Abstract
Background
Choline is an essential nutrient that is pivotal to proper brain development. Research in animal models suggests that perinatal choline deficiency influences neuron development in the hippocampus and cortex, yet these observations require invasive techniques.
Objective
This study aimed to characterize the effects of perinatal choline deficiency on gray and white matter development with the use of noninvasive neuroimaging techniques in young pigs.
Methods
During the last 64 d of the 114-d gestation period Yorkshire sows were provided with a choline-sufficient (CS) or choline-deficient (CD) diet, analyzed to contain 1214 mg or 483 mg total choline/kg diet, respectively. Upon farrowing, pigs (Sus scrofa domesticus) were allowed colostrum consumption for ≤48 h, were further stratified into postnatal treatment groups, and were provided either CS or CD milk replacers, analyzed to contain 1591 or 518 mg total choline/kg diet, respectively, for 28 d. At 30 d of age, pigs were subjected to MRI procedures to assess brain development. Gray and white matter development was assessed through voxel-based morphometry (VBM) and tract-based spatial statistics (TBSS) to assess the effects of prenatal and postnatal dietary choline status.
Results
VBM analysis indicated that prenatally CS pigs exhibited increased (P < 0.01) gray matter in the left and right cortex compared with prenatally CD pigs. Analysis of white matter indicated that prenatally CS pigs exhibited increased (P < 0.01) white matter in the internal capsule, putamen–globus pallidus, and right cortex compared with prenatally CD pigs. No postnatal effects (P > 0.05) of choline status were noted for VBM analyses of gray and white matter. TBSS also showed no significant effects (P > 0.05) of prenatal or postnatal choline status for diffusion values along white matter tracts.
Conclusions
Observations from this study suggest that prenatal choline deficiency results in altered cortical gray matter and reduced white matter in the internal capsule and putamen of young pigs. With the use of noninvasive neuroimaging techniques, results from our study indicate that prenatal choline deficiency greatly alters gray and white matter development in pigs, thereby providing a translational assessment that may be used in clinical populations.
Keywords: choline, choline deficiency, pig, neurodevelopment, gray matter, white matter, prenatal nutrition
Introduction
Choline is an essential nutrient that has many physiologic functions. Despite being characterized as an essential micronutrient in 1998 (1), nearly 90% of adults do not meet the adequate daily intake amounts of choline (2). It is recommended that pregnant women ingest 450 mg choline/d, with these numbers increasing to 550 mg/d during lactation (3). However, recent NHANES and independent reports suggest that women aged >20 y in the United States average ∼260 mg choline/d (4, 5). Although choline can be synthesized de novo, studies suggest that single nucleotide polymorphisms in the phosphatidylethanolamine N-methyltransferase (PEMT)
gene contribute to a reduced ability to endogenously synthesize choline, especially when dietary choline needs are not achieved (6). In addition, many vitamin and mineral supplements do not currently contain choline (5) and prenatal vitamins do not contain adequate amounts of choline, if any at all (7), further adding to the need for dietary choline ingestion throughout gestation. Although inadequate choline intake has been characterized in relatively high-income populations, such as in the United States, it is also thought to be prevalent in lower-income countries (8), rendering insufficient choline consumption a possible global concern.
Adequate choline ingestion throughout the perinatal period is critical because of the increased need to support fetal brain development (9–11). One study suggests that women whose choline intake was in the highest quartile during pregnancy had children who performed better on cognitive tests at 7 y of age, highlighting the long-term implications of choline consumption during pregnancy (12). The cognitive influences of choline may be due to its role in many physiologic aspects of brain development, including myelination, neurotransmitter synthesis, cell membrane composition, and neuron morphology (9, 13). Choline deficiency during the prenatal period, from embryonic days 12 to 17, in mice has previously been shown to alter hippocampal development by increasing apoptosis and reducing neuronal progenitor cell proliferation on embryonic day 17 (14). Rats born to choline-deficient (CD) dams exhibited reduced cross-sectional areas in region-specific neurons, thus suggesting regional specificity of choline effects on neuron morphology (13). Furthermore, a recent study found that prenatal choline deficiency in mice, from embryonic days 11 to 17, resulted in reduced numbers of upper-layer cortical neurons, altered epidermal growth factor signaling, and decreased cortical neural progenitor cells at embryonic day 17 as well as at 4 mo of age (15). Although many of these studies provide mechanistic characterization of the influence of choline on the developing brain, they also necessitate invasive techniques that are not suitable for elucidating choline's influence in human infants. Thus, there is a need to identify noninvasive methods of characterizing the influence of choline on brain development, which may help explain observations in rodent models.
