Pharmacol 386:155–166. [DOI] [PubMed] [Google Scholar]
- Hida H, Mouri A, Mori K, Matsumoto Y, Seki T, Taniguchi M, Yamada K, Iwamoto K, Ozaki N, Nabeshima T, Noda Y(2015)Blonanserin ameliorates phencyclidine-induced visual-recognition memory deficits: the complex mechanism of blonanserin action involving D₃-5-HT₂A and D₁-NMDA receptors in the mpfc. Neuropsychopharmacology 40:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H, Yamada K, Kamada D, Shibata Y, Katsuki A, Yoshimura R, Nakamura J(2014)Effect of blonanserin on cognitive and social function in acute phase japanese schizophrenia compared with risperidone. Neuropsychiatr Dis Treat 10:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, et al. . 2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S(2000)Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 157:514–520. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J(1999)Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther 288:774–781. [PubMed] [Google Scholar]
- McCormick PN, Kapur S, Graff-Guerrero A, Raymond R, Nobrega JN, Wilson AA(2010)The antipsychotics olanzapine, risperidone, clozapine, and haloperidol are D2-selective ex vivo but not in vitro. Neuropsychopharmacology 35:1826–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Agid O, Borlido C, Suridjan I, Rusjan P, Houle S, Remington G, Wilson AA, Kapur S(2011)Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr Res 131:63–68. [DOI] [PubMed] [Google Scholar]
- Murasaki M, Nishikawa H, Ishibashi T(2008). Dopamine-serotonin antagonist: receptor binding profile of a novel antipsychotic blonanserin. Jpn J Clin Psychopharmacol 11:845–854. In Japanese. [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, Mulsant B, Pollock B, Graff-Guerrero A(2013)The potential role of dopamine D₃ receptor neurotransmission in cognition. Eur Neuropsychopharmacol 23:799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G(1993)Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry 33:227–235. [DOI] [PubMed] [Google Scholar]
- Saruwatari J, Yasui-Furukori N, Inoue Y, Kaneko S(2010)Effect of dose timing in relation to food intake on systemic exposure to blonanserin. Eur J Clin Pharmacol 66:899–902. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T.(2000)The role of D1-dopamine receptors in working memory-guided movements mediated by frontal cortical areas. Parkinsonism Relat Disord 7:9–19. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Bonaventure P, Leysen JE(1996)Endogenous dopamine limits the binding of antipsychotic drugs to D3 receptors in the rat brain: a quantitative autoradiographic study. Histochem J 28:791–799. [DOI] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Mugnaini M, Griffante C, Wilson AA, Merlo-Pich E, Houle S, Gunn R, Rabiner EA, Laruelle M(2010)Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry 68:392–399. [DOI] [PubMed] [Google Scholar]
- Seeman P.(2002)Atypical antipsychotics: mechanism of action. Can J Psychiatry 47:27–38. [PubMed] [Google Scholar]
- Tadori Y, Forbes RA, McQuade RD, Kikuchi T(2011)Functional potencies of dopamine agonists and antagonists at human dopamine D₂ and D₃ receptors. Eur J Pharmacol 666:43–52. [DOI] [PubMed] [Google Scholar]
- Takano A, Suhara T, Ichimiya T, Yasuno F, Suzuki K(2006)Time course of in vivo 5-HTT transporter occupancy by fluvoxamine. J Clin Psychopharmacol 26:188–191. [DOI] [PubMed] [Google Scholar]
- Tateno A, Arakawa R, Okumura M, Fukuta H, Honjo K, Ishihara K, Nakamura H, Kumita S, Okubo Y(2013)Striatal and extrastriatal dopamine D2 receptor occupancy by a novel antipsychotic, blonanserin: a PET study with [11C]raclopride and [11C]FLB 457 in schizophrenia. J Clin Psychopharmacol 33:162–169. [DOI] [PubMed] [Google Scholar]
- Tenjin T, Miyamoto S, Miyake N, Ogino S, Kitajima R, Ojima K, Arai J, Teramoto H, Tsukahara S, Ito Y, Tadokoro M, Anai K, Funamoto Y, Kaneda Y, Sumiyoshi T, Yamaguchi N(2012)Effect of blonanserin on cognitive function in antipsychotic-naïve first-episode schizophrenia. Hum Psychopharmacol 27:90–100. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN(2011)Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 54:264–277. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, Wilson AA(2006)High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry 59:389–394. [DOI] [PubMed] [Google Scholar]