Cities often warm at faster rates during the day and across space. The mechanisms underlying how organisms respond to these climatic changes in cities are largely unknown. This study finds evidence that the heat tolerance of an acorn-dwelling ant species has evolved rapidly to cope with these faster rates of urban warming.
Keywords: Thermal tolerance, microclimate, physiology, phenotypic plasticity, evolution, urban heat island
Abstract
Because cities contain high levels of impervious surfaces and diminished buffering effects of vegetation cover, urbanized environments can warm faster over the day and exhibit more rapid warming over space due to greater thermal heterogeneity in these environments. Whether organismal physiologies can adapt to these more rapid spatio-temporal changes in temperature rise within cities is unknown, and exploring these responses can inform not only how plastic and evolutionary mechanisms shape organismal physiologies, but also the potential for organisms to cope with urban development. Here, we examined how plasticity in thermal tolerance under faster and slower rates of temperature change might evolve in response to the more rapid spatio-temporal temperature rise in cities. We focused on acorn ants, a temperature-sensitive, ground-dwelling ant species that makes its home inside hollowed out acorns. We reared acorn ant colonies from urban and rural populations under a common garden design in the laboratory and assessed the thermal tolerances of F1 offspring workers using both fast (1°C min−1) and slow (0.2°C min−1) rates of temperature change. Relative to the rural population, the urban population exhibited higher heat tolerance when the temperature was increased quickly, providing evidence that temperature ramp-rate plasticity evolved in the urban population. This result was correlated with both faster rates of diurnal warming in urban acorn ant nest sites and greater spatial heterogeneity in environmental temperature across urban foraging areas. By contrast, rates of diurnal cooling in acorn ant nest sites were similar across urban and rural habitats, and correspondingly, we found that urban and rural populations responded similarly to variation in the rate of temperature decrease when we assessed cold tolerance. Our study highlights the importance of considering not only evolutionary differentiation in trait means across urbanization gradients, but also how trait plasticity might or might not evolve.
Introduction
Phenotypic changes in populations across rural-to-urban gradients are evident across a wide range of taxa (Alberti et al., 2017), but the mechanisms governing these shifts in phenotype are not well understood. A key mechanistic question is whether urban-driven phenotypic shifts are entirely the result of plasticity, or whether there is a role for evolutionary change (Diamond and Martin, 2016; Merilä and Hendry, 2014). The argument for the dominance of plastic responses to cities seems plausible given the rapid timeframe over which urbanization processes typically occur, and the generally long timeframes over which evolution is thought to occur. Yet, there is more and more evidence for rapid evolution in cities (Johnson and Munshi-South, 2017). Disentangling the contributions of phenotypic plasticity and evolutionary change under urbanization is certainly an important research priority, as these mechanisms can occur over different timescales and operate under different constraints (Sgrò et al., 2016) with sometimes divergent implications for vulnerability to global change (Franks et al., 2014). But there is perhaps an underappreciated degree of subtlety here that can be overlooked in a framework that merely parses phenotypic variation into plastic and evolved components. And that subtlety has to do with the fact that plasticity itself can evolve (Ghalambor et al., 2007).
Why might we expect plasticity to evolve in cities? One likely candidate for evolved plasticity lies in the physiological tolerance of temperature change, particularly as rates of temperature change over time and space differ substantially among urban and rural habitats. Although cities are often warmer on average (by several °C) compared with nearby rural habitats (Imhoff et al., 2010), diurnal rates of temperature change, especially the rate of warming from nighttime to peak daytime temperatures, and the rate of warming across space are often more rapid in urban areas, owing to the diminished buffering effects of vegetation cover in developed areas (Adams and Smith, 2014). As a consequence, we might then expect urban populations to evolve a stronger plastic response in heat tolerance to rapid warming, i.e. that urban populations will achieve relatively higher heat tolerance when environmental temperature is increased quickly compared with rural populations. Shifts in estimated thermal tolerance as a function of the rate of temperature change (the “ramp rate”) are well documented for a number of ectothermic species (Allen et al., 2016; Castañeda et al., 2015; Chown et al., 2009; Moyano et al., 2017). However, the nature of this variation has generally been explored in context of the putative trade-offs between the ecological relevance of slower ramp rates versus the minimization of confounding acclimation effects not due to temperature stress (i.e. starvation and desiccation stress) associated with faster ramp rates (Terblanche et al., 2011). Yet, in natural settings, organisms may experience slower and faster rates of temperature change, though few studies have quantified relevant rates of temperature change in the field (Woods et al., 2015), and even fewer have examined the evolution of tolerance plasticity in response to different rates of environmental temperature change (but see Allen et al. (2016) for recent comparative work demonstrating interspecific variation in tolerance plasticity across different temperature ramp rates).
Here we use cities to explore how environmental variation in the rate of temperature change among urban and rural populations alters the plastic expression of thermal tolerance, and how this plasticity might evolve. Our study organism, the acorn ant (Temnothorax curvispinosus) is highly sensitive to temperature, including in development rate (Penick et al., 2017), running speed (Maclean et al., 2017) and thermal tolerance (Diamond et al., 2013). Previously, we found evidence for evolved differences in heat and cold tolerance of acorn ants across an urbanization gradient such that urban populations exhibited increases in heat tolerance and losses in cold tolerance relative to rural populations (Diamond et al., 2017); however, we did not explore whether plasticity had evolved, specifically whether urban acorn ants were able to better tolerate more rapid changes in environmental temperature. Like many ectothermic species, the thermal tolerances of ants appear to be sensitive to the rate of temperature change (Ribeiro et al., 2012).
In this study, we quantified ecologically relevant rates of temperature change during the growing season for urban and rural acorn ants in the field, and linked these environmental differences with heat tolerance responses to faster and slower rates of temperature change in the laboratory. Through field-based recordings of environmental temperature and a laboratory common garden experiment, we evaluated the evidence for: (i) whether acorn ant nest sites warm more rapidly over time in urban habitats; (ii) whether the magnitude of spatial temperature change across the foraging area is greater in urban habitats; and (iii) whether differences in the rate of temperature change over time and space among urban and rural habitats are consistent with physiological responses to rate of temperature change. Specifically, we examined whether there has been evolutionary divergence in temperature ramp-rate plasticity such that urban populations of acorn ants are better able to tolerate more rapid increases in temperature change over space and time compared with rural populations. Although we focused on heat tolerance and spatio-temporal changes in environmental temperature during daytime hours when ants are most active within the growing season, we also examined plasticity in cold tolerance responses to different rates of temperature decrease and temporal changes in rates of cooling from peak daytime temperatures to nighttime within the growing season. The diurnal temperature change experienced by acorn ants over winter when ants are largely inactive and confined to the nest environment is likely the most relevant to link with ramp-rate plasticity in cold tolerance, though rates of cooling from day to night during the growing season may nonetheless provide a proxy. Although relevant physiological and environmental data are sparse, we hypothesized that urban populations might be less able to tolerate more rapid decreases in temperature compared with rural populations. This expectation is based on widespread signatures of nighttime-biased warming in many cities, which could lead to diminished rates of temperature decrease over diurnal timescales (Imhoff et al., 2010). Alternatively, snow cover could homogenize rates of temperature decrease (Mitrus, 2013) that acorn ants experience in urban and rural habitats, potentially leading to a lack of population differentiation in cold tolerance plasticity across different rates of temperature decrease.
