Abstract
Background
Silicon (Si) is known to have numerous beneficial effects on plants, alleviating diverse forms of abiotic and biotic stress. Research on this topic has accelerated in recent years and revealed multiple effects of Si in a range of plant species. Available information regarding the impact of Si on plant defence, growth and development is fragmented, discipline-specific, and usually focused on downstream, distal phenomena rather than underlying effects. Accordingly, there is a growing need for studies that address fundamental metabolic and regulatory processes, thereby allowing greater unification and focus of current research across disciplines.
Scope and Conclusions
Silicon is often regarded as a plant nutritional ‘non-entity’. A suite of factors associated with Si have been recently identified, relating to plant chemistry, physiology, gene regulation and interactions with other organisms. Research to date has typically focused on the impact of Si application upon plant stress responses. However, the fundamental, underlying mechanisms that account for the manifold effects of Si in plant biology remain undefined. Here, the known effects of Si in higher plants relating to alleviation of both abiotic and biotic stress are briefly reviewed and the potential importance of Si in plant primary metabolism is discussed, highlighting the need for a unifying research framework targeting common underlying mechanisms. The traditional approach of discipline-specific work on single stressors in individual plant species is currently inadequate. Thus, a holistic and comparative approach is proposed to assess the mode of action of Si between plant trait types (e.g. C3, C4 and CAM; Si accumulators and non-accumulators) and between biotic and abiotic stressors (pathogens, herbivores, drought, salt), considering potential pathways (i.e. primary metabolic processes) highlighted by recent empirical evidence. Utilizing genomic, transcriptomic, proteomic and metabolomic approaches in such comparative studies will pave the way for unification of the field and a deeper understanding of the role of Si in plants.
Keywords: Abiotic stress, biotic stress, herbivory, omics, pathogens, primary metabolism
INTRODUCTION
The many benefits of silicon (Si) to plants are now well recognized (Epstein, 2009; Debona et al., 2017). As the eighth most abundant element in the universe and second most abundant in the Earth’s crust, Si is not lacking in quantity (Epstein, 1999); yet plant-available forms of Si can be limiting (Savant et al., 1997). As early as the 19th century the importance of Si was apparent to botanists (de Saussure, 1804; Hall and Morison, 1906; Guntzer et al., 2012). Despite the fact that Si is now recognized as a beneficial nutrient (IPNI, 2015), it is deemed to be non-essential to plant growth (Richmond and Sussman, 2003).
Recent experimentation has demonstrated that Si exhibits extraordinary effects on plant growth and development unequalled by any other non-essential plant nutrient, highlighting the current underappreciation of this ubiquitous element (Detmann et al., 2012; Van Bockhaven et al., 2015b). In 1969, Lewin and Reimann suggested that Si played an important metabolic role in living organisms due to its relative abundance in nature. Epstein (1994) further argued that Si played a key role in plant growth, mechanical strength and resistance to pathogens and herbivory, and as such merited greater awareness of its role in plant biology. Further, Epstein (2009) pointed out that most plants contain substantial amounts of Si, suggesting this is unlikely to be a product of stochastic nutrient uptake, just as evolutionary processes have selected for uptake of other elemental nutrients, such as potassium (K). Moreover, Si uptake by plants can be adaptive, in response to adverse environmental conditions, be they abiotic or biotic (Hartley, 2015), and as such is not necessarily essential, but arguably fundamental.
Although the essentiality of this element to plants is still debated, there have been significant advances in our understanding of the uptake of Si in higher plants. The comprehensive compilation of the Si concentrations within 735 plant species facilitated the assessment of a given species’ ability to absorb Si (Hodson et al., 2005). The identification of Si transporters in rice (Oryza sativa) (Ma et al., 2006) (Fig. 1) and subsequently in a variety of other species, including maize (Zea mays) (Mitani et al., 2009), barley (Hordeum vulgare) (Chiba et al., 2009), wheat (Triticum aestivum) (Montpetit et al., 2012), horsetail (Equisetum arvense) (Grégoire et al., 2012), pumpkin (Cucurbita moschata) (Mitani et al., 2011) and soybean (Glycine max) (Deshmukh et al., 2013; Deshmukh and Bélanger, 2015; Ma and Yamaji, 2015), has facilitated greater knowledge of the uptake and transport of Si within plant tissues, as there is significant variation in Si uptake between plant species, and indeed between cultivars. Plants are typically characterized as hyper-accumulators, accumulators, passive and non-accumulating species. Additionally, our awareness of the beneficial effects of supplementing plants with soluble forms of Si has evolved considerably, particularly in regard to plant resistance to stress (Debona et al., 2017), yet relatively few studies have explored whether Si may play an important role in plant primary metabolism.
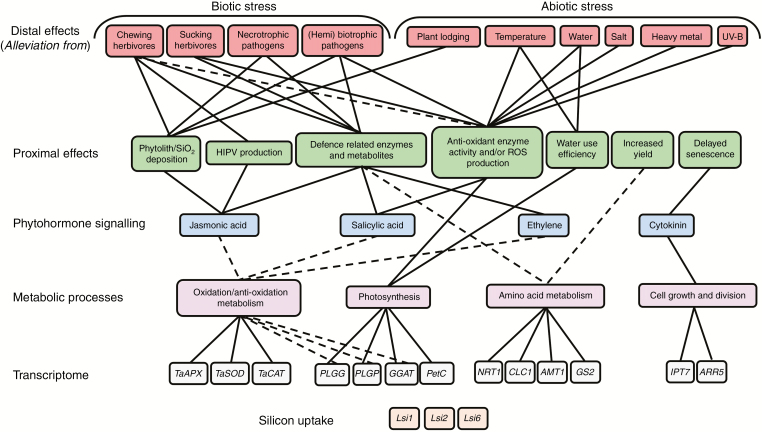
Fig. 1.
A hierarchical summary of the major distal effects of silicon (Si) on abiotic and biotic plant stresses and some of the progressively more fundamental proximate and underlying phenomena associated with stress alleviation. Effects on the transcriptome highlight examples of genes (associated with metabolic processes indicated) with altered transcription in response to Si (e.g. regulation of TaAPX, a gene involved in oxidation metabolism, has been shown to be altered by Si). Identified silicon transporter genes essential for silicon uptake in plants are also shown (Lsi1, Lsi2, Lsi6). Solid lines show linkages for which there is empirical support and dashed lines show potential links or interactions.
