Abstract
Background
Antiretroviral therapy (ART) reduces HIV transmission, but treated patients may again become infectious. We used a mathematical model to determine whether ART as prevention is more effective if viral load (VL) is routinely monitored and patients lost to follow-up (LTFU) traced.
Methods
We simulated ART cohorts to parameterize a deterministic transmission model calibrated to Malawi. We investigated the following strategies for improving treatment and retention: monitoring VL every 12 or 24 months, tracing patients LTFU, or a generic strategy leading to uninterrupted treatment. We tested 3 scenarios, where ART scale-up continues at current (Universal ART), reduced (Failed scale-up), or accelerated speed (Test&Treat).
Results
In the Universal ART scenario, between 2017 and 2020 (2050), monitoring VL every 24 months prevented 0.5% (0.9%), monitoring every 12 months prevented 0.8% (1.4%), tracing prevented 0.3% (0.5%), and uninterrupted treatment prevented 5.5% (9.9%) of HIV infections. Failed scale-up resulted in 25% more infections than the Universal ART scenarios, whereas Test&Treat resulted in 7%–8% less.
Conclusions
Test&Treat reduces transmission of HIV, despite individual cases of treatment failure and ART interruption. Whereas viral load monitoring and tracing have only a minor impact on transmission, interventions that aim to minimize treatment interruptions can further increase the preventive effect of ART.
Keywords: antiretroviral therapy, HIV, loss to follow-up, mathematical model, monitoring, transmission
Antiretroviral therapy (ART) suppresses the HIV-RNA concentration (viral load [VL]) in people living with HIV (PLHIV), reducing transmission risk [1, 2]. Since 2016, the World Health Organization (WHO) has recommended that all PLHIV begin ART immediately [3]. Preventing transmission through treatment—“treatment as prevention” (TasP)—was an argument for expanding eligibility for ART to wider groups of patients and ultimately to all PLHIV. An intensive TasP intervention called “Test&Treat” screens the population regularly for HIV and immediately starts all patients who test positive on ART. In 2014, UNAIDS launched its global “90-90-90” target, with the goal of substantially reducing transmission. The benefits of TasP and Test&Treat have been widely discussed, but the evidence is not conclusive. Some studies have suggested that successful Test&Treat programs could eradicate the epidemic, but others predict only minor benefit on the population level [4–6].
Treatment failures, poor adherence, and frequent dropout from care impair the effect of TasP [7–9]. Viral load monitoring and tracing patients lost to follow-up (LTFU) can support viral suppression in treated patients. The WHO has recommended routine VL monitoring as its preferred monitoring strategy since 2013. For several years, VL monitoring in sub-Saharan Africa was available only in South Africa and Botswana, and a few research sites. New testing technologies have made routine VL monitoring easier, but coverage remains limited [10]. Patients LTFU are frequently traced in sub-Saharan Africa through phone calls or home visits to those who do not return to pick up their antiretrovirals [11].
We have developed mathematical models to test the effect of VL monitoring [12] and tracing patients LTFU [13] on reducing potential transmission of HIV. We found that these interventions could prevent patients on ART from transmitting the infection, but our analyses only evaluated a patient’s potential for transmitting the virus. The future course of the HIV epidemic depends on other important factors, such as transmission from untreated patients, behavioral preferences, and contact patterns. In this study, we took the next step and developed a transmission model to assess the potential effect of VL monitoring and tracing on the HIV epidemic.
METHODS
Setting
We modeled the HIV epidemic of Malawi. In 2016, the estimated adult HIV prevalence in Malawi was 9.2%, and about 680 000 patients (65% of all PLHIV) were on ART [14]. Until recently, Malawi relied on clinical monitoring and occasional CD4 counts to monitor treatment response, but since 2011, the Ministry of Health has recommended monitoring VL at regular 24-month intervals [15]. In 2016, 19% of all patients on ART in Malawi had had at least 1 VL test. Several sites trace patients who miss appointments [16].
Mathematical Model
The model consists of an individual-based simulation of disease progression and a deterministic transmission model.