Neuroimaging provides a sensitive and noninvasive means for characterizing brain development in humans and animal models alike. Previous studies have used MRI techniques to evaluate the influence of early-life nutrition on markers of myelination using diffusion tensor imaging and changes in neurometabolites using magnetic resonance spectroscopy in the biomedical piglet model (16). In fact, we previously established that neuroimaging can sensitively quantify volumetric differences in pigs whose mothers were provided CD diets throughout gestation (17). Given the fact that other animal studies have observed differences in neuron morphology and cortical development, this study is a follow-up analysis from our initial neuroimaging assessment identifying the effects of dietary choline deficiency on brain volumes and diffusion measures (17). Herein, we aimed to assess gray and white matter development in the context of prenatal and postnatal dietary choline deficiency in pigs. We hypothesized that pigs from sows that were provided a CD diet would exhibit region-specific decreases in gray and white matter of the brain at 4 wk of age. As such, we used voxel-based morphometry (VBM) and tract-based spatial statistics (TBSS) to quantify differences in brain gray and white matter development as a result of prenatal and postnatal choline deficiency in pigs.
Methods
Animals and housing
For the prenatal dietary choline intervention, pregnant Yorkshire sows (n = 12) were fed either a choline-sufficient (CS; n = 6) or CD (n = 6) diet starting at day 50 of gestation, which is described in further detail below. The standard gestation period for sows is 114 d; thus, the dietary intervention was provided for the last 64 d of gestation. Throughout gestation, sows were housed indoors in standard agricultural gestation and farrowing crates as described previously (18). As stated previously, the timing of choline deficiency was chosen to minimize chances of spontaneous fetal loss due to the dietary intervention, and sows were not induced in this study due to unseasonably warm weather, resulting in farrowing between 111 and 114 d of gestation (18).
The methods provided below were previously described (17, 18) and are repeated in detail below to maintain continuity of methods descriptions within a study. A total of 32 naturally farrowed domestic pigs (Sus scrofa domesticus; n = 16/sex) from the 12 sows that were provided the experimental gestation diets were obtained at 2 d of age. Each neonatal pig was placed in an artificial rearing system as previously described (17). Individual caging units (1.03 m deep × 0.77 m wide × 0.81 m high), designed for neonatal pigs, included ad libitum access to water, supplemental heat, and a towel and rubber toy for enrichment. Light in the rearing facility was kept on a 12-h light-dark cycle, with light maintained from 0600 to 1800. Ambient local temperatures in the cages were maintained between 24° and 36°C using heat lamps. Upon placement into the artificial rearing system, neonatal pigs were administered 5 mL Clostridium perfringens antitoxin C+D per the manufacturer's recommendations (Colorado Serum Company). Neonatal pigs exhibiting sickness behavior, defined as diarrhea for >48 h, were placed on an electrolyte solution and administered a single dose of sulfamethoxazole and trimethoprim oral suspension (50 and 8 mg/mL, respectively; Hi-Tech Pharmacal) for 3 consecutive days.
Feeding methods were previously described (17, 18) and are repeated in detail below to maintain continuity of methods descriptions within a study. After placement into the animal care facility, all neonatal pigs were provided a custom soy-based milk replacer (Test Diets) formulated to meet all nutrient requirements (19), apart from choline (further details below), for the duration of the 28-d postnatal feeding study. Milk replacer powder was reconstituted (200 g milk replacer powder/L tap water) fresh ≥4 times/d, with feeding rates and intervals increased on the basis of growth performance of postnatally CS pigs. Milk replacer was supplied at a rate of 285 mL/kg body weight from 3 to 9 d of age, 300 mL/kg body weight from 10 to 13 d of age, 325 mL/kg body weight from 14 to 15 d of age, and 350 mL/kg body weight from 16 to 29 d of age (based on daily recorded pig body weights). Feedings occurred every 3–4 h starting at 0800 and ending at 2300 daily. All animal care and experimental procedures were in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois at Urbana–Champaign Institutional Animal Care and Use Committee.
Dietary treatments
Dietary treatments were previously described (17, 18, 20) and are repeated in detail below to maintain continuity of methods descriptions within a study. For the last 64 d of the 114-d gestation period, sows (n = 12) were fed either CS (n = 6; 1214 mg analyzed total choline/kg diet) or CD (n = 6; 483 mg analyzed total choline/kg diet) gestation diets (19). Sows were administered the experimental gestation diet from day 50 of the 114-d gestation period until 48 h postfarrowing. Sows were provided restricted access to feed to maintain appropriate body condition per standard agricultural procedures; a detailed description of sow diets was previously described (20).