Materials and methods
Acorn ant collections
The acorn ant, T. curvispinosus, is widely distributed across the eastern United States, and commonly occurs in both urban and rural habitats. Colonies typically contain 50–200 workers and several queens at maturity. While queen lifespan can be quite high: from 5 up to 15 years (Keller, 1998), worker lifespan is comparatively short, on the order of one month (Herbers, 1989; Herbers and Johnson, 2007; Modlmeier et al., 2013). The entire colony resides within pre-formed cavities in leaf litter, typically oak acorns, hickory nuts and small twigs; queens and immature workers are confined to the nest environment, while mature workers frequently leave the nest to forage on detritus (Herbers, 1989). As a consequence of their above-ground nesting habit, acorn ant colonies experience little buffering from changes in air temperature, compared with other species that diurnally and seasonally burrow underground to thermoregulate (Herbers and Johnson, 2007). Owing to their small body size, T. curvispinosus foraging distances are also quite small, often 1 m or less, and dispersal distances are similarly restricted, typically less than a few meters (Diamond et al., 2012; Pratt, 2008). This small dispersal distance makes the species excellent for the study of urban thermal physiology, as highly structured populations can be found over small geographic scales (Stuart, 1987).
We collected acorn ant colonies from four urban and three rural sites across Cleveland, OH, USA (42°N latitude) in June 2016 (Supplementary Table 1). To identify urban and rural habitats, we used percent developed impervious surface area (ISA) (Imhoff et al., 2010), such that urban sites were categorized as between 40 and 60% ISA, and rural sites were categorized as 0% ISA (note that ISA values were based on the 90 m radius around a particular site). Given that acorn ants are a cavity-nesting species and are reliant upon a supply of acorns for nest construction—acorn ants change their nest several times throughout the year (Alloway et al., 1982; Herbers and Johnson, 2007)—our urban sites were effectively forested patches surrounded by developed areas. By contrast, rural sites were situated within large stands of continuous forest. Although the urbanization process may entail a variety of changes including to vegetation, habitat structure and the level of environmental contaminants, our comparison of forested patches within cities with rural forested areas allowed us to minimize the influence of these confounding variables while focusing on the temperature signal of urbanization.
Thermal landscapes
We measured the temperature profiles in both acorn ant nest sites and the foraging areas surrounding individual nests. Because nests are static in space, we were most interested in the diurnal temperature changes in rural versus urban habitats for the nest environment. Within foraging areas, we were most interested in the spatial variation in temperature across the foraging area, i.e. how a foraging worker would experience temperature differences as they moved throughout the landscape.
For June through July 2017 (this time of year captures peak activity season for acorn ants (Penick et al., 2017)), we recorded temperature every 30 min from nine temperature loggers (iButtons, Maxim Integrated Thermochron data loggers DS1921G-F5) arrayed in a 2 m × 2 m square grid, each centered on a known acorn ant nest. We recorded from three rural grids and four urban grids (Fig. 1b). While some temperature loggers were situated in more shaded microhabitats and others were situated in light gaps, all temperature loggers were buffered against direct solar radiation by being encapsulated within 15 × 1.9 cm2 (l × w) sections of white PVC piping. The PVC piping was left open on either end to allow for air flow and laid flat against the ground to capture air temperature near the acorn ant nest and forager height. The central temperature logger within the grid and directly adjacent to the acorn ant nest was assayed for temporal changes in temperature, whereas the entire grid was used to assay spatial variation in temperature experienced by foraging ants. Although all temperature loggers were deployed in the same manner within each grid (i.e. housed within a PVC pipe shield), we only analyzed the central temperature logger next to the known nest site for temporal changes in temperature to best capture the microsite selection of nest locations by acorn ants. Because this approach limited our sample size for nest site temperature data, we added nest site temperature data recorded June–July in 2016 (using the same design of an iButton temperature logger housed within PVC piping) from one additional urban site and one additional rural site (the total number of nest site temperature recordings was: nrural = 4, nurban = 5).
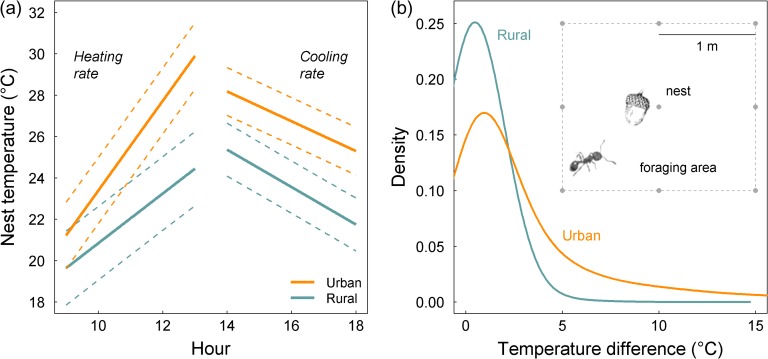
Figure 1:
The rate of diurnal temperature change in urban and rural acorn ant nest sites and the magnitude of spatial temperature change across foraging areas. (a) Predicted values (mean and standard error) from models of rates of nest site warming (9 a.m. to 1 p.m.) and nest site cooling (2 p.m. to 6 p.m.) in urban and rural habitats during peak activity season (June–July). (b) Kernel density distributions (smoothed histograms) of the hourly pairwise differences (during the peak foraging interval, 9 a.m. to 1 p.m.) among foraging area temperatures for urban and rural habitats, again during peak activity season (June–July).
The use of this standardized temperature logger design (iButtons housed within PVC piping) allowed us to make direct comparisons of environmental temperature across urban and rural habitats. Even if the temperature logger design does not capture precise nest and foraging area temperature (e.g. owing to differences in the size, shape and moisture levels of an acorn ant nest or how foraging ant body temperature responds to environmental temperature differences), it still captures the relative differences in spatio-temporal rates of environmental temperature change across urban and rural habitats.