Research into the effects of Si on plant resistance to abiotic (Cooke and Leishman, 2016) and biotic (Fauteux et al., 2005; Reynolds et al., 2009, 2016) stress has improved our knowledge of the impacts of Si application on plants at the molecular (Ma and Yamaji, 2015), physiological (Detmann et al., 2012) and ecological (Cooke et al., 2016) levels. Interest in this research topic is apparent in the number of recent review articles published on the alleviation of plant stress by Si (Cooke and Leishman, 2016; Coskun et al., 2016; Imtiaz et al., 2016; Reynolds et al., 2016; Debona et al., 2017; Kim et al., 2017; Luyckx et al., 2017; Sakr, 2017; Etesami and Jeong, 2018). Yet none of these draw sufficient attention to the potential for Si to interact with fundamental plant metabolic processes. Indeed, studies performed have typically considered species-specific and focused aspects of Si–plant stress interactions. Therefore there is an increasingly important knowledge gap regarding the underlying factors associated with the disparate effects of Si. In this review, we highlight the gap in our fundamental understanding by briefly summarizing the proposed mechanisms by which Si increases plant resistance to, and alleviation of, stress, and also review the evidence for a role of Si in plant growth and metabolism. While individual studies have added to the body of knowledge of the broader impacts of Si on plant development and defence, including interactions with pathogens and herbivores, the mechanisms underpinning these effects remain little understood. We propose a broad framework for the study of the effects of Si in plants, and highlight how research can progress to achieve a unified understanding of the role of Si in plant biology.
MECHANISMS ALLEVIATING ABIOTIC STRESS
The application of Si has been shown to alleviate the negative effects of numerous abiotic stresses, including salt, water, heat, cold, UV-B, heavy metals and mechanical stress (lodging) (Fig. 1). The recent meta-analysis by Cooke and Leishman (2016) on the alleviation of abiotic stress by Si highlighted that most studies have focused on single species and single-stress models. Still, the authors found overall consistency in responses across plant families and stress types, but there was a lack of studies which looked to compare effects between species or stress types. Several potential mechanisms associated with stress alleviation in higher plants have been identified: increased structural reinforcement (Meunier et al., 2017), altered photosynthetic rate (Perez et al., 2013; Mihalicová Malcovská et al., 2014; Sanglard et al., 2014; Rahman et al., 2015; Kang et al., 2016), changes in stomatal conductance (Hattori et al., 2005) and enhanced water use efficiency (Kurdali and Al-Chammaa, 2013). Other possible mechanisms may also include osmotic adjustments through increased water potential and water content (Gong and Chen, 2012; Ming et al., 2012), reductions in oxidative stress (Shen et al., 2010; Ali et al., 2016; Kim et al., 2017), alterations in mineral uptake and accumulation (Li et al., 2015; Pavlovic et al., 2016) and alterations to phytohormone concentrations (Hamayun et al., 2010; Kim et al., 2014) (Fig. 1). Concerning the latter, the ethylene phytohormonal pathway was recently shown to be implicated in the alleviation of salt stress by Si through reduced oxidative damage (Liang et al., 2015). Significantly, without ethylene, Si not only failed to enhance plant resistance to salt stress but caused an increase in oxidative damage and cell death.
Reduction of oxidative damage via decreased production of reactive oxygen species (ROS) and/or increased activity of antioxidant metabolism appears to play an important role in Si-induced abiotic stress alleviation (Zhu et al., 2004; Liang et al., 2008; Miao et al., 2010; Ali et al., 2016; Kim et al., 2017). Reactive oxygen species (e.g. O2−, H2O2) are derivatives of oxygen that are highly reactive and lead to oxidative destruction of cells. The production of these destructive molecules increases when plants are exposed to stress. Van Bockhaven et al. (2013) suggest the importance of the photorespiration component of photosynthesis, alongside oxidation/antioxidation metabolism, to Si-induced stress tolerance. The activity of photorespiration and antioxidative enzymes as well as expression of key photorespiratory genes have been linked to increased abiotic stress tolerance (Romero-Puertas et al., 2007; Rojas et al., 2012). Moreover, evidence suggests that Si-enhanced abiotic stress tolerance is linked to accumulation of photorespiratory enzymes (Nwugo and Huerta, 2011). Of course Si not only acts on C3 plants (where photorespiration is a major component of the photosynthetic processes) (Vaculík et al., 2012; Frew et al., 2016a), highlighting that if Si-driven photorespiration is a mechanism, it may not necessarily be a comprehensively unifying one. Nevertheless, this suggests Si may impact primary metabolic processes in higher plants, rather than its role being confined solely to plant responses to external stress.
The role of plant antioxidant metabolism in Si-enhanced abiotic stress alleviation has also been shown at the transcriptomic level. Ma et al. (2016) found Si reduced H2O2 accumulation and increased expression of antioxidant enzyme genes (e.g. TaSOD, TaCAT) in wheat under drought stress (Fig. 1). Similarly, using targeted transcriptomic profiling, Farooq et al. (2016) observed that Si treatment increased the antioxidant capacity of rice plants under cadmium stress. Recent experimentation has assessed the impact of Si on plants subject to abiotic stress at the level of gene expression (Khattab et al., 2014; Liu et al., 2014; Yin et al., 2016), and it is now becoming apparent that Si may impact primary metabolism in higher plants (Detmann et al., 2012; Sanglard et al., 2014), but use to date of transcriptomics has been limited. The use of untargeted transcriptomics and metabolomics in future studies is likely to provide important insights into underlying mechanisms of stress alleviation.
MECHANISMS ALLEVIATING BIOTIC STRESS
Just as plants must contend with numerous abiotic stressors, so too are they subject to stress from other organisms. Supplementing plants with Si has been shown to increase plant resistance to mammalian, arthropod and molluscan herbivores, fungal and bacterial pathogens, viruses and nematodes (Griffin et al., 2015; Rodrigues et al., 2015; Reynolds et al., 2016). One of the earliest mechanisms identified and associated with plant resistance to pests is the physical defence conferred by Si deposition in plant tissues in the form of phytoliths (largely composed of SiO2) (McNaughton and Tarrants, 1983; Katz, 2015). Plants translocate Si from the soil solution as monosilicic acid, which naturally polymerizes to form phytoliths, which are irreversibly deposited within the plant (Epstein, 1994; Ma and Yamaji, 2015). The deposition of phytoliths increases plant rigidity and physical toughness (tensile strength) (Massey et al., 2007b) and acts as a physical barrier to fungal penetration (Kim et al., 2002). Silicon deposition can also wear down the feeding mouthparts, or mandibles, of insects (Kvedaras et al., 2009; Massey and Hartley, 2009; Jeer et al., 2017), reduce plant digestibility to both insect and mammalian herbivores (Massey et al., 2006; Massey and Hartley, 2006; Frew et al., 2016b), adversely impact herbivores (reducing growth and consumption rates) and also reduce predatory behaviour towards prey fed on high-Si diets (Ryalls et al., 2017). Importantly, silicification of plant tissue is inducible, with more heavily attacked plants typically accumulating more Si (McNaughton and Tarrants, 1983; Massey et al., 2007a; Hartley and DeGabriel, 2016). Nevertheless, Si also acts via mechanisms other than increased physical defences.
