Abstract
Small unruptured aneurysms are thought to have a low risk of rupture, but the management of such lesions is still controversial. A 73-year-old man with a small anterior communication artery aneurysm, 4 mm in diameter, while on follow-up, developed an aneurysmal subarachnoid hemorrhage 2 weeks after the detection of a newly emerged bleb on the surface of the aneurysm. In conclusion, the formation of a bleb should be considered as a warning sign of an impending rupture, and treatment should be provided even for patients with small aneurysms.
INTRODUCTION
Rupture of intracranial aneurysms causes aneurysmal subarachnoid hemorrhage (aSAH), resulting in high mortality and morbidity rates [1]. Due to the risk of complications, however, indications for treatment of unruptured intracranial aneurysms (UIAs) need to be carefully considered [2]. Some specialists state that UIAs require surgical treatment, regardless of their size or risk factors for rupture, as long as surgery is technically possible. Others, in contrast, assert that patients with a single UIA smaller than 5 mm in size, because of their very low rupture rate, should undergo observation [3]. Various risks of rupture, such as aneurysm size, location, shape, growth and ethnic factors, had been reported [3–6], but the indication for surgical treatment of UIAs is still debatable.
Here we report a case of a ruptured anterior communicating artery aneurysm, which was initially small in size, but later showed de novo bleb formation preceeding rupture.
CASE REPORT
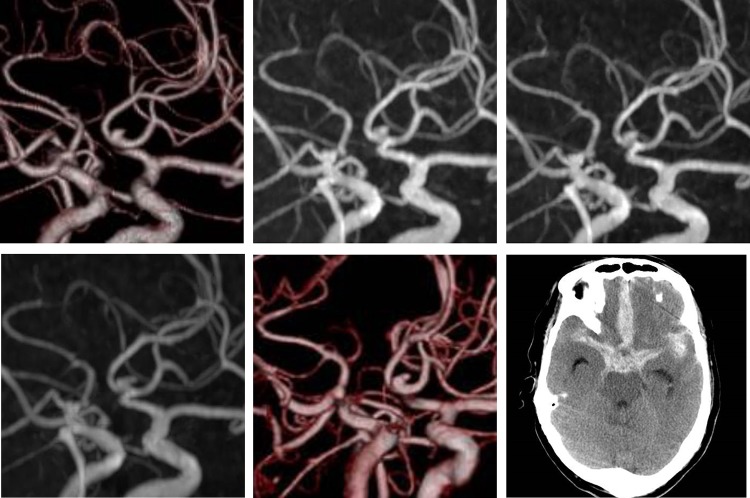
A 73-year-old man was diagnosed with a UIA of the anterior communicating artery (A-com), and had been followed up for 2 years at the outpatient department of the Institution. Computed tomography angiography (CTA) revealed an A-com UIA with a height of 2.7 mm and width of 2.1 mm (Fig. 1, upper-left). During the follow-up using magnetic resonance angiography (MRA), the UIA was considered to be stable (Fig. 1, upper-middle and right). Two years after the initial diagnosis, MRA demonstrated an irregular shape of the aneurysm (Fig. 1, lower-left), and further CTA examination revealed a newly emerged bleb on its surface (Fig. 1, lower-middle).
Figure 1:
Initial computed tomography angiogram of the unruptured cerebral aneurysm. A saccular aneurysm, 2.7 × 2.1 mm2 in size, is arising from the anterior communicating artery (upper-left). Follow-up magnetic resonance angiography. Images at 6 months (upper-middle) and 1 year (upper-right) after the initial diagnosis show the unchanged aneurysm. Two years after diagnosis, the shape of the aneurysm changed (lower-left). Computed tomography angiogram shows a de novo bleb (lower-middle). Computed tomography after the aneurysm rupture indicates aSAH (lower-right).
We considered the emergence of the bleb as a risk for early rupture, and planned the surgery. However, 2 weeks after the examination, while the patient was waiting for admission, he developed a sudden headache and was transported to our hospital. Computed tomograms taken on admission showed subarachnoid hemorrhage (Fig. 1, lower-right), and surgery was performed the same day. Surgical findings showed that the bleb was the rupture point. Postsurgical recovery was uneventful, but higher brain dysfunction remained.
DISCUSSION
The management of incidental small UIAs is controversial and many factors need to be considered in the decision-making process. We have already reported that even small aneurysms require treatment in certain cases [7]. Our case, in which rupture of the aneurysm occurred after the detection of the de novo bleb, suggests that bleb formation should be considered as a warning sing of an impending rupture.