At birth, neonatal pigs were allowed colostrum consumption for ≤48 h. At 2 d of age, pigs (n = 32) were further stratified into CS (n = 17; 1591 mg analyzed total choline/kg diet) or CD (n = 15; 518 mg analyzed total choline/kg diet) postnatal custom milk replacer treatments and were fed these diets for the remainder of the 28-d feeding study (19). This resulted in a 2 × 2 factorial arrangement with a total of 4 treatment groups: CS/CS, prenatal and postnatal CS; CS/CD, prenatal CS and postnatal CD; CD/CS, prenatal CD and postnatal CS; and CD/CD, prenatal and postnatal CD. All daily choline requirements referenced above were based on the most comprehensive evidence for the domestic pig, which is based on a per-kilogram body weight basis (19). Analysis of final choline content in gestation and milk replacer diets was performed by using AOAC 994.14 methods by an external laboratory (Eurofins US) (18).
Neuroimaging procedures
Neuroimaging procedures were previously described (17) and are repeated in detail below to maintain continuity of methods descriptions within a study. At 30 d of age, pigs were subjected to MRI scanning procedures. Before scanning, pigs were anesthetized via an intramuscular injection of Telazol (0.07 mg/kg body weight; Zoetis). Pigs were immediately transferred to the MRI machine in which they were maintained on an average of 2% isoflurane:98% oxygen for the duration of the 60-min scan. Pig heart rate and peripheral capillary oxygen saturation were monitored throughout the duration of the scanning session with the use of an MRI-compatible pulse oximeter. MRI procedures, along with postimaging analyses of brain region volume and diffusion tensor imaging, were previously described (21). Pigs were scanned with the use of a Siemens MAGNETOM Trio 3T Imager with a Siemens 12-channel head coil. Upon completion of MRI scans, pigs were allowed to recover from anesthesia, vital signs were monitored every 15 min until complete recovery, and pigs were subsequently transported back to the rearing facilities.
VBM
Detailed descriptions of VBM procedures were previously described (21–23) and are repeated in detail below to maintain continuity of methods. A T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) sequence was used to obtain anatomic images of the pig brain, with a 0.7-mm isotropic voxel, resulting in a 0.343-mm3 voxel size. The following sequence-specific parameters were used to acquire T1-weighted MPRAGE data: repetition time = 1900 ms, echo time = 2.49 ms, inversion time = 900 ms, 224 slices, field of view = 180 mm, and flip angle = 9°. Pig brain images were manually skull-stripped by using a Wacom Cintiq digital display as previously described (17). The “Segment” function of statistical parametric mapping 8 (SPM8; Wellcome Department of Clinical Neurology) and pig-specific previous probability tissue maps (24) were then used to segment the brains into gray matter and white matter. VBM analysis was performed to assess gray and white matter alterations with the use of SPM8 software. The Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra (DARTEL) toolbox was used with pig-specific specifications that included changing the bounding box of −30.1 to 30.1, −35 to 44.8, −28 to 31.5 and a voxel size of 0.7-mm isotropic (i.e., 0.343 mm3). After the nonlinear transformation of the data in the DARTEL procedure, flow fields were created and converted to warp files. The warp files generated were then applied to the pigs’ gray and white matter. The modulated data were smoothed with a 4-mm full-width half maximum, and were subjected to VBM procedures using the SPM toolbox with the use of methods previously described (21).
TBSS
Detailed descriptions of TBSS procedures were previously described (22, 25) and are repeated in detail below to maintain continuity of methods. Diffusion tensor imaging was used to assess white matter maturation and axonal tract integrity with the use of a b-value = 1000 s/mm2 across 30 directions and a 2-mm isotropic voxel (i.e., 8 mm3). Diffusion-weighted echoplanar imaging images were assessed in FMRIB Software Library (FSL) 5.0 for fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) with the use of methods that were previously described (17). The FSL 5.0 toolbox was used for TBSS assessment of FA data (26, 27). FA images, previously generated from diffusion data, were manually skull-stripped, and all FA data from individual pigs were aligned by using the FSL nonlinear registration tool FMRIB's nonlinear image registration tool (FNIRT). Upon alignment, the study-specific mean FA image was created and a mean FA skeleton representing the center of all common tracts was established. A threshold of 0.2 was determined to be sensitive for mean FA tract delineation and to ensure that only white matter was included in the analyzed FA skeleton. Once the study-specific mean FA skeleton was created, each pig's aligned FA data were projected onto the mean FA skeleton and the resulting voxel-wise cross-subject data were used for statistical analyses (26, 27). For all TBSS analyses involving registration to an atlas, the Piglet Brain Atlas (http://pigmri.illinois.edu/) was used in place of human brain templates (24).
The mean FA skeleton generated in the previous steps was used as “predetermined” white matter tracts. Subsequently the TBSS non-FA function was used to generate data on diffusion differences along the white matter tracts for other diffusion tensor measures (i.e., MD, AD, and RD). To analyze differences in these diffusion measures, nonlinear warps and skeleton projection values generated in the TBSS FA analysis were applied to each of the MD, AD, and RD images. Pig-specific alterations to the non-FA script included registration to pig brain atlas space, rather than MNI152 space, and registration with the use of a pig-specific internal capsule mask rather than a lower cingulum mask.