Common garden experiment
We collected urban and rural acorn ant colonies from the wild and reared them under common garden temperature treatments in the laboratory. After collection from the field, ant colonies were allowed to acclimate to laboratory conditions (~25°C, an intermediate temperature among our two temperature treatments) for ~24 h prior to being randomly assigned to two temperature treatments. Colonies from each source population (urban or rural) were reared under either a “warm” temperature regime mimicking peak urban foraging temperatures during the growing season (25°C nighttime temperature and 30°C daytime temperature) or a “cool” temperature regime mimicking peak rural foraging temperatures during the growing season (20°C nighttime temperature and 25°C daytime temperature) (see Diamond et al. (2017) for the development of rearing temperature regimes from environmental temperature data). Each treatment was kept under a standard long-day 14:10 L:D photoperiod, with the temperature change synced to the day–night cycle.
To generate F1 offspring for thermal tolerance testing, we reared colonies long-term, for a minimum of 5 weeks, in the laboratory common garden temperature treatments. The long acclimation period allowed sufficient time for turnover of workers within the colonies, i.e. the original workers died and the queens produced a new cohort of workers from eggs laid entirely within the common garden treatments, prior to assessment of thermal tolerance. This aspect of the experimental design allowed us to eliminate developmental acclimation effects from the field environment and some, but not all, potential maternal effects (Massamba-N’Siala et al., 2014). Each colony was maintained individually in a 120 mL plastic cup. Resource tubes with sugar water (25% solution) and plain tap water were provided to colonies along with a continuous supply of dead mealworms.
At the end of the laboratory acclimation period, we used a dynamic temperature ramping protocol to assess the critical thermal maximum and minimum (CTmax and CTmin), each defined as the loss of muscular coordination, which yields ecologically relevant metrics of heat and cold tolerance (Terblanche et al., 2011). Ant workers were placed individually into 1.5 mL Eppendorf tubes with a cotton plug in the lid. Temperatures were manipulated using a dry block incubator (Boekel Scientific Tropicooler). We split the workers of a single colony into two groups for assessing thermal tolerance: in one group, the temperature was increased or decreased at a rate of 1°C min−1 (the “fast” ramp rate, within the context of our study) and in the other group, the temperature was increased or decreased at a rate of 0.2°C min−1 (the ‘slow’ ramp rate). Each tube was pulled out of the incubator once at the beginning of each temperature interval to check for muscular coordination of the individual ant, homogenizing the time spent inside and outside the incubator for each individual. Initial temperature for the estimation of CTmax was 34°C, and was 16°C for CTmin. We assayed CTmax and CTmin using fast and slow rates of temperature change for a total of 30 colonies (number of colonies per population/temperature treatment/temperature rate: nrural/cool/fast = 7; nrural/warm/fast = 8; nurban/cool/fast = 8; nurban/warm/fast = 7; nrural/cool/slow = 7; nrural/warm/slow = 8; nurban/cool/slow = 8; nurban/warm/slow = 7). The mean number of workers assayed for each combination of CTmax and CTmin, fast and slow rate of temperature change, warm and cool temperature treatment and urban and rural source population was 8.77 (± 2.40, 1 SD).
Statistical analyses
All statistical analyses were performed in R (R Core Team, 2017). To examine how diurnal changes in nest site temperature profiles differed among urban and rural habitats, we constructed two linear mixed effects models using the lmer function from the {lme4} package (Bates et al., 2015)—one model to assess nest site warming rates and one to assess nest site cooling rates. The nest site warming rate model included temperature as the response and hour (9 a.m. to 1 p.m.), source population (urban or rural) and their interaction. We included random intercept terms to account for autocorrelation due to the temperature logger and day from which temperatures were recorded. The nest site cooling rate model was constructed in a similar fashion to the nest site warming rate model except that the hours under consideration ranged from 2 p.m. to 6 p.m. The 9 a.m. to 1 p.m. and 2 p.m. to 6 p.m. time intervals represent the linear portions of the nest site warming and cooling periods over the course of a day (Supplementary Fig. S1). We did not model rates of temperature change between 1 p.m. and 2 p.m. owing to site- and day-level variation in the hour at which maximum daytime temperatures were reached. Sliding the time interval window by 1–2 h did not qualitatively alter our results (for models of both rates of temperature increase and decrease).
We also examined how spatial differences in foraging area temperature profiles differed among urban and rural habitats. Because we were specifically interested in the magnitude of temperature change across the spatial grid of the foraging area, we computed the Euclidean pairwise difference between each of the nine grid-arrayed temperature loggers for each hour within the peak foraging interval (9 a.m. to 1 p.m.) across the temperature monitoring period, and examined the distributions of these temperature profiles using kernel density estimation. We then constructed a simple linear model to assess whether the maximum of the urban and rural kernel density curves for the differences in temperature across the foraging area were significantly different from one another.
To examine how urban and rural populations differed in their ability to tolerate slow and fast rates of temperature increase (and decrease), we constructed linear mixed effects models separately for CTmax and CTmin as responses. As predictors, we included the main effects, two- and three-way interactions of the source population (urban or rural), rate of temperature change (fast or slow) and temperature treatment (warm or cool). We also included a random intercept for colony identity to account for non-independence among workers from the same colony. In the case of significant interactions among our predictor variables, we performed post-hoc analyses using the lsmeans function from the {lsmeans} package (Lenth, 2016).
Finally, to gain insight into potential genetic correlations among tolerance responses to different rates of temperature change, we examined the colony mean correlations between thermal tolerances assessed using the fast and slow rates of temperature change. We performed separate correlation analyses for each combination of source population, temperature treatment and tolerance metric (heat or cold tolerance). Owing to small sample sizes for colony mean tolerances, we performed Spearman’s rank correlation analysis.
Results
Ramp-rate plasticity in heat tolerance and rates of environmental temperature change
Rates of temperature increase, both over time at acorn ant nest sites and over space across foraging areas were greater in urban environments compared with rural environments (Fig. 1). The linear mixed model of nest site temperature as a function of hour during the diurnal period of nest site warming, source population, and their interaction revealed a statistically significant interaction between hour and source population (estimate and SE: 0.968 ± 0.166, χ2 = 34.1, P < 0.0001, nobs = 1316, ngroups = 9 temperature loggers and 30 days), indicating the temperature rise per hour at the nest site was nearly a full degree Celsius greater in urban habitats compared with rural habitats. When we modeled the rate of temperature increase separately for each location, we found that the rate of temperature increase in the urban locations was 2.18 ± 0.130°C h−1 compared with 1.17 ± 0.0778°C h−1 in undeveloped forest habitat.
Likewise, we found that the magnitude of temperature change across the foraging area (estimated as the peak of the kernel density distribution of pairwise differences in environmental temperature among the temperature loggers within each foraging area grid) was greater in urban areas by nearly 0.5°C compared with rural areas (estimate and SE: 0.494 ± 0.110, F = 20.2, P = 0.00641, df = 5). We also computed the pairwise differences in environmental temperature among the temperature loggers within each foraging area while restricting comparisons to those temperature loggers exactly 1m away from one another (rather than all pairwise differences among the temperature loggers); these comparisons yielded remarkably similar results, so we present only the results of the all-pairwise difference analysis. In general, the rural temperature differences were more uniform among the different rural sites, whereas the urban temperature differences exhibited more site-level variation (Supplementary Fig. S2).