Disease Progression Simulation
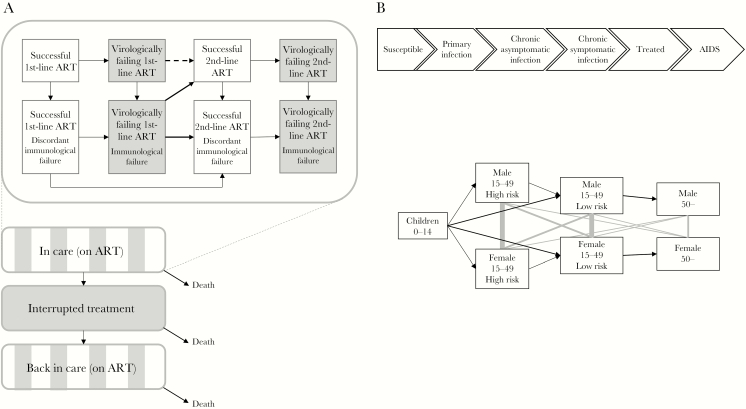
We used the R package gems to develop an individual-based simulation model for disease progression in patients who have started ART (“treatment model”) [17, 18]. We divided the patient’s time on ART into 32 states (Figure 1A; Supplementary Table 1) that accounted for virological and immunological treatment response (successful or failing), ART regimen (first- or second-line), and retention (on or off ART). We did not consider further treatment options beyond second-line. We separated HIV-related and HIV-unrelated mortality. We limited the number of off-ART episodes to 1 to simplify the structure. Patients were simulated for 10 years after initiating ART. Virological and immunological treatment response was based on previous analyses of routine data from sites in South Africa with 6-monthly VL and CD4 monitoring (Supplementary Table 2) [12, 19, 20]. The risk of virological failure corresponds to a cumulative risk of 5.7% 1 year and 12.9% 5 years after ART initiation. One year after ART initiation, the sensitivity of immunological criteria was 7% and specificity 12%; 5 years after ART initiation, the sensitivity and specificity were 26% and 45%, respectively. A resistance penalty factor was applied to increase the risk of failure depending on how long the patient had spent on a failing regimen or off ART. Parameters related to treatment interruptions and return to care with or without tracing were derived from data from Lighthouse and Martin Preuss Centre clinics in Lilongwe, Malawi [13]. Patients switched to second-line ART after either virological or immunological treatment failure, depending on the monitoring strategy.
Figure 1.
Schematic representation of the mathematical model. A, Flow of patients in the treatment model. White boxes represent stages with suppressed viral load, and gray boxes represent stages with continuously elevated viral load. “Discordant” immunological failure refers to a decline in CD4 cell count fulfilling the failure criteria under suppressed viral load; this condition will not reverse upon switch to second-line therapy. The flow described on the upper half is applicable to patients on ART, including those who returned after ART interruption. While progressing along the stages of treatment response (upper graph), the patients may also interrupt and restart treatment or die (lower graph). B, Transmission model. The upper graph shows the course of the HIV infection, and the lower graph the flow through age, sex, and risk group. Black arrows show flows between compartments, and gray lines show sexual contact patterns. Abbreviation: ART, antiretroviral therapy.
The output of the simulations is a matrix of the entry times to all states for each patient. For each simulated patient, we sampled the VL values for different stages (virologically successful, failing, or off ART). We then calculated the mean log10 viral load over time in each strategy to inform the transmission model [12]. We ran the model for the following nine strategies:
i. no VL monitoring, no tracing;
ii. no VL monitoring, active tracing;
iii. no VL monitoring, uninterrupted treatment;
iv. VL monitoring at 24-month intervals, no tracing;
v. VL monitoring at 24-month intervals, active tracing;
vi. VL monitoring at 24-month intervals, uninterrupted treatment;
vii. VL monitoring at 12-month intervals, no tracing;
viii. VL monitoring at 12-month intervals, active tracing;
ix. VL monitoring at 12-month intervals, uninterrupted treatment.
“No VL monitoring” means that treatment failure is again determined by clinical symptoms and CD4 counts only. The WHO recommends VL monitoring at 12-month intervals, but VL monitoring at 24-month intervals is current practice in Malawi. Treatment failure is confirmed 3 months after detection, and the patient is switched to second-line therapy after a random delay. In strategies with no tracing, patients LTFU may only return spontaneously. With active tracing, all patients LTFU are traced 3 weeks after missing their appointment and return to care at a higher rate than without tracing [16]. Strategies with uninterrupted treatment represent the ideal but unrealistic scenario that treatment is never interrupted. This scenario sets the theoretical limit for improving retention. In all scenarios, we assumed that patients retained in care adhered to treatment, as in the data that were used for parameterization [12, 13, 19, 20].
Transmission Model
We developed a deterministic transmission model (“transmission model”) to represent the HIV epidemic in Malawi between 1975 and 2050. The model consists of 40 compartments (Figure 1B) representing HIV status (susceptible; primary, asymptomatic chronic, or symptomatic chronic infection; ART; AIDS), age (children <15 years, adults 15–49 years, adults ≥50 years), sex (not distinguished for children), and risk behavior (high or low, except for children, older adults, and AIDS patients). “Acute infection” represents early HIV infection, when the risk of onward transmission is highest [21]. During “asymptomatic chronic infection,” CD4 cell counts are expected to be >350 cells/μL. In “symptomatic chronic infection,” CD4 cell count is below 350 cells/μL and the patient is in WHO clinical stage ≥2. Although there is no strict dependency between symptoms and clinical stage, these definitions roughly correlate [22]. “ART” represents patients who ever started ART, including also patients who interrupted ART. “AIDS” represents the last year of untreated HIV, where the patient has a CD4 cell count below 200 cells/μL or severe AIDS-defining opportunistic infections.
The model is solved numerically. We calculated infectiousness from the estimated per-act transmission probability and assumed frequency of unprotected sex acts (Table 1). Infectiousness was multiplied by 20 during the acute stage, and by a factor that depended on the monitoring and retention strategy during ART. This factor was estimated for each scenario from the treatment model. We also allowed infectiousness to decrease over time. The progression of patients across the stages of HIV was estimated from the literature.
Table 1.