Statistical analysis
VBM
VBM analyses were conducted separately to determine differences between prenatal choline status (i.e., CS compared with CD sow diet) or differences between postnatal choline status (i.e., CS compared with CD milk replacer). As such, 2-sample permutation t tests were performed on a voxel-by-voxel basis for gray and white matter differences between CS and CD pigs. A family-wise error correction (Gaussian Random Field Theory; P < 0.05) was applied to account for multiple comparisons, and an additional threshold criterion of ≥20-edge connected voxels was used to determine significant clusters. All P values reported herein are P-corrected.
TBSS
For TBSS analysis, data were analyzed separately to determine differences between prenatal choline status (i.e., CS compared with CD sow diet) or differences between postnatal choline status (i.e., CS compared with CD milk replacer). A nonparametric permutation inference function called “randomize” was used within the FSL toolbox and run as a 2-sample t test with 500 permutations to compare the effects of early-life choline deficiency. Multiple comparisons were also accounted for within the randomize function. The resulting statistical analyses were conducted for FA, AD, MD, and RD data.
Results
Neonatal pig growth
Sow choline status did not influence pig birth weight, with prenatally CS pigs averaging 1.59 kg and prenatally CD pigs averaging 1.52 kg. Moreover, there was no difference in body weight between the 4 dietary treatment groups after the 28-d postnatal feeding study. These findings were previously published and are provided to the reader for context of overall pig body growth. A more detailed description of pig growth can be found in Getty and Dilger (18).
VBM
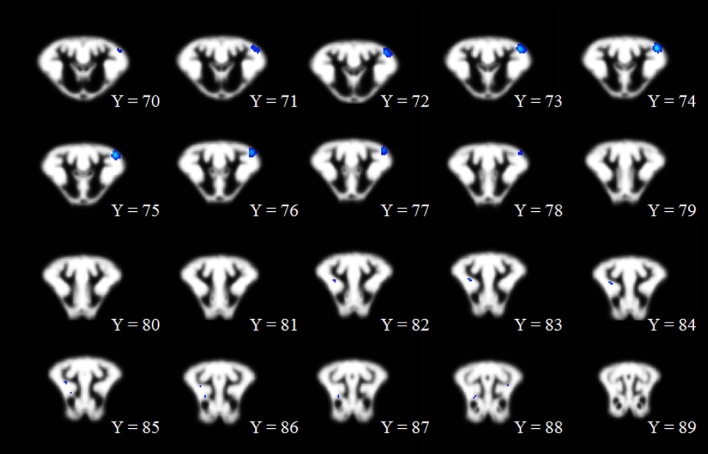
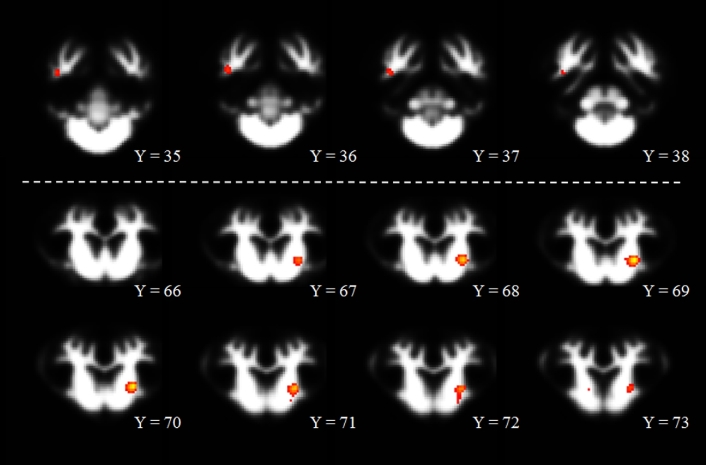
A comparison of gray matter showed localized clusters in which prenatal CS pigs exhibited increased (P < 0.01) gray matter compared with prenatal CD pigs (Table 1, Figure 1; CS > CD). These differences appeared in 4 separate clusters that were located in the left and right cortex, with the largest cluster located in the left cortex containing 312 voxels. Notably, the Pig Brain Atlas does not differentiate between particular regions of the cortex; however, upon visual inspection, these clusters appear to be located in the frontal and prefrontal cortices. The converse comparison in which prenatal CD pigs exhibited increased gray matter compared with prenatal CS pigs (i.e., CD > CS) yielded no significant (P > 0.05) clusters. A comparison of white matter showed localized clusters in which prenatal CS pigs exhibited increased (P < 0.001) white matter compared with prenatal CD pigs (Table 1, Figure 2; CS > CD). These differences appeared in 3 separate clusters that were located in the putamen–globus pallidus, right cortex, and internal capsule, with the largest cluster located in the putamen–globus pallidus containing 203 voxels. The converse comparison in which prenatal CD pigs exhibited increased white matter compared with prenatal CS pigs (i.e., CD > CS) yielded no significant (P > 0.05) clusters. Assessment of gray and white matter differences between postnatal CS and postnatal CD pigs yielded no significant differences (P > 0.05) for the 4 possible comparisons (i.e., gray matter CS > CD; gray matter CD > CS; white matter CS > CD; white matter CD > CS). Notably, the regions identified above were determined by inserting the local maxima X, Y, and Z coordinates listed in Table 1 into the Pig Brain Atlas and cross-referencing the brain region that was identified via the atlas.