Silicon also affects the concentrations of an array of metabolites related to plant defence (Chérif et al., 1992; Rémus-Borel et al., 2005; Rahman et al., 2015; Debona et al., 2017), including increased defence enzyme activities (e.g. chitinase, β-1,3-glucanase, phenylalanine ammonia-lyase, polyphenol oxidase), in a number of plant–pathogen systems, including necrotrophic, biotrophic and hemibiotrophic pathogens (the latter two hereafter referred together as (hemi)biotrophic) (Chérif et al., 1994; Liang et al., 2005; Cai et al., 2008). For example, Si-induced increases in flavonoids, peroxidases and chitinase have been observed in response to some necrotrophic pathogens (Chérif et al., 1994; Fortunato et al., 2013).
Interactions between Si and plant defence signal transduction pathways, specifically the key phytohormone signalling pathways, have recently been investigated. Upon attack or infection, plants typically produce a complex and specific blend of salicylic acid (SA) (generally associated with (hemi)biotrophic pathogens), jasmonic acid (JA) (which is associated with necrotrophic pathogens and insect herbivores) and ethylene (which is typically regarded as ‘fine-tuning’ the JA defence response) (Glazebrook, 2005; Wu and Baldwin, 2010).
Just as phytohormone signalling is critical to Si-enhanced resistance to abiotic stress (Liang et al., 2015), phytohormones have been shown to be critical to Si-mediated plant resistance to biotic stress. By using JA-deficient rice mutants, Ye et al. (2013) identified that the JA pathway was critical for Si-enhanced resistance to insect herbivores. This hypothesis was further supported by several additional studies investigating the ability of Si to enhance JA-dependent defence mechanisms, including indirect attraction of insect herbivore natural enemies (Kvedaras et al., 2010) by altering the composition of the herbivore-induced plant volatiles (HIPVs) produced under herbivore attack (Liu et al., 2017). Vivancos et al. (2015) found that although Si upregulated SA-dependent defence genes upon infection from a biotrophic fungal pathogen, induction of the SA pathway was not necessary for Si to enhance resistance. Interestingly, Van Bockhaven et al. (2015a) observed that the effects of Si in enhancing resistance to a necrotrophic fungal pathogen (Cochliobolus miyabeanus) were independent of both the JA and SA pathways. Rather, they suggested that Si prevented the pathogen from hijacking the plant ethylene pathway such that Si application deactivated pathogen ethylene production. Together these findings suggest that Si plays a complex but critical role in alleviation of plant biotic stress via effects on multiple phytohormone signalling pathways (Fig. 1).
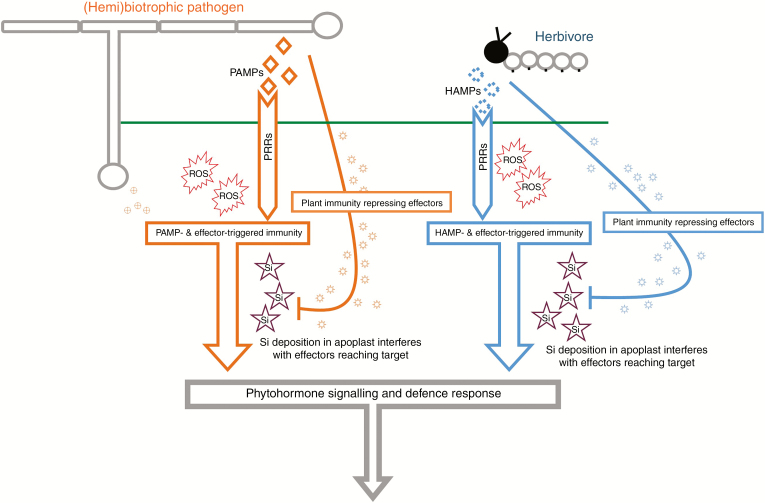
Silicon may also play a role in overcoming the ability of some (hemi)biotrophic pathogens and insects to supress plant-induced defences. The activation of defence phytohormone signalling is stimulated upon plant recognition of a biotic threat. This normally occurs via recognition of conserved molecular patterns, which vary depending on the biotic threat, e.g. pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs) and herbivore-associated molecular patterns (HAMPs) (Dodds and Rathjen, 2010; Erb et al., 2012). Recognition of these molecules by pattern recognition receptors (PRRs), often in concert with recognition of other pathogen/insect effector proteins, can trigger the plant defence response (known as PAMP-triggered immunity [PTI] or effector-triggered immunity [ETI]) appropriate to the biotic challenge (Boller and Felix, 2009; Erb et al., 2012). Although necrotrophic pathogens are not known to produce effector proteins (Glazebrook, 2005), both (hemi)biotrophic pathogens and herbivores are known to produce effector proteins that are able to suppress PTI and ETI, effectively suppressing the plant immune response (Musser et al., 2002; Giraldo and Valent, 2013). Vivancos et al. (2015) hypothesized that Si deposition in the plant apoplast is likely to interfere with (hemi)biotrophic pathogen effectors reaching their target sites, thereby preventing the pathogen from inhibiting the plant defence response. This hypothesis could also apply to insect herbivores, as they produce similar effector proteins (Hogenhout and Bos, 2011). Thus, Si potentially overcomes plant defence suppression, allowing a full defence response to be initiated upon recognition of a biotic threat (Fig. 2). Significantly, in terms of seeking some unification of effects across biotic stressors, one of the earliest cellular responses following recognition of PAMPs or HAMPs is ROS production.
Fig. 2.
Simplified summary of plant recognition of biotic stressors, (hemi)biotrophic pathogens and insect herbivores, and the subsequent cascade of events leading to plant-induced defence response. Pathogen- and herbivore-associated molecular patterns (PAMPs and HAMPs) are recognized by pattern recognition receptors (PRRs). Reactive oxygen species (ROS) are generated upon recognition of PAMPs and HAMPs. Recognition of PAMPs and HAMPs, in conjunction with recognition of other endogenous pathogen/herbivore-produced effectors, leads to PAMP/HAMP-triggered immunity and effector-triggered immunity (ETI), which induces phytohormone signalling to produce an induced plant defence response. (Hemi)biotrophic pathogens and insects can produce effectors that suppress ETI, inhibiting the plant defence response. Deposition of Si in the apoplast potentially interferes with immunity-repressing effectors reaching their target sites, thereby allowing the plant to mount an induced defence response to combat the biotic threat (Vivancos et al., 2015).