Prior Parameter Values of the Transmission Model: Fixed Parameters With Identical Values in All Scenarios and Strategies
| Demographic Parameters | Value | Source |
|---|---|---|
| Birth rate, default value,a y-1 | 0.16 | [24] |
| Non-HIV related mortality: children aged <15 y, y-1 | 0.0142 | [25] |
| Non-HIV related mortality: males aged 15–<50 y, y-1 | 0.0059 | [25] |
| Non-HIV related mortality: females aged 15–<50 y, y-1 | 0.0051 | [25] |
| Non-HIV related mortality: males aged 50 y and above, y-1 | 0.0478 | [25] |
| Non-HIV related mortality: females aged 50 y and above, y-1 | 0.0422 | [25] |
| Mixing and sexual behavior | ||
| Proportion of young males engaging in high-risk behavior | 0.10 | Assumption |
| Proportion of young females engaging in high-risk behavior | 0.05 | Assumption |
| Mean duration of high-risk behavior among males, y | 25 | Assumption |
| Mean duration of high-risk behavior among females, y | 10 | [26, 27], assumption |
| Mean number of unprotected sex acts/y with regular partner | 50 | Assumption |
| Mean number of unprotected sex acts/y with casual partners: low-risk individuals | 1 | Assumption |
| Mean number of unprotected sex acts/y with casual partners: high-risk individuals | 100 | Assumption |
| Mixing (proportion of casual partners sampled exclusively from own risk group) | 0.5 | Assumption |
| Sexual transmission | ||
| Per-act transmission probability, male-to-female (chronic untreated infection), default valuea | 0.00155 | [2] |
| Per-act transmission probability, female-to-male (chronic untreated infection), default valuea | 0.00079 | [2] |
| Risk ratio for transmission probability during acute infection | 20 | [28] |
| Mother-to-child transmission | ||
| Probability of mother-to-child transmission if the mother is acutely infected | 0.313 | [29], assumption |
| Probability of mother-to-child transmission if the mother is chronically infected | 0.250 | [29, 30] |
| Probability of mother-to-child transmission if the mother is treated | 0.050 | [29], assumption |
| Natural progression of HIV | ||
| Mean duration of acute infection, y | 0.25 | [28] |
| Mean duration of asymptomatic stage, y | 4.8 | [31] |
| Mean duration of symptomatic stage before AIDS, y | 5.2 | [31] |
| HIV related mortality during symptomatic stage, y-1 | 0.1 | Assumption |
| HIV related mortality during AIDS, y-1 | 1 | |
| Treatment | ||
| Introduction of ART, y | 2003 | [23] |
| Eligibility at CD4 <350 cells/μL | 2011 | [23] |
| Universal ART eligibility | 2015 | |
| Introduction of “Option B+” | 2011 | [23] |
| Initial conditions in 1975b | ||
| Total population size | 5302000 | [32] |
| Male-to-female ratio among adults aged 15–<50 y | 1:1 | [32] |
| Male-to-female ratio among adults aged 50 y and above | 47:53 | [32] |
| Proportion of children aged <15 y | 0.469 | [32] |
| Proportion of people aged 50 y and above | 0.025 | [32] |
Abbreviation: ART, antiretroviral therapy; y, year.
aDefault value was adjusted during the calibration using a constant coefficient (Supplementary Table 5 for values).
bHIV prevalence and risk behavior in 1975 were determined in calibration (Supplementary Table 5).
We calculated the ART initiation rate at based on the diagnosis rate, availability, and eligibility criteria for ART and the expected progression of the patient’s CD4 cell count (Table 1). Antiretroviral therapy was generally unavailable until 2003, provided to symptomatic patients only between 2003 and 2015, and increasingly to asymptomatic patients after 2015. The rate of ART initiation among symptomatic patients increased in 2011 to match the change in CD4-based eligibility criteria (from <250 to <350 cells/μL). A separate ART initiation rate was applied to women from 2011 on to take into account the “Option B+” strategy of treating all pregnant and breastfeeding women [23].
We ran the simulation for 1975–2016 with the best available parameter estimates to calibrate the model and compared the model’s outputs with observed data, including total population size and number of patients on ART each year from 2010. We also compared our results to the estimates of the UNAIDS EPP/Spectrum model on prevalence and number of annual new infections. We then calibrated the following parameters: per-act transmission probability, birth rate, and year and magnitude of decrease in infectiousness.
We considered 3 possible scenarios for treatment access from 2017 (see Table 2 for input parameters):
Table 2.
Parameters of the Transmission Model: Parameters With Values Depending on Time Period and ART Initiation Scenario
| 2003–2010 | 2005–2014 | 2015–2016 | Failed Scale-up | Universal ART | Test&Treat | |
|---|---|---|---|---|---|---|
| Rate of starting ART, asymptomatic, adults | 0 | 0 | 0.5 | 0.1 | 0.5 | 1 |
| Rate of starting ART, symptomatic, adults | 0.05 | 2 | 1 | 1 | 1 | 1 |
| Rate of starting ART, asymptomatic, children | 0 | 10 | 10 | 10 | 10 | 10 |
| Rate of starting ART, symptomatic, children | 0.3 | 10 | 10 | 10 | 10 | 10 |
| Rate of starting ART due to PMTCT, asymptomatic, women | 0 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Rate of starting ART due to PMTCT, symptomatic, women | 0.15 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Rate of AIDS for patients on treatment | 0.05 | 0.05 | 0.05 | 0.01 | 0.01 | 0.01 |
All rates are per person-year.