Various studies have reported that size and location of the UIA are considered as risk factors of aneurysm rupture [3, 6]. Irregular aneurysm shape was also shown to be an independent risk factor [4, 6]. Even if the presence or development of a bleb in a UIA was suspected to carry risk of rupture [3, 4], the risk of a newly emerged bleb itself with subsequent aSAH, as in our case, has not been reported. Recent studies have shown some other factors pertaining to risk of UIA rupture. Inoue and colleagues reported that the annual rupture risk of growing UIAs was higher than that of nongrowing UIAs (18.5%) [8]. Other previous study demonstrated that the period of UIA growth carried a high rupture risk during conservative follow-up with CTA. Additionally, Sato and Yoshimoto [9] brought to light a discrepancy between etiologic data and clinical practice, showing that while small aneurysms theoretically carry a very low risk of rupture, small ruptured aneurysms are commonly observed. The authors suggested the presence of a ‘high-risk period’ after the formation of an intracranial aneurysm [9]. Taking the findings of these reports together, the ‘high-risk period’ seems to be the period after the formation of an aneurysm and during its growth. However, it is yet to be established whether growth or de novo bleb formation of UIAs contributes more to aneurysm rupture. In our case, the rupture occurred 2 weeks after the detection of the de novo bleb; therefore, we suggest that bleb formation in a UIA may also signal the beginning of a ‘high-risk period’ for subsequent rupture.
According to the American Heart Association/American Stroke Association guidelines, several factors, including patient age and aneurysm location and size, should be taken into account when considering surgical clipping as the mode of treatment for UIA. Although small UIAs are reported to carry low risk of rupture [3, 6], the majority of patients with aSAHs have aneurysms with a diameter of <10 mm [10]. This suggests the necessity of treatment for small but high-risk UIAs, ergo it is necessary to determine authentic risk factors for treatment indication.
In a previous report we suggested the importance of appropriate timing for treatment of small asymptomatic UIAs from the viewpoint of functional recovery [7]. Preventive surgery might be considered in selected young patients in cases of certain ‘higher-risk’ aneurysm locations, expanding aneurysms, or in patients with a family history of aneurysmal hemorrhage, as well as in those who cannot continue their normal lives knowing that they harbor a UIA. We suggest that aneurysms with a de novo bleb formation should also be included in this list. Indication for treatment of small aneurysms is still a difficult issue, but bleb formation could contribute to the treatment decision-making process.
In conclusion, we should consider multiple factors to assess the risk of UIA rupture, since prophylactic treatment has non-negligible complications. The formation of a de novo bleb in a UIA could be a predictor of early rupture; hence, treatment should be considered following bleb formation in small UIAs.
ACKNOWLEDGEMENTS
We thank Alexander Zaboronok, MD, PhD, Assistant Professor of the Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, for critical revision of the article and helpful suggestions, and Thomas Mayers, Medical English Communications Center, Faculty of Medicine, University of Tsukuba, for native English revision.
CONFLICT OF INTEREST STATEMENT
All authors have no conflict of interest, and have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
REFERENCES
- 1. Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 2011;10:349–56. [DOI] [PubMed] [Google Scholar]
- 2. Wiebers DO, Whisnant JP, Huston J III, Meissner I, Brown RD Jr, Piepgras DG, et al. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103–10. [DOI] [PubMed] [Google Scholar]
- 3. Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVestudy, Japan. Stroke 2010;41:1969–77. [DOI] [PubMed] [Google Scholar]
- 4. Lindgren AE, Koivisto T, Björkman J, von Und Zu Fraunberg M, Helin K, Jääskeläinen JE, et al. Irregular shape of intracranial aneurysm indicates rupture risk irrespective of size in a population-based cohort. Stroke 2016;47:1219–26. [DOI] [PubMed] [Google Scholar]
- 5. Murayama Y, Takao H, Ishibashi T, Saguchi T, Ebara M, Yuki I, et al. Risk analysis of unruptured intracranial aneurysms: prospective 10-year cohort study. Stroke 2016;47:365–71. [DOI] [PubMed] [Google Scholar]
- 6. UCAS Japan Investigators. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012;366:2474–82. [DOI] [PubMed] [Google Scholar]
- 7. Yanaka K, Matsumaru Y, Mashiko R, Hyodo A, Sugimoto K, Nose T. Small unruptured cerebral aneurysms presenting with oculomotor nerve palsy. Neurosurgery 2003;52:553–7. [DOI] [PubMed] [Google Scholar]
- 8. Inoue T, Shimizu H, Fujimura M, Saito A, Tominaga T. Annual rupture risk of growing unruptured cerebral aneurysms detected by magnetic resonance angiography. J Neurosurg 2012;117:20–5. [DOI] [PubMed] [Google Scholar]
- 9. Sato K, Yoshimoto Y. Risk profile of intracranial aneurysms: rupture rate is not constant after formation. Stroke 2011;42:3376–81. [DOI] [PubMed] [Google Scholar]
- 10. Kassell NF, Torner JC. Size of intracranial aneurysms. Neurosurgery 1983;12:291–7. [DOI] [PubMed] [Google Scholar]