As with Si-induced alleviation of abiotic stress, ROS and enhanced antioxidant metabolism is a commonly reported mechanism through which Si is proposed to act to alleviate biotic stresses (Van Bockhaven et al., 2013). This occurs via reduction of oxidative damage to the plant by enhancement of antioxidant metabolism and/or ROS-initiated defence responses (Domiciano et al., 2015; Yang et al., 2017). Generation of ROS and antioxidant metabolism have been associated with pathogen (bacterial and fungal) infection (Debona et al., 2014; Domiciano et al., 2015) and in response to damage from chewing and sucking insects (Han et al., 2016; Yang et al., 2017). Formation of ROS can negatively and directly impact biotic stressors (Ramputh et al., 2002); however, ROS have a variety of signalling roles in various defence signalling pathways with phytohormones, including JA and SA (Leon et al., 1995; Glazebrook, 2005; Torres, 2010). Furthermore, ROS can also activate plant defence genes and the associated accumulation of defence metabolites, including phytoalexins and allelochemicals (Thoma et al., 2003).
Van Bockhaven et al. (2013, 2015a, b) suggested that primary plant metabolism, specifically photorespiration and the production of ROS, plays an important role in the broad-spectrum effects of Si on biotic stress alleviation, although these roles are currently not clearly defined. These authors also highlighted that for diatoms (algal phytoplankton) Si is essential for life processes, including DNA replication (Okita and Volcani, 1978; Martin-Jézéquel et al., 2000), and that combining knowledge on how Si interacts with cellular metabolism in algae and other primitive plants may therefore provide important insight into the role of Si in angiosperms. The nature of ROS production as a by-product of fundamental life processes and the implication of an interaction of Si with oxidation/antioxidant metabolism in numerous studies relating to both abiotic and biotic plant stresses suggest this is a promising avenue of research towards determination of the fundamental role(s) of Si in higher plants.
The utilization of transcriptomic techniques such as microarrays alongside more targeted assays such as real-time quantitative PCR (qPCR) are critical in developing an understanding of how Si impacts plant gene expression. Fauteux et al. (2006) found that pathogen infection upregulated defence genes and downregulated primary metabolism genes, but following the application of Si downregulated genes were not as severely impacted, while they found little evidence to suggest an impact of Si without pathogen stress. Similarly, Chain et al. (2009) and Van Bockhaven et al. (2015b) found that Si application nearly eradicated the effects of pathogen stress on the plant transcriptome. In contrast with studies related to pathogen stress, where some transcriptomic work has been reported, limited research has evaluated the impact of Si on enhanced plant resistance to insect herbivores. Therefore, further studies on the interactions of Si with the transcriptome of different plant species varying in their Si uptake ability (e.g. accumulators, non-accumulators) under different forms of insect herbivory (e.g. chewers, suckers) should provide valuable insight into how Si alters plant gene expression in response to insect stressors, and help unify this branch of the field with plant pathology.
EFFECTS OF SILICON ON UNSTRESSED PLANTS
The effects of Si on the alleviation of abiotic and biotic stress are now well recognized, but it was generally considered that Si had little or no effect on plant metabolism in unstressed situations (Ma, 2004). That view may no longer be empirically supported and this has profound implications for the effects of Si on plants, given that positive response to Si application may not be limited to plants under stress. Rather the direct impact of Si extends to more fundamental metabolic processes. Fauteux et al. (2005) found that in the absence of stress Si application altered regulation of only two genes in Arabidopsis thaliana. Though this species does not actively accumulate Si (Vivancos et al., 2015), beneficial effects of Si in this model plant have been demonstrated (Ghanmi et al., 2004; Fauteux et al., 2006; Khandekar and Leisner, 2011). Recent pot-based experiments on the impact of Si application on sugarcane (Saccharum spp. hybrid) growth and defence against an insect herbivore revealed significant increases in plant growth even in the absence of herbivory (Frew et al., 2016a). In wheat, Chain et al. (2009) found that Si amendment affected the regulation of 47 genes in unstressed plants, while Brunings et al. (2009) reported the altered regulation of 221 genes in unstressed rice plants, of which 28 were associated with defence and stress, while the remainder were associated with primary metabolic processes or had unknown functions. In rice, Van Bockhaven et al. (2015b) found that Si altered the expression of genes associated with cell wall biosynthesis and glycolysis, and downregulated nitrogen and amino acid metabolism, as well as the metabolism of the ethylene, JA and SA defence hormones.
A study by Detmann et al. (2012) explored mechanisms by which Si had a positive effect on unstressed rice plants. By analysing photosynthetic gas exchange parameters alongside transcriptomic and metabolomic profiling, the authors concluded that Si increased photosynthetic efficiency and ultimately altered rice primary metabolism through stimulating amino acid remobilization (Fig. 1). Fleck et al. (2011) found that Si substantially altered the root anatomy of unstressed rice plants as well as the regulation of 265 genes, including a 25-fold upregulation of a specific protein-encoding gene that, it was suggested, may play a central role in the perception of a Si signal of an unknown nature. Further to this, Si was recently shown to delay leaf senescence by activation of the cytokinin pathway in both Si-accumulating and non-accumulating plant species (Markovich et al., 2017).
It is important to note that few environments, if any, are completely stress-free. The very concept of ‘stress’ could be misleading, as even basic metabolic processes can impart stress on plants, for example oxidative stress as a by-product of essential metabolic processes such as photosynthesis or respiration (Apel and Hirt, 2004). Consequently, the ability of Si to alleviate stress is very likely a fundamental component of plant life-processes.
THE ROLE OF SILICON IN PLANT BIOLOGY
Our understanding of the role of Si in plant biology is dominated by the multiple effects of Si on stress alleviation, and although some effects of Si on plant metabolism and gene expression have been demonstrated, the mechanisms whereby Si acts on plant growth and development remain ambiguous. Thus, we suggest a paradigm shift in the research approach to understanding the role of Si in plants. The notion of Si as a ‘non-essential nutrient’ has been challenged (Takahashi et al., 1990; Epstein, 1994, 1999, 2009; Cooke and Leishman, 2011). It is clear that Si has notable effects on numerous plant species, even those not classified as Si accumulators, under different environmental conditions (Li et al., 2015), highlighting that the nature and magnitude of the effects of Si are not necessarily concentration-dependent (Katz, 2014). The multiple effects of Si, including altered expression of defence enzymes and metabolites, increased phytolith deposition, changes in transpiration rates, CO2 assimilation and increased activity of antioxidant enzymes, all contribute to stress alleviation (Fig. 1). There is now increasing evidence that may suggest a critical role for Si in plant growth, primary metabolism and development (Detmann et al., 2012; Van Bockhaven et al., 2013; Markovich et al., 2017), but further research is required.