TABLE 1.
Voxel-based morphometry assessment of gray and white matter differences between prenatally CS and CD pigs1
| Cluster | Peak | Local maxima coordinates3 | |||||
|---|---|---|---|---|---|---|---|
| Tissue type, comparison, and anatomic region2 | Voxels, n | P | P | Pseudo t | X | Y | Z |
| Gray | |||||||
| CS > CD | |||||||
| Left cortex | 312 | <0.001 | <0.001 | 9.54 | −14.0 | 16.8 | 13.3 |
| Right cortex | 22 | 0.003 | 0.002 | 7.87 | 9.1 | 23.8 | 4.2 |
| Right cortex | 21 | 0.004 | 0.007 | 7.36 | 7.0 | 25.9 | −1.4 |
| Left cortex | 42 | 0.001 | 0.018 | 6.88 | −17.5 | −2.8 | 14.7 |
| CD > CS | — | — | — | — | — | ||
| White | |||||||
| CS > CD | |||||||
| Putamen-GP | 203 | <0.001 | 0.001 | 8.59 | −8.4 | 13.3 | 1.4 |
| Right cortex | 53 | <0.001 | 0.014 | 7.05 | 12.6 | −9.8 | 4.9 |
| Internal capsule | 31 | 0.001 | 0.027 | 6.73 | 4.9 | 16.1 | 0.0 |
| CD > CS | — | — | — | — | — | ||
Voxel-based morphometry analysis of gray and white matter differences in the prenatal CS and CD pig brains at postnatal day 28. P values reported are family-wise error-corrected (P < 0.05), and a minimum cluster size of 20 voxels was used to determine voxel clusters listed in the table. CD, choline deficient; CS, choline sufficient; GP, globus pallidus.
Brain regions based on visual inspection of the cluster location and cross-referenced with the Piglet Brain Atlas (24).
Local maxima coordinates: X increases from left (−) to right (+), Y increases from posterior (−) to anterior (+), and Z increases from inferior (−) to superior (+).
FIGURE 1.
Voxel-based morphometric assessment in which prenatally CS pigs exhibited greater gray matter than did prenatally CD pigs. The range of dark blue to light blue indicates the degree of statistical difference, ranging from pseudo t values of 6.80 to 9.55, respectively. All clusters represented in the figure are significant at a P-corrected value of P < 0.01. Notably, there were no significant clusters in which prenatally CD pigs exhibited greater gray matter than prenatally CS pigs. Within the figure, the changes from Y = 70 to Y = 89 indicate movement from anterior to posterior, respectively, through the brain image slices. CD, choline-deficient; CS, choline-sufficient.
FIGURE 2.
Voxel-based morphometric assessment in which prenatally CS pigs exhibited greater white matter than did prenatally CD pigs. The range of red to yellow indicates the degree of statistical difference, ranging from pseudo t values of 6.70 to 8.60, respectively. All clusters represented in the figure are significant at a P-corrected value of P < 0.001. Notably, there were no significant clusters in which prenatally CD pigs exhibited greater white matter than prenatally CS pigs. Within the figure, the changes from Y = 35 to Y = 73 indicate movement from posterior to anterior, respectively, through brain image slices. CD, choline-deficient; CS, choline-sufficient.
TBSS
No differences due to prenatal choline status were observed for diffusion values along predetermined white matter tracts. Assessment of white matter tracts in which rates were higher in prenatally CD pigs than in prenatally CS pigs (prenatal: CD > CS) indicated no significant results for FA (P = 0.28), AD (P = 0.65), MD (P = 0.63), and RD (P = 0.41). The converse assessment of areas along white matter tracts in which prenatally CS pigs exhibited greater diffusion values than prenatally CD pigs (prenatal: CS > CD) also indicated no significant differences for FA (P = 0.17), AD (P = 0.23), MD (P = 0.27), and RD (P = 0.29). No differences due to postnatal choline status were observed for diffusion values along predetermined white matter tracts. Assessment of white matter tracts in which rates were higher in postnatally CD pigs than in postnatally CS pigs (postnatal: CD > CS) indicated no significant results for FA (P = 0.36), AD (P = 0.45), MD (P = 0.74), and RD (P = 0.91). The converse assessment of areas along white matter tracts in which postnatally CS pigs exhibited greater diffusion values than postnatally CD pigs (postnatal: CS > CD) also indicated no significant differences for FA (P = 0.95), AD (P = 0.89), MD (P = 0.59), and RD (P = 0.52). Supplemental Figure 1 shows the white matter skeleton that was used for TBSS assessment. Notably, the diffusion tensor values assessed above are commonly used to determine alterations in water movement within tissue, and changes in these values are related to changes in brain microstructure (28–30).