Abbreviations: ART, antiretroviral therapy; PMTCT, prevention of mother-to-child transmission.
1) Failed scale-up: Recommendations to treat all PLHIV are not successfully implemented, and treatment remains restricted to the sickest patients. Women can start ART earlier because “Option B+” has already been implemented.
2) Universal ART: The policy introduced in 2015 continues, and ART can be initiated during the asymptomatic stage, although at a lower rate than in symptomatic patients because of barriers to testing.
3) Test&Treat: Intensive screening is added to the Universal ART scenario, and ART initiation rate increases.
We ran each of the 3 access scenarios for all 9 monitoring and retention strategies, (i)–(ix). We reported the number of expected new infections and AIDS-related deaths in 2020, 2030, and 2050 and calculated the relative reduction in new infections compared with strategy (i) of the corresponding access scenario for 2 time windows: 2017–2020 and 2017–2050.
Sensitivity Analyses
We conducted 2 sensitivity analyses to assess the impact of uncertainty around the parameters (Supplementary Table 3). In the first analysis, we assumed that the risk of virological failure would considerably increase over time. Second, we conducted an analysis where all-cause mortality was reduced from 2017 onwards, and treated patients could no longer proceed to AIDS.
RESULTS
The mean (log10 scale) viral load of the patients simulated in the treatment model for 10 years ranged from 63 to 104 copies/mL across the scenarios (Supplementary Table 4). The corresponding per-act transmission risk from patients who started ART was 14–17 times lower than the risk from chronically infected, untreated patients. The per-act transmission probability determined via calibration of the transmission model was 20% higher than the literature-based prior (Supplementary Table 5).
The transmission model’s pre-2017 results were in line with observed data and the UNAIDS EPP/Spectrum predictions. Prevalence among adults aged 15–49 years followed the upper limit of the UNAIDS estimates (Supplementary Figure 1) [14]. The total number of PLHIV in 2016 was about 10% lower than predicted by UNAIDS. The largest discrepancy was in annual new infections, which our model predicted to be about 20% higher until 2013. In the last few years, the new infections declined rapidly, going below the UNAIDS lower limit in 2016. According to our model, 695 000 patients were on ART in 2016, in line with observed estimates (680 000). In 2016, we predicted 23 100 AIDS-related deaths, whereas UNAIDS predicted 24 000.
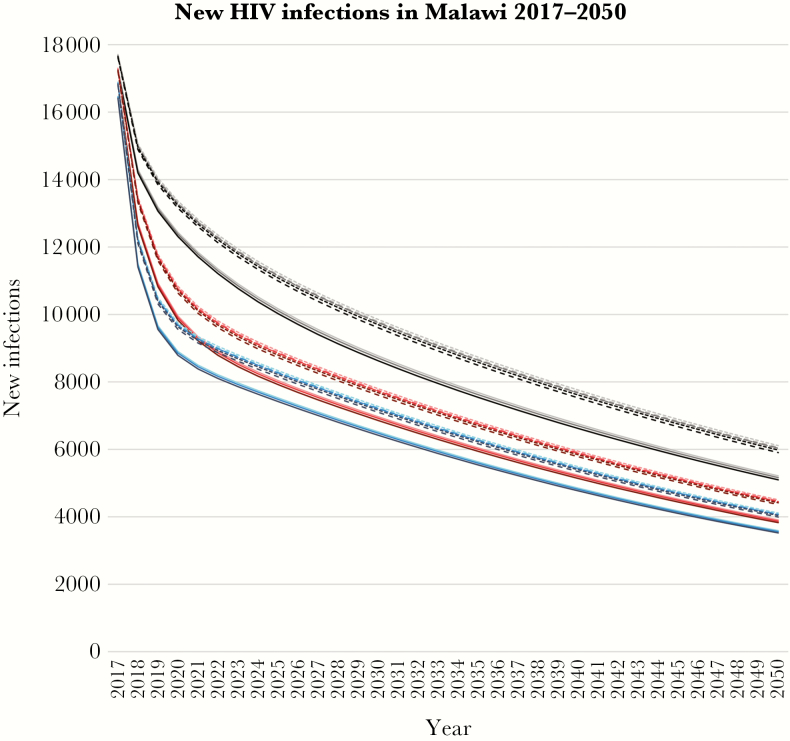
In all scenarios, prevalence continued to decline. Prevalence among adults aged 15–49 years was 7.8%–7.9% in 2020, 4.4%–4.8% in 2030, and 1.4%–1.7% in 2050, depending on the scenario (Supplementary Figure 2). The number of annual new infections also decreased rapidly, ranging 8800–13 400 in 2020, 6400–9900 in 2030, and 3500–6100 in 2050 across the scenarios (Figure 2, Table 3). The number of AIDS deaths was 12 000 in 2020, 7400–7900 in 2030, and 4600–5400 in 2050.
Figure 2.
Number of annual predicted new HIV infections in Malawi between 2017 and 2050. Gray/black, Failed scale-up; pink/red, Universal ART; blue, Test&Treat. Light color, no viral load monitoring; intermediate color, 24-monthly viral load monitoring; dark color, 12-monthly viral load monitoring. Dotted curves, no tracing; dashed curves, tracing patients lost to follow-up; solid curves, no treatment interruptions.