Looking forward, there are emerging pathways that can facilitate our understanding of Si, an element that has a diverse array of beneficial effects on plants. The impact of Si nutrition on gene regulation and the differential effects observed between species have only partially been revealed to date. In some high Si-accumulating species, Si could play an important role in plant primary metabolism (Van Bockhaven et al., 2015b). The current foundation of knowledge from studies in rice and in non-Si-accumulating species that respond to Si application, including A. thaliana (Fauteux et al., 2006; Markovich et al., 2017) and tomato (Solanum lycopersicum) (Ghareeb et al., 2011; Li et al., 2015), provide a starting point for future mechanistic studies. These systems, along with those high Si-accumulators such as rice, barley and wheat, should be employed incrementally across multiple experiments to evaluate the impacts of Si in unstressed situations as well as under single and multiple abiotic and biotic stresses. These should focus, in particular, on those primary metabolic processes revealed in recent research to potentially interact with Si (e.g. oxidation metabolism). As highlighted, Si may interact with primary photosynthetic processes (photorespiration), but this requires clarification as these interactions are likely to differ between C3-, C4- and CAM-type plants due to the differential physiological and enzymatic carbon fixation processes employed. The form in which Si is delivered to the plant may also strongly impact its effects on plant metabolism. The delivery mode of Si, in terms of formulation (e.g. calcium silicate, sodium silicate), should be further evaluated with emphasis on optimal translocation and accumulation in planta, as uptake and concentration at the site of action may prove critical to impacts on plant defence and growth. As a parallel priority, it is also important to investigate the role of Si in plant community structure and its ecological impact on mutualists and plant–plant interactions, including allelopathic effects of invasive species.
Appropriately designed, cross-disciplinary, incremental studies could reveal far-reaching effects on multiple fields, including plant pathology, entomology and agronomy, as well as plant biochemistry, ecology and evolutionary biology. Consideration of the impacts of plant breeding on Si uptake ability and the potential impacts on agronomy and agriculture is also worthy of note (Simpson et al., 2017), as is the production of transgenic plant species with increased Si uptake capacity (i.e. introduction of Si transporter genes [Lsi1, Lsi2]). Untargeted omics, utilizing multivariate analyses, in parallel with targeted profiling of significant markers integral to those primary metabolic processes already highlighted (photosynthesis, oxidation), should be used purposefully, with initial comparisons of specific plant trait groups (Si accumulator and non-accumulator; C3 and C4 plants), or of specific stresses ((hemi)biotrophic pathogens and chewing insect herbivores). Such comparative analyses will facilitate interpretation of effects and interactions, but potentially highlight next steps and contribute to elucidation of the fundamental role of Si in plant growth and development.
Silicon deposition may interfere with (hemi)biotrophic pathogen/herbivore effector proteins that suppress plant ETI (Fig. 2), which highlights another potentially unifying mechanism underpinning Si-enhanced plant resistance to some biotic stressors (Vivancos et al., 2015), and, considering the close association of this process with ROS production and defence phytohormone signalling, may also shed light on the ability of Si to alleviate abiotic stress. As a first step, this could be addressed using targeted transcriptomics evaluating the regulation of genes associated with ETI in (hemi)biotrophic pathogen/herbivore-stressed plants under high- and low-Si environments. As we have highlighted in this article, there is evidence from the last decade that Si may have an important, yet undefined, role in plant primary metabolism, specifically related to oxidation metabolism, or generation of ROS, that spans beyond biotic stress alleviation. The use of untargeted and targeted omics studies selectively comparing stress types could reveal new insights and confirm whether Si does indeed impact primary metabolism. Subsequent meta-analyses will also prove critical in identifying unifying pathways and mechanisms by which Si acts on plant metabolism.
Despite past efforts to raise awareness of the importance of Si (Epstein, 1994, 1999, 2009; Cooke and Leishman, 2011), coupled with selected recent findings on its role in gene regulation in plant development and defence, many plant scientists remain indifferent to or unaware of the potential roles of this interesting and unique element. Therefore, to address this future challenge we suggest the thoughtful and focused design of multidisciplinary experimentation among collaborators, leading to a better understanding of the underpinning role of Si in higher plant growth and development, and therefore its ability to enhance plant resistance to stress.
ACKNOWLEDGEMENTS
This work was supported by a Charles Sturt University Faculty of Science Research Fellowship. The authors sincerely thank Mrs A. C. Johnson for assistance with manuscript preparation.
LITERATURE CITED
- Ali A, Haq T ul, Mahmood R, Jaan M, Abbas MN. 2016. Stimulating the anti-oxidative role and wheat growth improvement through silicon under salt stress. Silicon 1–4. doi:10.1007/s12633-015-9378-4. [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373–399. [DOI] [PubMed] [Google Scholar]
- Van Bockhaven J, De Vleesschauwer D, Höfte M. 2013. Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. Journal of Experimental Botany 64: 1281–1293. [DOI] [PubMed] [Google Scholar]
- Van Bockhaven J, Spíchal L, Novák O et al. 2015. a Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytologist 206: 761–773. [DOI] [PubMed] [Google Scholar]
- Van Bockhaven J, Steppe K, Bauweraerts I et al. 2015. b Primary metabolism plays a central role in moulding silicon-inducible brown spot resistance in rice. Molecular Plant Pathology 16: 811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology 60: 379–406. [DOI] [PubMed] [Google Scholar]
- Brunings AM, Datnoff LE, Ma JF et al. 2009. Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Annals of Applied Biology 155: 161–170. [Google Scholar]
- Cai K, Gao D, Luo S, Zeng R, Yang J, Zhu X. 2008. Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiologia Plantarum 134: 324–333. [DOI] [PubMed] [Google Scholar]
- Chain F, Côté-Beaulieu C, Belzile F, Menzies JG, Bélanger RR. 2009. A comprehensive transcriptomic analysis of the effect of silicon on wheat plants under control and pathogen stress conditions. Molecular Plant-Microbe Interactions 22: 1323–1330. [DOI] [PubMed] [Google Scholar]