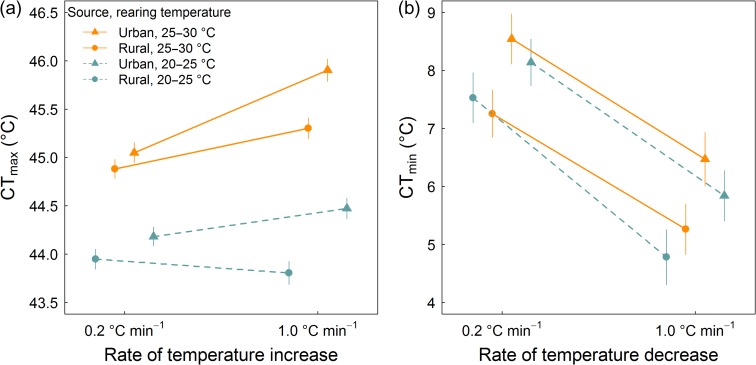
We found that urban populations of acorn ants are better at responding to rapid increases in temperature compared with rural acorn ant populations, that is, they achieve relatively higher CTmax values when environmental temperature is increased quickly compared with rural populations (Fig. 2a). In support, our statistical models revealed a significant interaction between the rate of temperature increase and source population such that urban ants tested using a fast rate of temperature increase during the thermal tolerance assay had the greatest CTmax values (Table 1). Post-hoc tests allowed us to further explore the nature of this interaction: we found that the magnitude of the difference between CTmax responses to fast versus slow rates of temperature increase was greater for urban populations than for rural populations. Indeed, we found no significant difference between rural population CTmax responses to fast versus slow rates of temperature increase (Table 2). Relatedly, we found that the population differentiation between urban and rural populations in CTmax was much more apparent under faster rates of temperature increase than under slower rates of temperature increase. In fact, while the difference between urban and rural population CTmax was statistically significant under the fast rate of temperature increase, it was marginally non-significant under the slow rate of temperature increase (Table 2).
Figure 2:
Plasticity of thermal tolerance assessed using slower and faster rates of temperature change for urban and rural acorn ant populations reared under common garden under cool and warm temperature regimes: (a) heat tolerance, CTmax; and (b) cold tolerance, CTmin. Predicted values (mean and standard error) from linear mixed effects models of tolerance as a function of source population (urban, rural), rearing temperature (20–25°C, 25–30°C), rate of temperature change during the thermal tolerance assay (0.2°C min−1, 1.0°C min−1) and their interaction are shown.
Table 1:
Statistical model summaries (estimates, standard errors, likelihood ratio test statistics and P-values) of CTmax and CTmin as a function of the rate of temperature change during the thermal tolerance trials, whether the source population was urban or rural, the temperature under which the colony was reared during the common garden experiment, and the two- and three-way interactions involving these variables. Significant P-values at the 0.05 level are indicated in bold
| Tolerance type | Term | Estimate | SE | χ 2 | P |
|---|---|---|---|---|---|
| CTmax | Ramp rate | 0.0932 | 0.166 | 21.4 | <0.0001 |
| n obs = 528 | Source population | 0.615 | 0.176 | 21.7 | <0.0001 |
| n groups = 30 | Rearing temperature | 1.44 | 0.175 | 190 | <0.0001 |
| Ramp × Source | −0.343 | 0.225 | 7.58 | 0.00592 | |
| Ramp × Rearing | −0.473 | 0.224 | 12.8 | 0.000354 | |
| Source × Rearing | 0.0341 | 0.245 | 0.164 | 0.686 | |
| Ramp × Source × Rearing | −0.177 | 0.315 | 0.316 | 0.574 | |
| CTmin | Ramp rate | 2.74 | 0.452 | 112 | <0.0001 |
| n obs = 526 | Source population | 1.06 | 0.644 | 7.35 | 0.00669 |
| n groups = 30 | Rearing temperature | 0.482 | 0.646 | 0.508 | 0.476 |
| Ramp × Source | −0.448 | 0.610 | 0.177 | 0.674 | |
| Ramp × Rearing | −0.755 | 0.613 | 1.28 | 0.258 | |
| Source × Rearing | 0.149 | 0.906 | 0.368 | 0.544 | |
| Ramp × Source × Rearing | 0.530 | 0.858 | 0.382 | 0.537 |
Note that the baseline factor level for source population is the rural group and for the ramp rate is the fast group (1°C min−1).
Table 2:
Post-hoc tests (estimates, standard errors, test statistics and P-values) for the significant interactions from models of CTmax (Table 1). Significant P-values at the 0.05 level are indicated in bold
| Interaction | Difference | Group | Estimate | SE | t | P |
|---|---|---|---|---|---|---|
| Ramp rate × Source population | Rural—urban population | Fast ramp | −0.633 | 0.122 | −5.19 | <0.0001 |
| Rural—urban population | Slow ramp | −0.200 | 0.107 | −1.88 | 0.0716 | |
| Fast—slow ramp | Rural population | 0.139 | 0.112 | 1.24 | 0.215 | |
| Fast—slow ramp | Urban population | 0.572 | 0.110 | 5.18 | <0.0001 | |
| Ramp rate × Rearing temperature | Cold—warm temperature | Fast ramp | −1.46 | 0.122 | −12.0 | <0.0001 |
| Cold—warm temperature | Slow ramp | −0.900 | 0.107 | −8.45 | <0.0001 | |
| Fast—slow ramp | Cold temperature | 0.0742 | 0.112 | 0.663 | 0.508 | |
| Fast—slow ramp | Warm temperature | 0.637 | 0.110 | 5.78 | <0.0001 |
Similarly, we found that CTmax responses to fast versus slow rates of temperature increase depended on rearing temperature, as indicated by a significant interaction between the rate of temperature increase and rearing temperature (Table 1). Post-hoc tests further showed that the difference between CTmax responses to fast versus slow rates of temperature increase was most pronounced at the warmer rearing temperature (Table 2).
Ramp-rate plasticity in cold tolerance and rates of environmental temperature change
In contrast to our results for CTmax, we saw no evidence of an interaction between the rate of temperature change during the thermal tolerance assay and source population for CTmin, although cold tolerance was generally improved for rural acorn ant populations and when cold tolerance was assessed using a faster rate of temperature change (Fig. 2b and Table 1).