Table 3.
Number of Predicted HIV Infections in Malawi in Different Scenarios
| Failed Scale-up | Universal ART | Test&Treat | |||||||
|---|---|---|---|---|---|---|---|---|---|
| New Infections 2020 | Total New Infections 2017–2020 | Total New Infections 2017–2050 | New infections2020 | Total New Infections 2017–2020 | Total New Infections 2017–2050 | New Infections 2020 | Total New Infections 2017–2020 | Total New Infections 2017–2050 | |
| i) CD4 monitoring, no tracing | 13363 | 60182 (ref) | 326790 (ref) | infections | 53419 (ref) | 260908 (ref) | 9751 | 49422 (ref) | 241548 (ref) |
| ii) CD4 monitoring, tracing | 13314 | 60023 (0.3%) | 324965 (0.6%) | 2020 | 53262 (0.3%) | 259498 (0.5%) | 9705 | 49268 (0.3%) | 240265 (0.5%) |
| iii) CD4 monitoring, no interruptions | 12438 | 57218 (4.9%) | 293353 (10.2%) | 9955 | 50503 (5.5%) | 235083 (9.9%) | 8904 | 46553 (5.8%) | 218062 (9.7%) |
| iv) 24-m VL monitoring, no tracing | 13284 | 59927 (0.4%) | 323868 (0.9%) | 10762 | 53168 (0.5%) | 258650 (0.9%) | 9678 | 49175 (0.5%) | 239495 (0.9%) |
| v) 24-m VL monitoring, tracing | 13289 | 59942 (0.4%) | 324039 (0.8%) | 10766 | 53182 (0.4%) | 258782 (0.8%) | 9682 | 49190 (0.5%) | 239615 (0.8%) |
| vi) 24-m VL monitoring, no interruptions | 12384 | 57044 (5.2%) | 291420 (10.8%) | 9903 | 50331 (5.8%) | 233591 (10.5%) | 8854 | 46384 (6.1%) | 216705 (10.3%) |
| vii) 12-m VL monitoring, no tracing | 13232 | 59760 (0.7%) | 321959 (1.5%) | 10712 | 53003 (0.8%) | 257175 (1.4%) | 9630 | 49013 (0.8%) | 238153 (1.4%) |
| viii) 12-m VL monitoring, tracing | 13154 | 59511 (1.1%) | 319122 (2.3%) | 10638 | 52758 (1.2%) | 254983 (2.3%) | 9559 | 48772 (1.3%) | 236160 (2.2%) |
| ix) 12-m VL monitoring, no interruptions | 12312 | 56815 (5.6%) | 288888 (11.6%) | 9835 | 50106 (6.2%) | 231637 (11.2%) | 8789 | 46163 (6.6%) | 214927 (11.0%) |
Percentages in parentheses refer to the reduction compared with scenario (i) (first row) of the corresponding access scenario (Failed scale-up, Universal ART, or Test&Treat).
Abbreviations: ART, antiretroviral therapy; VL, viral load.
Of the factors that differed between scenarios, results were most sensitive to the overall treatment and testing scenario. In the Universal ART scenario, our model predicted 50 100–53 400 new infections between 2017 and 2020, or 231 600–260 900 between 2017 and 2050. In the Failed scale-up scenario, the ranges were 56 800–60 200 until 2020 (13% higher than with Universal ART) or 288 900–326 800 until 2050 (25% higher than with Universal ART). With Test&Treat, the ranges dropped to 46 200–49 400 until 2020 or 214 900–241 500 until 2050, about 7%–8% lower than with Universal ART.
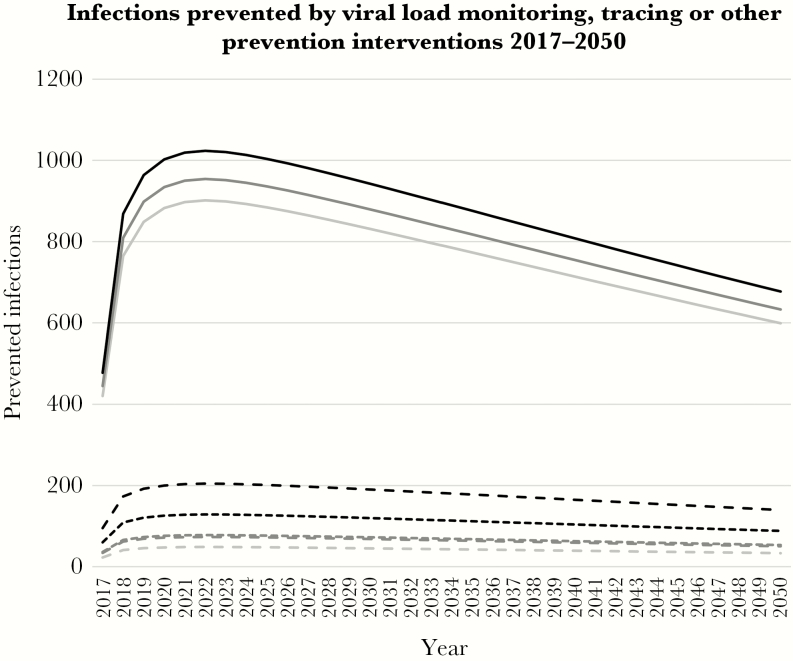
The differences between monitoring and retention strategies were smaller (Figure 3, Table 3). Assuming the Universal ART scenario, current retention, no tracing, and no VL monitoring, 53 400 patients were infected between 2017 and 2020, or 260 900 between 2017 and 2050. Monitoring VL at 24-month intervals lowered these numbers by 0.5% to 53 200 until 2020, or by 0.9% to 258 700 until 2050; and, at 12-month intervals, by 0.8% to 53 000 until 2020, or by 1.4% to 257 200 until 2050. The relative differences remained the same across all scenarios of treatment scale-up and retention/tracing.