- Chérif M, Benhamou N, Menzies JG, Bélanger RR. 1992. Silicon induced resistance in cucumber plants against Pythium ultimum. Physiological and Molecular Plant Pathology 41: 411–425. [Google Scholar]
- Chérif M, Asselin A, Bélanger RR. 1994. Defense responses induced by soluble silicon in cucumber roots infected by Pythium spp. Phytopathology 84: 236–242. [Google Scholar]
- Chiba Y, Mitani N, Yamaji N, Ma JF. 2009. HvLsi1 is a silicon influx transporter in barley. Plant Journal 57: 810–818. [DOI] [PubMed] [Google Scholar]
- Cooke J, Leishman MR. 2011. Is plant ecology more siliceous than we realise?Trends in Plant Science 16: 61–68. [DOI] [PubMed] [Google Scholar]
- Cooke J, Leishman MR. 2016. Consistent alleviation of abiotic stress with silicon addition: a meta-analysis. Functional Ecology 30: 1340–1357. [Google Scholar]
- Cooke J, DeGabriel JL, Hartley SE. 2016. The functional ecology of plant silicon: geoscience to genes. Functional Ecology 30: 1270–1276. [Google Scholar]
- Coskun D, Britto DT, Huynh WQ, Kronzucker HJ. 2016. The role of silicon in higher plants under salinity and drought stress. Frontiers in Plant Science 7. doi:10.3389/fpls.2016.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debona D, Rodrigues FA, Rios JA, Nascimento KJT, Silva LC. 2014. The effect of silicon on antioxidant metabolism of wheat leaves infected by Pyricularia oryzae. Plant Pathology 63: 581–589. [Google Scholar]
- Debona D, Rodrigues FA, Datnoff LE. 2017. Silicon’s role in abiotic and biotic plant stresses. Annual Review of Phytopathology 55: 85–107. [DOI] [PubMed] [Google Scholar]
- Deshmukh R, Bélanger RR. 2015. Molecular evolution of aquaporins and silicon influx in plants. Functional Ecology 30: 1277–1285. [Google Scholar]
- Deshmukh RK, Vivancos J, Guérin V et al. 2013. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Molecular Biology 83: 303–315. [DOI] [PubMed] [Google Scholar]
- Detmann KC, Araújo WL, Martins SCV et al. 2012. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytologist 196: 752–762. [DOI] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant-pathogen interactions. Nature Reviews Genetics 11: 539–548. [DOI] [PubMed] [Google Scholar]
- Domiciano GP, Cacique IS, Chagas Freitas C et al. 2015. Alterations in gas exchange and oxidative metabolism in rice leaves infected by Pyricularia oryzae are attenuated by silicon. Phytopathology 105: 738–747. [DOI] [PubMed] [Google Scholar]
- Epstein E. 1994. The anomaly of silicon in plant biology. Proceedings of the National Academy of Sciences of the USA 91: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. 1999. Silicon. Annual Review of Plant Biology 50: 641–664. [DOI] [PubMed] [Google Scholar]
- Epstein E. 2009. Silicon: its manifold roles in plants. Annals of Applied Biology 155: 155–160. [Google Scholar]
- Erb M, Meldau S, Howe GA. 2012. Role of phytohormones in insect-specific plant reactions. Trends in Plant Science 17: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami H, Jeong BR. 2018. Silicon (Si): review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicology and Environmental Safety 147: 881–896. [DOI] [PubMed] [Google Scholar]
- Farooq MA, Detterbeck A, Clemens S, Dietz K-J. 2016. Silicon-induced reversibility of cadmium toxicity in rice. Journal of Experimental Botany 67: 3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauteux F, Rémus-Borel W, Menzies JG, Bélanger RR. 2005. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiology Letters 249: 1–6. [DOI] [PubMed] [Google Scholar]
- Fauteux F, Chain F, Belzile F, Menzies JG, Bélanger RR. 2006. The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. Proceedings of the National Academy of Sciences of the USA 103: 17554–17559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck AT, Nye T, Repenning C, Stahl F, Zahn M, Schenk MK. 2011. Silicon enhances suberization and lignification in roots of rice (Oryza sativa). Journal of Experimental Botany 62: 2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato AA, da Silva WL, Rodrigues FÁ. 2013. Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporum f. sp. cubense. Phytopathology 104: 597–603. [DOI] [PubMed] [Google Scholar]
- Frew A, Allsopp PG, Gherlenda AN, Johnson SN. 2016a Increased root herbivory under elevated atmospheric carbon dioxide concentrations is reversed by silicon-based plant defences. Journal of Applied Ecology 54: 1310–1319. [Google Scholar]
- Frew A, Powell JR, Sallam N, Allsopp PG, Johnson SN. 2016b Trade-offs between silicon and phenolic defenses may explain enhanced performance of root herbivores on phenolic-rich plants. Journal of Chemical Ecology 42: 768–771. [DOI] [PubMed] [Google Scholar]
- Ghanmi D, McNally DJ, Benhamou N, Menzies JG, Bélanger RR. 2004. Powdery mildew of Arabidopsis thaliana: a pathosystem for exploring the role of silicon in plant–microbe interactions. Physiological and Molecular Plant Pathology 64: 189–199. [Google Scholar]
- Ghareeb H, Bozsó Z, Ott PG, Repenning C, Stahl F, Wydra K. 2011. Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiological and Molecular Plant Pathology 75: 83–89. [Google Scholar]
- Giraldo MC, Valent B. 2013. Filamentous plant pathogen effectors in action. Nature Reviews. Microbiology 11: 800–814. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43: 205–227. [DOI] [PubMed] [Google Scholar]
- Gong H, Chen K. 2012. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiologiae Plantarum 34: 1589–1594. [Google Scholar]
- Grégoire C, Rémus-Borel W, Vivancos J, Labbé C, Belzile F, Bélanger RR. 2012. Discovery of a multigene family of aquaporin silicon transporters in the primitive plant Equisetum arvense. Plant Journal 72: 320–330. [DOI] [PubMed] [Google Scholar]
- Griffin M, Hogan B, Schmidt O. 2015. Silicon reduces slug feeding on wheat seedlings. Journal of Pest Science 88: 17–24. [Google Scholar]
- Guntzer F, Keller C, Meunier J-D. 2012. Benefits of plant silicon for crops: a review. Agronomy for Sustainable Development 32: 201–213. [Google Scholar]
- Hall AD, Morison CGT. 1906. On the function of silica in the nutrition of cereals. Part I. Proceedings of the Royal Society of London B 77: 455–477. [Google Scholar]
- Hamayun M, Sohn E-Y, Khan SA, Shinwari ZK, Khan AL, Lee I-J. 2010. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Pakistan Journal of Botany 42: 1713–1722. [Google Scholar]
- Han Y, Li P, Gong S, Yang L, Wen L, Hou M. 2016. Defense responses in rice induced by silicon amendment against infestation by the leaf folder Cnaphalocrocis medinalis. PLoS ONE 11: e0153918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SE. 2015. Round and round in cycles? Silicon-based plant defences and vole population dynamics. Functional Ecology 29: 151–153. [Google Scholar]
- Hartley SE, DeGabriel JL. 2016. The ecology of herbivore-induced silicon defences in grasses. Functional Ecology 30: 1311–1322. [Google Scholar]
- Hattori T, Inanaga S, Araki H et al. 2005. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiologia Plantarum 123: 459–466. [Google Scholar]