The lack of interaction between source population and the rate of temperature change was consistent with the lack of interaction between source population and rate of nest site cooling. The linear mixed model of nest site temperature as a function of hour during the diurnal period of nest site cooling, source population, and their interaction did not detect a significant interaction between hour and source population (estimate and SE: 0.184 ± 0.134, χ2 = 1.84, P = 0.175, though as expected urban habitats were warmer on average 0.243 ± 2.70, χ2 = 3.84, P = 0.0499 and temperatures in both habitats decreased over the course of the afternoon −0.905 ± 0.101, χ2 = 142, P < 0.0001; for each model nobs = 1316, ngroups = 9 temperature loggers and 30 days). These results indicate that the rate of temperature decrease per hour at nest sites was the same across urban and rural environments.
Genetic correlations between fast and slow-ramped thermal tolerance
Spearman’s rank correlations between mean colony thermal tolerance assessed under slower and faster rates of temperature change were generally weak and statistically non-significant. For CTmax, ρurban/warm = 0.352, P = 0.439; ρrural/warm = −0.271, P = 0.516; ρurban/cool = −0.220, P = 0.601; ρrural/cool = 0.132, P = 0.778; and for CTmin, ρurban/warm = −0.655, P = 0.111; ρrural/warm = 0.132, P = 0.756; ρurban/cool = −0.707, P = 0.0501; ρrural/cool = −0.127, P = 0.786.
Discussion
Urban development can alter the thermal landscape, in many cases leading to more rapid changes in temperature over time and space (Imhoff et al., 2010). Evolutionary differentiation among urban and rural populations in terms of their mean thermal tolerance has been described in some systems (Brans et al., 2017; Diamond et al., 2017), and urban-driven evolutionary change in sub-lethal performance traits like growth rate, which captures the plastic response of growth across a range of rearing temperatures, has likewise been demonstrated (McLean et al., 2005; Tüzün et al., 2017). But whether the plastic response of thermal tolerance to different rates of temperature change evolves in urban environments is unclear. In this study, we examined how plasticity in thermal tolerance across faster and slower rates of temperature change (the “ramp rate”) evolves in response to the more rapid rate of temperature change experienced in cities. Using a common garden experimental design with acorn ants from urban and rural populations, we found that, relative to rural population ants, urban population ants exhibited greater heat tolerance under the fast rate of temperature change, and this result was correlated with both faster rates of diurnal temperature rise in urban acorn ant nest sites and more rapid spatial changes in temperature across urban foraging areas. These results underscore the importance of linking ramp rate plasticity in thermal tolerance traits with ecologically relevant variation in the rate of temperature change in the field, and further demonstrate the need to examine not only evolutionary differentiation in mean trait values, but also trait plasticity.
The sensitivity of thermal tolerances to the nature of temperature stress—whether static or dynamic, and in the latter case, whether the temperature change is faster or slower—is well established for a number of ectothermic species (Allen et al., 2016; Castañeda et al., 2015; Chown et al., 2009; Moyano et al., 2017; Overgaard et al., 2012; Terblanche et al., 2011). For the dynamic assessment of thermal tolerance, where a temperature stress is applied incrementally over time until the loss of muscular coordination (the critical temperatures, CTmax and CTmin) or until death (the lethal thermal limits), the relative merits of faster or slower rates of temperature change have been debated for some time (Terblanche et al., 2011). The debate is also far from an academic one: the differences in tolerance estimates using faster or slower rates of temperature change can be substantial, on the order of several degrees Celsius. Faster rates of temperature change typically lead to upward bias in the magnitude of tolerance, either improved heat tolerance or improved cold tolerance, potentially as a consequence of minimizing confounding effects including starvation and desiccation (Chown et al., 2009; Rezende et al., 2011; Santos et al., 2011, 2012). The tradeoff with faster rates of temperature change mitigating potential confounding variables is that they have been criticized as having less ecological relevance. By contrast, while slower rates of temperature change may be more ecologically relevant, they may also be confounded non-temperature aspects of physiological stress (but see Overgaard et al., 2012). A major assumption here is that slow rates of temperature change are the most ecologically relevant—for decades the most commonly used ramp rate was ~0.3°C min−1 as a compromise between fast and slow rates of temperature change, though positioned more toward the slower rate of temperature change (reviewed in Allen et al., 2016). Recent syntheses show that while slow rates of temperature change are appropriate in many scenarios, there is considerable variation in the rate of environmental temperature change in nature (Terblanche et al., 2011). Below we explore the nature of environmental temperature variation and challenge the assumption of the ecological relevance of slow rates of temperature change, particularly in urbanized landscapes.
First, however, our results provide clear evidence of the substantial influence of ramp rate on tolerance estimates, irrespective of how well ramp rates used during laboratory assays match environmental rates of temperature change. We generally find support for upward bias in the estimates, such that faster rates of temperature change yield improved heat tolerance (higher CTmax values) and cold tolerance (lower CTmin values). The effect of ramp rate was greater for CTmin than for CTmax (Table 1), though this is consistent with other findings from ectothermic species which show more limited plasticity in heat tolerance under differences in mean rearing temperature (Gunderson and Stillman, 2015; Sørensen et al., 2016). Additionally, we found contrasting roles for the interactive effects of rearing temperature and ramp rate on CTmin and CTmax. While we found that the effect of ramp rate was independent of rearing temperature for CTmin, we found that the relationship between CTmax and the rate of temperature increase depended on the acclimation temperature (Table 1). Specifically, the increase in CTmax under faster rates of temperature change was greater in the warmer acclimation temperature (Fig. 2a and Table 2). Although few tests have been performed, the interactive effect of ramp rate and acclimation temperature on thermal tolerance is supported by work in other arthropod systems (Allen et al., 2016; Castañeda et al., 2015). Indeed, Allen et al. 2016, further show that heat tolerance response to different rates of temperature change varies among species, demonstrating that ramp rate plasticity in thermal tolerance can evolve. This finding suggests ramp rate plasticity may evolve over long timescales, but leaves open the question of whether ramp rate plasticity in thermal tolerance can evolve rapidly over contemporary timescales.
Cities provide natural laboratories to explore the rapid evolution of tolerance plasticity, specifically how urban-driven environmental differences in the rate of temperature change among populations shape the tolerance response to different rates of temperature change during the tolerance assessment. Many cities exhibit substantial heat island effects wherein urban environments are warmer (by several °C) than nearby undeveloped areas owing to high levels of impervious surfaces like roads, sidewalks and buildings that absorb and trap heat, and diminished vegetation cover which can mitigate the effects of temperature rise (Imhoff et al., 2010). For the acorn ants used in our study, we found faster rates of diurnal temperature rise at urban nest sites during peak diurnal activity periods in the morning and early afternoon (Fig. 1a). We also found at least the potential for acorn ants to experience more rapid rates of spatial temperature change across urban foraging areas that had sparser canopy cover and thus increased amounts of warmer, light gap microclimates (Fig. 1b). These more rapid spatio-temporal environmental temperature increases in urban habitats were associated with greater plasticity in urban acorn ant heat tolerance across slower to faster rates of temperature change. Specifically, the urban population ants exhibited significantly greater increases in heat tolerance from the slower to faster rate of thermal tolerance assessment compared with rural population ants (Fig. 2a and Table 1). Together, these results provide evidence for evolved plasticity among urban and rural populations in the response of their heat tolerance to different rates of temperature change, and are consistent with the direction of environmental temperature variation (more rapid spatio-temporal variation in urban habitats).