Figure 3.
Number of annual new HIV infections prevented by routine viral load monitoring, tracing patients lost to follow-up, or other retention support interventions in the Universal ART scenario. Light gray, no viral load monitoring; dark gray, 24-monthly viral load monitoring; black, 12-monthly viral load monitoring. Dotted curves, no tracing; dashed curves, tracing patients lost to follow-up; solid curves, no treatment interruptions.
In the Universal ART scenario without viral load monitoring, actively tracing patients LTFU reduced the average number of new infections in 2017–2020 by 0.3% from 53 400 to 53 300, or in 2017–2050 by 0.5% from 260 900 to 259 500 (Figure 3, Table 3). When treatment interruptions were eliminated, the number of new infections decreased in 2017–2020 by 5.5% to 50 500, or in 2017–2050 by 9.9% to 235 100. The relative benefit of tracing and improved retention was similar across scenarios of treatment scale-up and monitoring.
The total number of PLHIV also decreased over time. In 2020, we predicted 839 200–849 500 PLHIV, and in 2050, 465 300–538 000 PLHIV. Because of the decreasing number of PLHIV, the total number of patients on treatment barely increased, even with accelerated ART scale-up, and started to decrease after 2019. In 2020, 778 100–794 900 patients were on ART, dropping in 2050 to 457 700–519 400. Decreased incidence and faster ART scale-up lowered the number of patients on ART, which, in 2050, was 8% higher with Failed scale-up, and 2% lower with Test&Treat, than with Universal ART.
Sensitivity Analyses
If the risk of virological failure increased over time, monitoring strategy no longer had an influence on the number of new infections (Supplementary Table 6). If we assumed lower mortality, the absolute number of new infections increased, but the relative benefit of the monitoring and retention support strategies did not differ from the main analysis (Supplementary Table 7).
DISCUSSION
In this modeling study, we found that the number of new HIV infections will likely continue to decrease rapidly in all testing, treatment, retention, and monitoring scenarios. The most important factors associated with the speed of decrease were the rates of ART initiation and treatment interruptions. VL monitoring and tracing lost patients reduced new infections, but only minimally. Interventions designed to keep patients in care without interruptions could be much more beneficial.
The results of VL monitoring were expected. In an earlier study, we predicted that routine VL monitoring could prevent up to one-third of transmissions from treated patients [12]. But the proportion of new infections attributable to patients on ART is low, so it is unsurprising that the overall effect of VL monitoring was small. Our results are in line with other modeling studies. For example, in 2014, Braithwaite et al. noted that VL monitoring was more cost-effective if its effect on transmission was considered, but extending ART eligibility, as the WHO recommended, had much more impact [33]. Some potential advantages we did not include in our model could also increase the benefit of VL monitoring. Patients whose ART is failing despite good adherence may believe they are not infectious, and thus be likely to engage in unprotected sex [34]. Patients on failing ART may also carry resistant strains of HIV. Their spread could limit the potency of available firstline regimens, increasing long-term mortality [35].
Loss to follow-up remains a major problem in sub-Saharan Africa. Although much LTFU can be explained by undocumented deaths and transfers between facilities, about one-third of lost patients have probably stopped treatment or are taking ART irregularly [16]. There is a broad range of reasons contributing to interrupting treatment [36]. Especially during the introduction of the Option B+, there were also concerns about retention rates among women who start ART in the asymptomatic stage. Experience from this program has, however, shown that long-term retention is feasible even among patients who start ART in an early stage of the infection [37]. In a prior study, we found that tracing lost patients did not substantially reduce expected transmission from patients who had started ART [13]. This study confirmed our finding. Tracing rarely locates patients who have moved or are traveling, or who provided an incorrect address or phone number. Many patients refuse to return to care, or interrupt treatment again later [16]. If treatment interruptions could be eliminated, the overall number of new infections would drop by 5% in the next few years, but perfect retention is unrealistic. Retention may be increased by further decentralizing treatment services, providing larger supplies of ART per visit, or SMS reminders [16, 38–40].
A recent survey found that, in Malawi, 89% of diagnosed PLHIV were on ART, but only 73% of all PLHIV had been diagnosed. Moreover, 91% of patients tested for VL in Malawi were virally suppressed [41]. Our results suggest that continuing screening to find more PLHIV in Malawi is the most effective strategy for meeting the ambitious 90-90-90 target. Our model predicted that, by 2020, in all scenarios, at least 90% of all PLHIV would be on ART. We may slightly overestimate the number of PLHIV on ART, as our estimate includes patients who have interrupted ART. But it is clear the 90-90-90 target can realistically be met in Malawi.