- Hodson MJ, White PJ, Mead A, Broadley MR. 2005. Phylogenetic variation in the silicon composition of plants. Annals of Botany 96: 1027–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenhout SA, Bos JI. 2011. Effector proteins that modulate plant–insect interactions. Current Opinion in Plant Biology 14: 422–428. [DOI] [PubMed] [Google Scholar]
- Imtiaz M, Rizwan MS, Mushtaq MA et al. 2016. Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: a review. Journal of Environmental Management 183: 521–529. [DOI] [PubMed] [Google Scholar]
- IPNI 2015. Silicon. Nutri-facts, No. 14. http://www.ipni.net/publication/nutrifacts-na.nsf/0/A7B4AB4D35C153BF85257ECE006E0E34/$FILE/NutriFacts-NA-14.pdf. Accessed 25 July 2017. [Google Scholar]
- Jeer M, Telugu UM, Voleti SR, Padmakumari AP. 2017. Soil application of silicon reduces yellow stem borer, Scirpophaga incertulas (Walker) damage in rice. Journal of Applied Entomology 141: 189–201. [Google Scholar]
- Kang J, Zhao W, Zhu X. 2016. Silicon improves photosynthesis and strengthens enzyme activities in the C3 succulent xerophyte Zygophyllum xanthoxylum under drought stress. Journal of Plant Physiology 199: 76–86. [DOI] [PubMed] [Google Scholar]
- Katz O. 2014. Beyond grasses: the potential benefits of studying silicon accumulation in non-grass species. Frontiers in Plant Science 5. doi:10.3389/fpls.2014.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz O. 2015. Silica phytoliths in angiosperms: phylogeny and early evolutionary history. New Phytologist 208: 642–646. [DOI] [PubMed] [Google Scholar]
- Khandekar S, Leisner S. 2011. Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. Journal of Plant Physiology 168: 699–705. [DOI] [PubMed] [Google Scholar]
- Khattab HI, Emam MA, Emam MM, Helal NM, Mohamed MR. 2014. Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biologia Plantarum 58: 265–273. [Google Scholar]
- Kim SG, Kim KW, Park EW, Choi D. 2002. Silicon-induced cell wall fortification of rice leaves: a possible cellular mechanism of enhanced host resistance to blast. Phytopathology 92: 1095–1103. [DOI] [PubMed] [Google Scholar]
- Kim Y-H, Khan AL, Kim D-H et al. 2014. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biology 14: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-H, Khan AL, Waqas M, Lee I-J. 2017. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Frontiers in Plant Science 8. doi:10.3389/fpls.2017.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdali F, Al-Chammaa M. 2013. Growth and nitrogen fixation in silicon and/or potassium fed chickpeas grown under drought and well watered conditions. Journal of Stress Physiology & Biochemistry 9: 385–406. [Google Scholar]
- Kvedaras OL, Byrne MJ, Coombes NE, Keeping MG. 2009. Influence of plant silicon and sugarcane cultivar on mandibular wear in the stalk borer Eldana saccharina. Agricultural and Forest Entomology 11: 301–306. [Google Scholar]
- Kvedaras OL, An M, Choi YS, Gurr GM. 2010. Silicon enhances natural enemy attraction and biological control through induced plant defences. Bulletin of Entomological Research 100: 367–371. [DOI] [PubMed] [Google Scholar]
- Leon J, Lawton MA, Raskin I. 1995. Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiology 108: 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin J, Reimann BEF. 1969. Silicon and plant growth. Annual Review of Plant Physiology 20: 289–304. [Google Scholar]
- Li H, Zhu Y, Hu Y, Han W, Gong H. 2015. Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiologiae Plantarum 4: 1–9. [Google Scholar]
- Liang YC, Sun WC, Si J, Römheld V. 2005. Effects of foliar- and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathology 54: 678–685. [Google Scholar]
- Liang Y, Zhu J, Li Z et al. 2008. Role of silicon in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environmental and Experimental Botany 64: 286–294. [Google Scholar]
- Liang X, Wang H, Hu Y et al. 2015. Silicon does not mitigate cell death in cultured tobacco BY-2 cells subjected to salinity without ethylene emission. Plant Cell Reports 34: 331–343. [DOI] [PubMed] [Google Scholar]
- Liu P, Yin L, Deng X, Wang S, Tanaka K, Zhang S. 2014. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. Journal of Experimental Botany 65: 4747–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhu J, Zhang P et al. 2017. Silicon supplementation alters the composition of herbivore induced plant volatiles and enhances attraction of parasitoids to infested rice plants. Frontiers in Plant Science 8doi:10.3389/fpls.2017.01265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx M, Hausman J-F, Lutts S, Guerriero G. 2017. Silicon and plants: current knowledge and technological perspectives. Frontiers in Plant Science 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Sun D, Wang C et al. 2016. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. Journal of Plant Growth Regulation 35: 1–10. doi:10.1007/s00344-015-9500-2. [Google Scholar]
- Ma JF. 2004. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Science and Plant Nutrition 50: 11–18. [Google Scholar]
- Ma JF, Yamaji N. 2015. A cooperative system of silicon transport in plants. Trends in Plant Science 20: 435–442. [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N et al. 2006. A silicon transporter in rice. Nature 440: 688–691. [DOI] [PubMed] [Google Scholar]
- Markovich O, Steiner E, Kouřil Š, Tarkowski P, Aharoni A, Elbaum R. 2017. Silicon promotes cytokinin biosynthesis and delays senescence in Arabidopsis and Sorghum. Plant, Cell & Environment 40: 1189–1196. [DOI] [PubMed] [Google Scholar]
- Martin-Jézéquel V, Hildebrand M, Brzezinski MA. 2000. Silicon metabolism in diatoms: implications for growth. Journal of Phycology 36: 821–840. [Google Scholar]
- Massey FP, Hartley SE. 2006. Experimental demonstration of the antiherbivore effects of silica in grasses: impacts on foliage digestibility and vole growth rates. Proceedings of the Royal Society of London B: Biological Sciences 273: 2299–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey FP, Hartley SE. 2009. Physical defences wear you down: progressive and irreversible impacts of silica on insect herbivores. Journal of Animal Ecology 78: 281–291. [DOI] [PubMed] [Google Scholar]
- Massey FP, Ennos AR, Hartley SE. 2006. Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder. Journal of Animal Ecology 75: 595–603. [DOI] [PubMed] [Google Scholar]
- Massey FP, Ennos AR, Hartley SE. 2007a Herbivore specific induction of silica-based plant defences. Oecologia 152: 677–683. [DOI] [PubMed] [Google Scholar]
- Massey FP, Ennos AR, Hartley SE. 2007b Grasses and the resource availability hypothesis: the importance of silica-based defences. Journal of Ecology 95: 414–424. [Google Scholar]
- McNaughton SJ, Tarrants JL. 1983. Grass leaf silicification: natural selection for an inducible defense against herbivores. Proceedings of the National Academy of Sciences of the USA 80: 790–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JD, Barboni D, Anwar-ul-Haq M et al. 2017. Effect of phytoliths for mitigating water stress in durum wheat. New Phytologist 215: 229–239. [DOI] [PubMed] [Google Scholar]