Discussion
Choline is an essential nutrient that is important for many aspects of brain structural and functional development (10). Research in rodent models of perinatal choline deficiency reported mechanisms through which altered choline status influences neurodevelopment; however, these methods of evaluation necessitate invasive techniques. Thus, the aim of this study was to use noninvasive neuroimaging techniques to characterize alterations in gray and white matter development due to perinatal choline deficiency. Herein, we report that prenatal dietary choline deficiency exerts a greater influence on gray and white matter development than postnatal dietary choline deficiency. These findings are novel because they use clinically translatable, noninvasive neuroimaging techniques in the well-established biomedical pig model to show alterations in gray and white matter development as a result of prenatal choline deficiency. Moreover, our results indicate that postnatal choline deficiency does not have as great of an effect on gray and white matter development as deficiency during the prenatal period.
We previously reported that pigs exhibit smaller total brain volumes when exposed to choline deficiency during the prenatal period (17). Notably, our previous analysis assessed relative regional volumes without identifying if gray or white matter had a greater influence on volumetric changes. Thus, to further characterize specific influences of dietary choline on gray or white matter development, we used VBM to assess differences in gray and white matter on a voxel-wise basis. Our results indicate that pigs exposed to prenatal choline deficiency exhibited reduced gray matter in both cortices compared with prenatal CS pigs. These findings corroborate our previous report of reduced total brain volumes and smaller absolute volumes of the left and right cortex (17). Notably, there were no gray matter clusters in which prenatal CD pigs exhibited greater gray matter than prenatal CS pigs. Interestingly, these findings corroborate research in rodent models that indicates that choline deficiency alters neural development. Mice that were born to CD dams exhibited increased hippocampal apoptosis and reduced neural progenitor cell proliferation (14). Furthermore, a separate study in mice indicated decreased cortical neural progenitor cells and fewer upper layer cortical neurons in offspring from CD dams (15). Results from Wang et al. (15) suggest that the apparent disruption in cortical development is the result of decreased epidermal growth factor receptor signaling in the CD mice. A separate study suggests that prenatal choline deficiency in rats results in medial septal neurons with smaller cross-sectional areas compared with control rats (13). Although our findings were not in the same brain regions as those observed by McKeon-O'Malley et al. (13), the observed decrease in gray matter in prenatally CD pigs may be the result of smaller neuron sizes. Although cellular analysis was not a primary outcome in this study, our neuroimaging results provide a noninvasive characterization of altered cortical development in pigs that were exposed to choline deficiency throughout gestation, and corroborate our previous findings of reduced absolute cortical volumes in prenatally CD pigs (17).
In pigs, cortical development reaches its maximal growth rate at 4 wk of age and is expected to continue for several weeks thereafter (31). In this study, we report differences in cortical gray matter at 4 wk of age; our previous analysis reported differences in absolute cortical volumes but no differences in relative volumes of the cortex (17). We speculate that our VBM results indicate the very early stages of altered cortical development in which neural progenitor cells may be altered or even reduced in number, thereby presenting as reduced gray matter in our VBM findings. With the use of the findings by Wang et al. (15) as evidence, we suggest that imaging the pigs at a later time point may result in reduced cortical volumes in the prenatally CD pigs, because it would be expected that cortical layers would not form properly, thus presenting as a reduction in cortical volume. Although histological analysis of brain samples was not an outcome of our study, future work should seek to characterize cortical development in the context of perinatal choline deficiency. Work of this nature is warranted to verify similar influences of dietary choline across animal models as well as providing substantiation for observations made through noninvasive techniques.
The assessment of white matter differences indicates an effect of prenatal choline status, in which prenatally CS pigs exhibit localized clusters of increased white matter compared with prenatally CD pigs. Although all gray matter differences were identified in cortical regions, the greatest differences in white matter appeared to be located in the putamen–globus pallidus and internal capsule. Notably, the location of all clusters was determined with the use of the Pig Brain Atlas, which characterizes 19 different brain regions. Although informative, the Pig Brain Atlas is not as granular as a human brain atlas; thus, a more specific atlas may help to better define the exact locations of these differences.