Cost-effectiveness calculations of VL monitoring and tracing patients LTFU should account for benefits to both individuals and the population. If VL test costs could be suppressed to $10 [10], testing each patient annually would cost about $6 million. This would prevent only about 100 new infections each year, thus costing about $60 000 to preventing a single infection. This is clearly more expensive than treating the infected patient for life. Annual VL testing may, however, also benefit the patient and reduce transmitted drug resistance. A more complete perspective is needed to assess cost-effectiveness.
Strengths and Limitations
We used a structurally simple, deterministic compartmental transmission model to produce results that closely match the data and projections returned by other established mathematical models. Our transmission model was informed by an individual-based simulation of the progression of HIV under ART, parameterized with routine cohort data. We expect that our results are generalizable to many Southern and Eastern African countries with similar epidemics and relatively high ART coverage [14, 41]. We also expect that the relative decrease in new infections with VL monitoring or tracing will be similar in other countries in this region. Our approach of linking a detailed simulation of disease progression to a relatively simple deterministic transmission model can also be adapted to other settings and questions.
Our study also had several limitations. The data we used to parameterize the model covered only a few years of follow-up and may thus not be accurate for long-term projections. We also did not consider other future changes in care, such as new antiretroviral regimens. New regimens may lead to substantially lower failure rates. If third-line ART becomes widely available in the future, it will likely further reduce the number of new infections, influencing also the relative benefit of the monitoring and retention interventions. Apart from a resistance penalty factor, first- and second-line failures were sampled independently, ignoring the possible individual factors that may result in failure through, for example, poor adherence. Several parameters had to be estimated using assumptions and calibration. We aimed to keep the transmission model as simple as possible and did not divide patients according to VL strata during treatment. Instead, we calculated average infectiousness for each stage of the disease, so our model may not be able to catch variation in infectiousness over time. We included only heterosexual transmission. Finally, we did not account for geographical variability, which can play a major role in the dynamics of the epidemic: district-level HIV prevalence in Malawi ranges from 3% in some northern districts to over 18% in the south.
There were also discrepancies between our and UNAIDS’ modeling estimates. In particular, our model estimated the annual new infections higher than UNAIDS. This resulted also in a slightly differing pattern in the total number of PLHIV. We predicted a moderate decrease since 2000, whereas UNAIDS suggests that the number remained stable or even increased slightly. However, the UNAIDS estimates are also based on models built on a limited amount of data. Our model’s projections on number of patients on ART matched closely with the reported data. However, our estimate also includes patients who have stopped ART.
Conclusions
Malawi’s response to the HIV epidemic has been successful, and we expect the number of new infections to continue to fall, regardless of the strategies of ART initiation, monitoring, and retention support. To reach the 90-90-90 goal, scaling-up ART and preventing treatment interruptions must be a priority. Though only a small number of infections can be prevented by viral load monitoring and tracing patients lost to follow-up, the population-level effects of these interventions should be included in future cost-effectiveness evaluations.
The substantial heterogeneity in the Malawian HIV epidemic across regions and population groups may require interventions targeted to specific groups or regions to increase treatment efficiency and acceptability. Test&Treat reduces transmission of HIV, despite individual cases of treatment failure and ART interruption, but the full benefits of the treatment as a prevention strategy cannot be reaped unless ART is improved with interventions that support retention and viral suppression.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Kali Tal for commenting on and editing the manuscript.
Financial support. This work was supported by the Swiss National Science Foundation (grant numbers PP00P3_163878, PDFMP3_137106).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cohen MS, Chen YQ, McCauley M et al. ; HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quinn TC, Wawer MJ, Sewankambo N et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. Accessed 31 October 2017.
- 4. Granich RM, Gilks CF, Dye C et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 5. Eaton JW, Johnson LF, Salomon JA et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 2012; 9:e1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwuji C, Orne-Gliemann J, Balestre E et al. The impact of universal test and treat on HIV incidence in a rural South African population: ANRS 12249 TasP trial, 2012–2016. Paper presented at: Programme and Abstracts of the 21st International AIDS Conference; 2016; Durban, South Africa FRAC0105LB. [Google Scholar]
- 7. Guy R, Wand H, McManus H et al. ; Australia HIV Observational Database (AHOD) and Treat Asia HIV Observation Database (TAHOD) Antiretroviral treatment interruption and loss to follow-up in two HIV cohorts in Australia and Asia: implications for ‘test and treat’ prevention strategy. AIDS Patient Care STDS 2013; 27:681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wanyama JN, Nabaggala MS, Wandera B et al. Significant rates of risky sexual behaviours among HIV-infected patients failing first-line ART: a sub-study of the Europe-Africa Research Network for the Evaluation of Second-line Therapy trial. Int J STD AIDS 2017; 29:287–97. [DOI] [PubMed] [Google Scholar]
- 9. Cohen MS, Smith MK, Muessig KE et al. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here?Lancet 2013; 382:1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis 2016; 62:1043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMahon JH, Elliott JH, Hong SY et al. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS One 2013; 8:e56047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Estill J, Aubrière C, Egger M et al. ; IeDEA Southern Africa Viral load monitoring of antiretroviral therapy, cohort viral load and HIV transmission in Southern Africa: a mathematical modelling analysis. AIDS 2012; 26:1403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Estill J, Tweya H, Egger M et al. Tracing of patients lost to follow-up and HIV transmission: mathematical modeling study based on 2 large ART programs in Malawi. J Acquir Immune Defic Syndr 2014; 65:e179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDSinfo Available at: aidsinfo.unaids.org. Accessed 31 October 2017.