- Miao B-H, Han X-G, Zhang W-H. 2010. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Annals of Botany 105: 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalicová Malcovská S, Ducaiová Z, Maslaáková I, Backor M. 2014. Effect of silicon on growth, photosynthesis, oxidative status and phenolic compounds of maize (Zea mays L.) grown in cadmium excess. Water, Air and Soil Pollution 225: 1–11. [Google Scholar]
- Ming DF, Pei ZF, Naeem MS, Gong HJ, Zhou WJ. 2012. Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. Journal of Agronomy and Crop Science 198: 14–26. [Google Scholar]
- Mitani N, Yamaji N, Ma JF. 2009. Identification of maize silicon influx transporters. Plant and Cell Physiology 50: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ago Y, Iwasaki K, Ma JF. 2011. Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant Journal 66: 231–240. [DOI] [PubMed] [Google Scholar]
- Montpetit J, Vivancos J, Mitani-Ueno N et al. 2012. Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Molecular Biology 79: 35–46. [DOI] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H et al. 2002. Herbivory: caterpillar saliva beats plant defences. Nature 416: 599–600. [DOI] [PubMed] [Google Scholar]
- Nwugo CC, Huerta AJ. 2011. The effect of silicon on the leaf proteome of rice (Oryza sativa L.) plants under cadmium-stress. Journal of Proteome Research 10: 518–528. [DOI] [PubMed] [Google Scholar]
- Okita TW, Volcani BE. 1978. Role of silicon in diatom metabolism IX: differential synthesis of DNA polymerases and DNA-binding proteins during silicate starvation and recovery in Cylindrotheca fusiformis. Biochimica et Biophysica Acta 519: 76–86. [DOI] [PubMed] [Google Scholar]
- Pavlovic J, Samardzic J, Kostic L et al. 2016. Silicon enhances leaf remobilization of iron in cucumber under limited iron conditions. Annals of Botany 118: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CEA, Rodrigues FÁ, Moreira WR, DaMatta FM. 2013. Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae. Phytopathology 104: 143–149. [DOI] [PubMed] [Google Scholar]
- Rahman A, Wallis CM, Uddin W. 2015. Silicon-induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 105: 748–757. [DOI] [PubMed] [Google Scholar]
- Ramputh AI, Arnason JT, Cass L, Simmonds JA. 2002. Reduced herbivory of the European corn borer (Ostrinia nubilalis) on corn transformed with germin, a wheat oxalate oxidase gene. Plant Science 162: 431–440. [Google Scholar]
- Rémus-Borel W, Menzies JG, Bélanger RR. 2005. Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiological and Molecular Plant Pathology 66: 108–115. [Google Scholar]
- Reynolds OL, Keeping MG, Meyer JH. 2009. Silicon-augmented resistance of plants to herbivorous insects: a review. Annals of Applied Biology 155: 171–186. [Google Scholar]
- Reynolds OL, Padula MP, Zeng R, Gurr GM. 2016. Silicon: potential to promote direct and indirect effects on plant defense against arthropod pests in agriculture. Frontiers in Plant Science 7. doi:10.3389/fpls.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond KE, Sussman M. 2003. Got silicon? The non-essential beneficial plant nutrient. Current Opinion in Plant Biology 6: 268–272. [DOI] [PubMed] [Google Scholar]
- Rodrigues FA, Dallagnol LJ, Duarte HSS, Datnoff LE. 2015. Silicon control of foliar diseases in monocots and dicots. In: Rodrigues FA, Datnoff LE, eds. Silicon and plant diseases. Cham: Springer, 67–108. [Google Scholar]
- Rojas CM, Senthil-Kumar M, Wang K, Ryu C-M, Kaundal A, Mysore KS. 2012. Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 24: 336–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas MC, Corpas FJ, Rodríguez-Serrano M, Gómez M, del Río LA, Sandalio LM. 2007. Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. Journal of Plant Physiology 164: 1346–1357. [DOI] [PubMed] [Google Scholar]
- Ryalls JMW, Hartley SE, Johnson SN. 2017. Impacts of silicon-based grass defences across trophic levels under both current and future atmospheric CO2 scenarios. Biology Letters 13: 20160912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr N. 2017. The role of silicon (Si) in increasing plant resistance against insect pests review article. Acta Phytopathologica et Entomologica Hungarica: 1–19. doi:10.1556/038.52.2017.020. [Google Scholar]
- Sanglard LMVP, Martins SCV, Detmann KC et al. 2014. Silicon nutrition alleviates the negative impacts of arsenic on the photosynthetic apparatus of rice leaves: an analysis of the key limitations of photosynthesis. Physiologia Plantarum 152: 355–366. [DOI] [PubMed] [Google Scholar]
- de Saussure NT. 1804. Recherches chimiques sur la vegetation. Paris: Nyon. [Google Scholar]
- Savant NK, Datnoff LE, Snyder GH. 1997. Depletion of plant-available silicon in soils: a possible cause of declining rice yields. Communications in Soil Science & Plant Analysis 28: 1245–1252. [Google Scholar]
- Shen X, Zhou Y, Duan L, Li Z, Eneji AE, Li J. 2010. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. Journal of Plant Physiology 167: 1248–1252. [DOI] [PubMed] [Google Scholar]
- Simpson KJ, Wade RN, Rees M, Osborne CP, Hartley SE. 2017. Still armed after domestication? Impacts of domestication and agronomic selection on silicon defences in cereals. Functional Ecology: doi:10.1111/1365–2435.12935. [Google Scholar]
- Takahashi E, Ma JF, Miyake Y. 1990. The possibility of silicon as an essential element for higher plants. Comments on Agricultural and Food Chemistry 2: 99–102. [Google Scholar]
- Thoma I, Loeffler C, Sinha AK et al. 2003. Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant Journal 34: 363–375. [DOI] [PubMed] [Google Scholar]
- Torres MA. 2010. ROS in biotic interactions. Physiologia Plantarum 138: 414–429. [DOI] [PubMed] [Google Scholar]
- Vaculík M, Landberg T, Greger M, Luxová M, Stoláriková M, Lux A. 2012. Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Annals of Botany 110: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos J, Labbé C, Menzies JG, Bélanger RR. 2015. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Molecular Plant Pathology 16: 572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. 2010. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics 44: 1–24. [DOI] [PubMed] [Google Scholar]
- Yang L, Han Y, Li P, Li F, Ali S, Hou M. 2017. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Scientific Reports 7. doi:10.1038/s41598-017-04571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Song Y, Long J et al. 2013. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proceedings of the National Academy of Sciences of the USA 110: E3631–E3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wang S, Tanaka K et al. 2016. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant, Cell & Environment 39: 245–258. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Wei G, Li J, Qian Q, Yu J. 2004. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Science 167: 527–533. [Google Scholar]