Notably, rural populations of acorn ants are already accustomed to relatively abrupt temperature changes, such as moving from the nest environment which shields ants against direct solar radiation to the more exposed foraging environment, and between light gaps in the canopy cover; however, these temperature changes appear to be exaggerated in urban environments. We found that the magnitude of temperature change across the foraging area (estimated by the difference in the peak of the kernel density distributions of temperature) in rural habitats ranged from 0.361 to 0.586°C, and from 0.752 to 1.12°C in urban habitats. But what do these temperature differences mean to a foraging acorn ant? Acorn ant workers are small in body size (~2 mm), but a long-distance foraging trip of 1 m would only take 1.11–2.08 min depending on ambient thermal conditions (at 26°C, a common foraging temperature in both rural and urban habitats, acorn ant workers travel on average 8 mm s−1, and at 36°C, a warm-day foraging temperature, acorn ant workers travel on average 15 mm s−1, Maclean et al., 2017). As a consequence, foraging acorn ants experience quite rapid changes in temperature across space, particularly in cities, such that our fast rate of temperature change during the thermal tolerance assay, 1°C min−1, appears to reflect the temperature changes experienced by urban acorn ant foragers. By contrast, the 0.2°C min−1 rate of temperature change during the thermal tolerance assay appears to better reflect the temperature changes experienced by rural acorn ant foragers. The nest site temperature changes were more subdued than foraging area temperature changes, but were nonetheless twice as fast during peak activity hours (mid-morning to early afternoon) in urban habitats compared with rural habitats, i.e. 2.2°C versus 1.2°C h−1.
So far, we have established that temperature change in urban habitats is more rapid at nest sites and foraging areas of acorn ants, that foragers have sufficiently fast running speeds to at least potentially experience the large magnitude of temperature changes across the foraging area, and that plasticity in heat tolerance is correlated with these rapid temperature changes in urban habitats (Figs 1 and 2; Table 1; Maclean et al. 2017). But is the magnitude of plasticity in heat tolerance under different rates of temperature change ecologically meaningful? Under the warm laboratory temperature regime (30°C daytime, 25°C nighttime), the increase in heat tolerance from slower to faster rates to temperature increase was on the order of 1°C, a value comparable to other ectothermic species (fruit flies show an increase of 1°C and Argentine ants an increase of several°C from a rate of temperature increase of 0.1–0.5 min−1, Chown et al., 2009), and of sufficient magnitude to alter fitness outcomes in ants (Penick et al., 2017). It is unclear whether this greater plasticity in urban acorn ant responses to faster rates of temperature increase is adaptive; however, it is plausible the shift is adaptive, as an increased ability to tolerate more rapid temperature rise could allow workers to sustain foraging in the highly heterogeneous foraging areas in urban habitats. For many ant species, foraging rate is related to colony growth which subsequently allows for the production of sexual reproductives (alates) when colonies become sufficiently large (reviewed in Shik, 2008).
Another open question is the extent to which tolerance responses to slower and faster rates of temperature change are genetically correlated. The evidence so far, and mostly from studies on the genetic architecture of thermal tolerance in Drosophila systems, indicates that in some cases, basal tolerance (akin to the fast-ramp tolerance in our study, assuming this is a sufficiently fast rate of temperature change to preclude an acclimatory response and which therefore captures the intrinsic genetic contribution to tolerance) and induced tolerance (akin to the slow-ramp tolerance in our study, assuming this is a sufficiently slow rate of temperature change to allow for acclimation) responses are positively correlated (Hangartner and Hoffmann, 2016; van Heerwaarden and Sgrò, 2013). Though in other cases, basal and induced heat tolerance responses are uncorrelated, suggesting at least some degree of independence in the genetic architecture of these components of tolerance (Blackburn et al., 2014; Mitchell and Hoffmann, 2010). We have neither a selection experiment nor a quantitative genetics breeding design as used in these Drosophila studies. However, we were able to explore the potential for correlation among faster- and slower-ramped tolerance responses (including both heat and cold tolerance) using our split-colony experimental design which provides an estimate of genetic correlation between fast- and slow-ramped traits. We computed colony mean fast- and slow-ramped thermal tolerance within each combination of urban versus rural populations and cool versus warm temperature treatments. We performed separate correlation analyses for heat and cold tolerance. Our analyses revealed little evidence of a consistent positive trend between tolerances assessed under slower and faster rates of temperature change across any of these groups, suggesting a weak or nonexistent genetic correlation. However, caution must be exercised here given the limitations on sample size and experimental design to test the genetic architecture of tolerance to different rates of temperature change.
Beyond linking evolved plasticity in heat tolerance under different rates of warming with environmental variation in rates of warming and exploring the genetic architecture of different components of heat tolerance, there are other important implications of our findings concerning the nature of population differentiation in heat tolerance. Differentiation between urban and rural populations appeared to be much greater in the intrinsic genetic component of heat tolerance (captured by the fast temperature ramp) rather than the induced acclimatory component of heat tolerance (captured by the slow temperature ramp) (Tables 1 and 2). Indeed, the evolutionary differentiation in heat tolerance between urban and rural populations was only detectable (statistically significant) when using the fast temperature ramp; differentiation was much weaker and marginally non-significant when using the slow temperature ramp (Table 2). The corollary of this finding is that although both fast and slow ramped heat tolerances suggest the evolution of improved heat tolerance in urban populations, the interpretation of the magnitude of the population differentiation would be quite different if only the fast ramp or the slow ramp assessment of heat tolerance was used. Further, this pattern appears to be largely driven by urban population responses to differences in the rate of temperature change, as the heat tolerance of the rural population was insensitive to whether fast or slow rates of temperature change were used (Table 2; though there was a trend for improved heat tolerance of the rural population from the slow to fast ramp under the warm rearing temperature treatment, Fig. 2a). This diminished sensitivity of the rural population to variation in the rate of temperature change is perhaps unsurprising given the relative homogeneity of rural thermal environments over time and space (Fig. 1). In any case, these results broadly support the growing importance of considering multiple components of thermal tolerance. For example, in Drosophila, geographic clines in thermal tolerance can be concluded to be present or absent based solely on which type of thermal tolerance assay is used (Sgrò et al., 2010); similar to our results, Castañeda and colleagues (2015) detected significant population differentiation in CTmax across a latitudinal cline only when using intrinsic measures of heat tolerance, but found no cline when using ramping measures of heat tolerance. Although this would seem to suggest a preference for using intrinsic measures of heat tolerance, other studies have found tolerance to be improved under slow rates of temperature change (Sørensen et al., 2013; and similar exceptions exist for cold tolerance, see Powell and Bale, 2004). As populations can diverge in some components of tolerance but not others, and the nature of this divergence depends on the specific study system, the choice of tolerance assay should be considered carefully when undertaking comparative studies of thermal tolerance.