- 15. Malawi Ministry of Health. Clinical Management of HIV in Children and Adults Available at: http://apps.who.int/medicinedocs/documents/s18802en/s18802en.pdf. Accessed 31 October 2017.
- 16. Tweya H, Feldacker C, Estill J et al. Are they really lost? “True” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in urban Malawi. PLoS One 2013; 8:e75761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blaser N, Salazar Vizcaya L, Estill J et al. Gems: an R package for simulating from disease progression models. J Stat Softw 2015; 64:1–22. [PMC free article] [PubMed] [Google Scholar]
- 18. Salazar Vizcaya L, Blaser N, Gsponer T. Gems: Generalized multistate simulation model Available at: http://cran.r-project.org/web/packages/gems/index.html. Accessed 31 October 2017.
- 19. Estill J, Egger M, Blaser N et al. ; IeDEA Southern Africa Cost-effectiveness of point-of-care viral load monitoring of antiretroviral therapy in resource-limited settings: mathematical modelling study. AIDS 2013; 27:1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Estill J, Egger M, Johnson LF et al. ; IeDEA Southern Africa Collaboration Monitoring of antiretroviral therapy and mortality in HIV programmes in Malawi, South Africa and Zambia: mathematical modelling study. PLoS One 2013; 8:e57611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powers KA, Ghani AC, Miller WC et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 2011; 378:256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edathodu J, Ali B, Alrajhi AA. CD4 validation for the World Health Organization classification and clinical staging of HIV/AIDS in a developing country. Int J Infect Dis 2009; 13:243–6. [DOI] [PubMed] [Google Scholar]
- 23. Harries AD, Ford N, Jahn A et al. Act local, think global: how the Malawi experience of scaling up antiretroviral treatment has informed global policy. BMC Public Health 2016; 16:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The World Bank. World Bank Open Data Available at: data.worldbank.org. Accessed 31 October 2017.
- 25. Lopez AD, Mathers CD, Ezzati M et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006; 367:1747–57. [DOI] [PubMed] [Google Scholar]
- 26. Elmes J, Nhongo K, Ward H et al. The price of sex: condom use and the determinants of the price of sex among female sex workers in eastern Zimbabwe. J Infect Dis 2014; 210(Suppl 2):S569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musyoki H, Kellogg TA, Geibel S et al. Prevalence of HIV, sexually transmitted infections, and risk behaviours among female sex workers in Nairobi, Kenya: results of a respondent driven sampling study. AIDS Behav 2015; 19(Suppl 1):S46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blaser N, Wettstein C, Estill J et al. Impact of viral load and the duration of primary infection on HIV transmission: systematic review and meta-analysis. AIDS 2014; 28:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Semba RD, Miotti PG, Chiphangwi JD et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. Lancet 1994; 343:1593–7. [DOI] [PubMed] [Google Scholar]
- 31. Wandel S, Egger M, Rangsin R et al. Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex Transm Infect 2008; 84(Suppl 1):i31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2010 revision Available at: http://www.un.org/en/development/desa/publications/world-population-prospects-the-2010-revision.html. Accessed 31 October 2017.
- 33. Braithwaite RS, Nucifora KA, Toohey C et al. How do different eligibility guidelines for antiretroviral therapy affect the cost-effectiveness of routine viral load testing in sub-Saharan Africa?AIDS 2014; 28(Suppl 1):S73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cassell MM, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention?BMJ 2006; 332:605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bertagnolio S, Perno CF, Vella S, Pillay D. The impact of HIV drug resistance on the selection of first- and second-line ART in resource-limited settings. J Infect Dis 2013; 207(Suppl 2):S45–8. [DOI] [PubMed] [Google Scholar]
- 36. Tweya H, Gugsa S, Hosseinipour M et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health 2014; 19:1360–6. [DOI] [PubMed] [Google Scholar]
- 37. Haas AD, Tenthani L, Msukwa MT et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV 2016; 3:e175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nhavoto JA, Grönlund Å, Klein GO. Mobile health treatment support intervention for HIV and tuberculosis in Mozambique: perspectives of patients and healthcare workers. PLoS One 2017; 12:e0176051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vogt F, Kalenga L, Lukela J et al. Brief report: decentralizing ART supply for stable HIV patients to community-based distribution centers: program outcomes from an urban context in Kinshasa, DRC. J Acquir Immune Defic Syndr 2017; 74:326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCormick NM, Li N, Sando D et al. Implementation and operational research: risk factors of loss to follow-up among HIV-positive pediatric patients in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr 2015; 70:e73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ICAP at Columbia University. PHIA Project Available at: http://phia.icap.columbia.edu/. Accessed 31 October 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.