The internal capsule is one of the earliest myelinating brain regions (32) and the presence of dietary choline is necessary for myelin components such as phospholipids and sphingomyelin (11). Thus, this observed reduction in white matter at 4 wk of age in prenatally CD pigs may provide early indications of altered myelination. Our previous analysis did not observe diffusion differences in the internal capsule of these pigs (17), which may suggest that myelination was still occurring and was not altered enough to be detected through diffusion tensor imaging. Notably, diffusion tensor imaging is commonly used to assess alterations in water movement in tissue and is influenced by changes in myelination and axon growth and development and increased fiber packing (28–30). If white matter differences are evident at 4 wk of age, we speculate that imaging at later time points would illuminate differences in diffusion within the internal capsule.
Our results also indicate reduced white matter in the putamen–globus pallidus region of prenatally CD pigs. Although there are not many studies that suggest an influence of dietary choline on white matter in these regions, it is known that the putamen and the globus pallidus, among other brain regions, contain a high density of cholinergic neurons (33). Moreover, reduced acetylcholine concentrations (34) and impaired acetylcholine synthesis were observed in the striatum (i.e., brain region containing the caudate and putamen) of CD rats (35). A separate study noted decreased phosphatidylcholine concentrations in striatal cells that were stimulated in media containing low concentrations of choline, thereby suggesting the choline-containing phospholipids acted as a choline pool for acetylcholine synthesis (36). White matter is, in part, composed of myelin, and among many other components, myelin is composed of phospholipids and sphingomyelin (11). Thus, the observed reduction in white matter in this region, which is highly innervated with cholinergic neurons, may suggest a breakdown of phospholipid products due to choline deficiency to ensure proper acetylcholine synthesis. To further characterize this observation, future work should seek to quantify acetylcholine concentrations in this brain region in the context of a choline deficiency intervention and should assess myelination to identify the extent of white matter composition in this region.
Interestingly, our neuroimaging assessments indicated no significant effects of postnatal choline deficiency on aspects of gray and white matter development. These findings corroborate our previous findings, which indicate that the greatest effect of postnatal choline deficiency was on glycerophosphocholine-phosphocholine concentrations and not on brain volumes (17). Whole-brain and cortical brain growth in the pig are at the maximal rate of growth at ∼4 wk of age (31); thus, it is possible that postnatal dietary choline deficiency would not be evident until later in life. Our findings may suggest that choline deficiency exerts its greatest effects on brain development during the prenatal period. Research suggests that children born to mothers with higher gestational choline intake perform better on cognitive tasks later in life (12). Thus, these findings of altered gray and white matter development may offer a noninvasive characterization of alterations in brain structure that result in later-life cognitive deficits.
In conclusion, we report herein that prenatal choline deficiency influences cortical gray matter development and subcortical white matter development in young pigs. Although adequate choline intake is critical throughout the perinatal period, the lack of postnatal effects on gray and white matter development highlights the importance of choline during gestation. Because most prenatal supplements do not contain adequate choline and pregnant women often fall below the suggested daily intake amounts, our findings highlight the need for continued efforts to ensure proper choline concentrations are achieved in pregnant women.
Supplementary Material
Acknowledgments
We thank the University of Illinois Imported Swine Research Laboratory staff and Jennifer Rytych for their help in daily pig-rearing activities. We also thank the MRI technicians, Holly Tracy and Nancy Dodge, at the Beckman Institute for Advanced Science and Technology Imaging Center for their help in MRI data acquisition. The authors’ responsibilities were as follows—RND and CMG: designed the research; CMG: conducted the animal study; ATM: processed MRI data; ATM and RND: analyzed MRI data; and all authors: wrote the manuscript, and read and approved the final manuscript.
Notes
Supported by the USDA National Institute of Food and Agriculture, Hatch Project 1009051. Archer Daniels Midland donated the soy protein isolate used in the formation of the diets.