Although we found evidence of plasticity in heat tolerance of acorn ants tested under slower and faster rates of temperature increase, and that the magnitude of this plasticity differed among urban and rural populations, we found no evidence of plasticity in cold tolerance under slower and faster rates of temperature decrease. However, we did find evidence of evolved losses in mean cold tolerance among urban populations—it is unclear why cold tolerance is lost, and in part appears to reflect a correlated response with changes in heat tolerance (Diamond et al., 2017).
The explanation for no differences among urban and rural populations in their ramp-rate plasticity in cold tolerance is perhaps simpler: we did not observe significant differences in rates of cooling at the nest site (Fig. 1a). One caution with this interpretation is that our study was much more focused on growing season responses (ant colonies were reared in common garden under growing season temperature regimes), and the lack of plasticity in cold tolerance could simply reflect the fact that ants were not tested for cold tolerance while being reared under overwintering conditions. However, we might expect that the lack of ramp-rate plasticity in cold tolerance could be reinforced by the insulating effects of snow cover over winter (Williams et al., 2015). Indeed, snow cover may buffer acorn ants against differences in rates of air temperature change across urban and rural habitats.
One important limitation of our study is the lack of information on how acorn ant foragers use the foraging area in rural and urban habitats. Although the rate of temperature increase during peak activity periods at nest sites is clearly greater in urban habitats (though small modifications in temperature can be achieved by moving from the top to bottom of the nest, see Karlik et al. (2016)), it is possible that foragers may avoid extreme temperatures in the foraging environment by excluding these microclimates from their foraging routes. Even as ant foragers move through warmer sections of the foraging area, the difference between air temperature and body temperature (Paaijmans et al., 2013; Sunday et al., 2014) may also afford some degree of thermal buffering against extreme temperatures in urban environments. Nonetheless, the association between faster rates of temperature change in urban environments and the greater plasticity in heat tolerance of urban acorn ant populations suggests that acorn ants are likely incapable of completely avoiding these more rapidly changing urban thermal environments.
In this study, we have further moved the debate on the use of faster versus slower rates of temperature change during thermal tolerance assays from the laboratory to the field. Specifically, we have linked microclimatic variation in the rate of temperature increase experienced by urban acorn ants to greater evolved plasticity in urban populations of acorn ants to respond, via improved heat tolerance, to these rapid temperature changes. Forecasts of species responses to global change may therefore benefit from a more fine-scale consideration of how thermal environments vary over space and time, and how these environmental differences in the rate of temperature change shape the evolution of thermal tolerance plasticity.
Supplementary Material
Acknowledgements
We are grateful to the Squire Valleevue and Valley Ridge Farm and the Holden Forests and Gardens for access to field sites, and we thank Ryan Martin and two anonymous reviewers for helpful comments on a previous version of this article.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This work was partially supported by the Case Western Reserve University Department of Biology Oglebay Fund.
References
- Adams MP, Smith PL (2014) A systematic approach to model the influence of the type and density of vegetation cover on urban heat using remote sensing. Landsc Urban Plan 132: 47–54. [Google Scholar]
- Alberti M, Correa C, Marzluff JM, Hendry AP, Palkovacs EP, Gotanda KM, Hunt VM, Apgar TM, Zhou Y (2017) Global urban signatures of phenotypic change in animal and plant populations. Proc Natl Acad Sci 114: 8951–8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Chown SL, Janion-Scheepers C, Clusella-Trullas S (2016) Interactions between rates of temperature change and acclimation affect latitudinal patterns of warming tolerance. Conserv Physiol 4, 10.1093/conphys/cow053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway T, Buschinger A, Talbot M, Stuart R, Thomas C (1982) Polygyny and polydomy in three North American species of the ant genus Leptothorax mayr (Hymenoptera: Formicidae). Psyche (Stuttg) 89: 249–274. [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. [Google Scholar]
- Blackburn S, Kellermann V, Heerwaarden B, van, Sgrò CM (2014) Evolutionary capacity of upper thermal limits: beyond single trait assessments. J Exp Biol 217: 1918–1924. [DOI] [PubMed] [Google Scholar]
- Brans KI, Jansen M, Vanoverbeke J, Tüzün N, Stoks R, De Meester L (2017) The heat is on: genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob Change Biol 23: 5218–5227. [DOI] [PubMed] [Google Scholar]
- Castañeda LE, Rezende EL, Santos M (2015) Heat tolerance in Drosophila subobscura along a latitudinal gradient: contrasting patterns between plastic and genetic responses. Evolution 69: 2721–2734. [DOI] [PubMed] [Google Scholar]
- Chown SL, Jumbam KR, Sørensen JG, Terblanche JS (2009) Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct Ecol 23: 133–140. [Google Scholar]
- Diamond SE, Chick L, Perez A, Strickler SA, Martin RA (2017) Rapid evolution of ant thermal tolerance across an urban-rural temperature cline. Biol J Linn Soc 121: 248–257. [Google Scholar]
- Diamond SE, Martin RA (2016) The interplay between plasticity and evolution in response to human-induced environmental change. F1000Research 5: 2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond SE, Nichols LM, McCoy N, Hirsch C, Pelini SL, Sanders NJ, Ellison AM, Gotelli NJ, Dunn RR (2012) A physiological trait-based approach to predicting the responses of species to experimental climate warming. Ecology 93: 2305–2312. [DOI] [PubMed] [Google Scholar]
- Diamond SE, Penick CA, Pelini SL, Ellison AM, Gotelli NJ, Sanders NJ, Dunn RR (2013) Using physiology to predict the responses of ants to climatic warming. Integr Comp Biol 53: 965–974. [DOI] [PubMed] [Google Scholar]
- Franks SJ, Weber JJ, Aitken SN (2014) Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol Appl 7: 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor CK, McKAY JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21: 394–407. [Google Scholar]
- Gunderson AR, Stillman JH (2015) Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc R Soc B 282: 20150401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangartner S, Hoffmann AA (2016) Evolutionary potential of multiple measures of upper thermal tolerance in Drosophila melanogaster. Funct Ecol 30: 442–452. [Google Scholar]