Author disclosures: ATM, CMG, and RND, no conflicts of interest. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used:
- AD
axial diffusivity
- CD
choline-deficient
- CS
choline-sufficient
- FA
fractional anisotropy
- FSL
FMRIB Software Library
- MD
mean diffusivity
- RD
radial diffusivity
- TBSS
tract-based spatial statistics
- VBM
voxel-based morphometry
References
- 1. Institute of Medicine. Dietary Reference Intakes for folate, thiamin, riboflavin, niacin, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 2. Jensen HH, Batres-Marquez SP, Carriquiry A, Schalinske KL. Choline in the diets of the US population: NHANES, 2003–2004. FASEB J 2007;21:lb219. [Google Scholar]
- 3. Zeisel SH, Da Costa KA. Choline: an essential nutrient for public health. Nutr Rev 2009;67:615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chester DN, Goldman JD, Ahuja JK, Moshfegh AJ. Dietary intakes of choline: What We Eat in America, NHANES 2007–2008 [Internet]. Food Surveys Research Group Dietary Data Brief No. 9. 2011. Available from: http://ars.usda.gov/Services/docs.htm?docid=19476, last accessed January 2018. [Google Scholar]
- 5. Wallace TC, Fulgoni VL. Assessment of total choline intakes in the United States. J Am Coll Nutr 2016;35:108–12. [DOI] [PubMed] [Google Scholar]
- 6. Da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J 2006;20:1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeisel SH. Nutrition in pregnancy: the argument for including a source of choline. Int J Womens Health 2013;5:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gossell-Williams M, Fletcher H, McFarlane-Anderson N, Jacob A, Patel J, Zeisel S. Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med J 2005;54:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeisel SH. Nutritional importance of choline for brain development. J Am Coll Nutr 2004;23:621–6. [DOI] [PubMed] [Google Scholar]
- 10. Zeisel S, Niculescu M. Perinatal choline influences brain structure and function. Nutr Rev 2006;64:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caudill MA. Pre- and postnatal health: evidence of increased choline needs. J Am Diet Assoc 2010;110:1198–206. [DOI] [PubMed] [Google Scholar]
- 12. Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol 2013;177:1338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKeon-O'Malley C, Siwek D, Lamoureux JA, Williams CL, Kowall NW. Prenatal choline deficiency decreases the cross-sectional area of cholinergic neurons in the medial septal nucleus. Brain Res 2003;977:278–83. [DOI] [PubMed] [Google Scholar]
- 14. Craciunescu CN, Albright CD, Mar M, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr 2003;18:3614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Surzenko N, Friday WB, Zeisel SH. Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring. FASEB J 2016;30:1566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mudd AT, Dilger RN. Early-life nutrition and neurodevelopment: use of the piglet as a translational model. Adv Nutr 2017;8:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mudd AT, Getty C, Sutton B, Dilger R. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr Neurosci 2016;19:425–33. [DOI] [PubMed] [Google Scholar]
- 18. Getty CM, Dilger RN. Moderate perinatal choline deficiency elicits altered physiology and metabolomic profiles in the piglet. PLoS One 2015;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US National Research Council. Nutrient requirements of swine. 11th rev Washington (DC): The National Academies Press; 2012. [Google Scholar]
- 20. Mudd AT, Alexander LS, Johnson SK, Getty CM, Malysheva O V, Caudill MA, Dilger RN. Perinatal dietary choline deficiency in sows influences concentrations of choline metabolites, fatty acids, and amino acids in milk throughout lactation. J Nutr 2016;146:2216–23. [DOI] [PubMed] [Google Scholar]
- 21. Radlowski EC, Conrad MS, Lezmi S, Dilger RN, Sutton B, Larsen R, Johnson RW. A neonatal piglet model for investigating brain and cognitive development in small for gestational age human infants. PLoS One 2014;9:e91951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mudd A, Fil J, Knight L, Lam F, Liang Z-P, Dilger R. Early-life iron deficiency reduces brain iron conten t and alters brain tissue composition despite iron repletion: a neuroimaging assessment. Nutrients 2018;10(2):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mudd A, Alexander L, Berding K, Waworuntu R, Berg B, Donovan S, Dilger R. Dietary prebiotics, milk fat globule membrane and lactoferrin affects structural neurodevelopment in the young piglet. Front Pediatr 2016;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conrad MS, Sutton BP, Dilger RN, Johnson R. An in vivo three-dimensional magnetic resonance imaging-based averaged brain collection of the neonatal piglet (Sus scrofa). PLoS One 2014;9:107650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mudd AT, Waworuntu RV, Berg BM, Dilger RN. Dietary alpha-lipoic acid alters piglet neurodevelopment. Front Pediatr 2016;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(Suppl 1):S208–19. [DOI] [PubMed] [Google Scholar]
- 27. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–505. [DOI] [PubMed] [Google Scholar]
- 28. Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–46. [DOI] [PubMed] [Google Scholar]
- 29. Drobyshevsky A, Song S, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci 2005;25:5988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conrad MS, Dilger RN, Johnson RW. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev Neurosci 2012;34:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SCR, Murphy DGM. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci 2011;31:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mesulam M-M, Mash D, Hersh L, Bothwell M, Geula C. Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J Comp Neurol 1992;323:252–68. [DOI] [PubMed] [Google Scholar]
- 34. Wecker L, Schnidt DE. Central cholinergic function: relationship to choline administration. Life Sci 1979;25:375–84. [DOI] [PubMed] [Google Scholar]
- 35. Wecker L. Influence of dietary choline availability and neuronal demand on acetylcholine synthesis by rat brain. J Neurochem 1988;51:497–504. [DOI] [PubMed] [Google Scholar]
- 36. Ulus IH, Wurtman RJ, Mauron C, Blusztajn JK. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res 1989;484:217–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.