- Herbers J. (1989) Community structure in north temperate ants—temporal and spatial variation. Oecologia 81: 201–211. [DOI] [PubMed] [Google Scholar]
- Herbers J, Johnson C (2007) Social structure and winter survival in acorn ants. Oikos 116: 829–835. [Google Scholar]
- Imhoff ML, Zhang P, Wolfe RE, Bounoua L (2010) Remote sensing of the urban heat island effect across biomes in the continental USA. Remote Sens Environ 114: 504–513. [Google Scholar]
- Johnson MTJ, Munshi-South J (2017) Evolution of life in urban environments. Science 358: eaam8327. [DOI] [PubMed] [Google Scholar]
- Karlik J, Epps MJ, Dunn RR, Penick CA (2016) Life inside an acorn: how microclimate and microbes influence nest organization in Temnothorax ants. Ethology 122: 790–797. [Google Scholar]
- Keller L. (1998) Queen lifespan and colony characteristics in ants and termites. Insectes Soc 45: 235–246. [Google Scholar]
- Lenth RV. (2016) Least-square means: the R package lsmeans. J Stat Softw 69: 1–33. [Google Scholar]
- Maclean HJ, Penick CA, Dunn RR, Diamond SE (2017) Experimental winter warming modifies thermal performance and primes acorn ants for warm weather. J Insect Physiol 100: 77–81. [DOI] [PubMed] [Google Scholar]
- Massamba-N’Siala G, Prevedelli D, Simonini R (2014) Trans-generational plasticity in physiological thermal tolerance is modulated by maternal pre-reproductive environment in the polychaete Ophryotrocha labronica. J Exp Biol 217: 2004–2012. [DOI] [PubMed] [Google Scholar]
- McLean MA, Angilletta MJ, Williams KS (2005) If you can’t stand the heat, stay out of the city: thermal reaction norms of chitinolytic fungi in an urban heat island. J Therm Biol 30: 384–391. [Google Scholar]
- Merilä J, Hendry AP (2014) Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol Appl 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KA, Hoffmann AA (2010) Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct Ecol 24: 694–700. [Google Scholar]
- Mitrus S. (2013) Cost to the cavity-nest ant Temnothorax crassispinus (Hymenoptera: Formicidae) of overwintering aboveground. EJE 110: 177–179. [Google Scholar]
- Modlmeier AP, Foitzik S, Scharf I (2013) Starvation endurance in the ant Temnothorax nylanderi depends on group size, body size and access to larvae. Physiol Entomol 38: 89–94. [Google Scholar]
- Moyano M, Candebat C, Ruhbaum Y, Álvarez-Fernández S, Claireaux G, Zambonino-Infante J-L, Peck MA (2017) Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae. PLoS One 12: e0179928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J, Kristensen TN, Sørensen JG (2012) Validity of thermal ramping assays used to assess thermal tolerance in arthropods. PLoS One 7, 10.1371/journal.pone.0032758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC, Thomas MB (2013) Temperature variation makes ectotherms more sensitive to climate change. Glob Change Biol 19: 2373–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penick CA, Diamond SE, Sanders NJ, Dunn RR (2017) Beyond thermal limits: comprehensive metrics of performance identify key axes of thermal adaptation in ants. Funct Ecol 31: 1091–1100. [Google Scholar]
- Powell SJ, Bale JS (2004) Cold shock injury and ecological costs of rapid cold hardening in the grain aphid Sitobion avenae (Hemiptera: Aphididae). J Insect Physiol 50: 277–284. [DOI] [PubMed] [Google Scholar]
- Pratt S. (2008) Efficiency and regulation of recruitment during colony emigration by the ant Temnothorax curvispinosus. Behav Ecol Sociobiol 62: 1369–1376. [Google Scholar]
- R Core Team (2017) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- Rezende EL, Tejedo M, Santos M (2011) Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Funct Ecol 25: 111–121. [Google Scholar]
- Ribeiro PL, Camacho A, Navas CA (2012) Considerations for assessing maximum critical temperatures in small ectothermic animals: insights from leaf-cutting ants. PLoS One 7: e32083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Castañeda LE, Rezende EL (2011) Making sense of heat tolerance estimates in ectotherms: lessons from Drosophila. Funct Ecol 25: 1169–1180. [Google Scholar]
- Santos M, Castañeda LE, Rezende EL (2012) Keeping pace with climate change: what is wrong with the evolutionary potential of upper thermal limits? Ecol Evol 2: 2866–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgrò CM, Overgaard J, Kristensen TN, Mitchell KA, Cockerell FE, Hoffmann AA (2010) A comprehensive assessment of geographic variation in heat tolerance and hardening capacity in populations of Drosophila melanogaster from eastern Australia. J Evol Biol 23: 2484–2493. [DOI] [PubMed] [Google Scholar]
- Sgrò CM, Terblanche JS, Hoffmann AA (2016) What can plasticity contribute to insect responses to climate change? Annu Rev Entomol 61: 433–451. [DOI] [PubMed] [Google Scholar]
- Shik JZ. (2008) Ant colony size and the scaling of reproductive effort. Funct Ecol 22: 674–681. [Google Scholar]
- Sørensen JG, Kristensen TN, Overgaard J (2016) Evolutionary and ecological patterns of thermal acclimation capacity in Drosophila: is it important for keeping up with climate change? Curr Opin Insect Sci 17: 98–104. [DOI] [PubMed] [Google Scholar]
- Sørensen JG, Loeschcke V, Kristensen TN (2013) Cellular damage as induced by high temperature is dependent on rate of temperature change—investigating consequences of ramping rates on molecular and organismal phenotypes in Drosophila melanogaster. J Exp Biol 216: 809–814. [DOI] [PubMed] [Google Scholar]
- Stuart RJ. (1987) Transient nestmate recognition cues contribute to a multicolonial population structure in the ant, Leptothorax curvispinosus. Behav Ecol Sociobiol 21: 229–235. [Google Scholar]
- Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB (2014) Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc Natl Acad Sci 111: 5610–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL (2011) Ecologically relevant measures of tolerance to potentially lethal temperatures. J Exp Biol 214: 3713–3725. [DOI] [PubMed] [Google Scholar]
- Tüzün N, Op de Beeck L, Brans KI, Janssens L, Stoks R (2017) Microgeographic differentiation in thermal performance curves between rural and urban populations of an aquatic insect. Evol Appl 10: 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerwaarden B, Sgrò CM (2013) Multivariate analysis of adaptive capacity for upper thermal limits in Drosophila simulans. J Evol Biol 26: 800–809. [DOI] [PubMed] [Google Scholar]
- Williams C, Henry HAL, Sinclair BJ (2015) Cold truths: how winter drives responses of terrestrial organisms to climate change. Biol Rev 90: 214–235. [DOI] [PubMed] [Google Scholar]
- Woods HA, Dillon ME, Pincebourde S (2015) The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J Therm Biol 54: 86–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.