Abstract
Heat shock proteins (Hsps) are prominent proteins that greatly contribute to insect survival under stress conditions. In this study, we cloned two Hsp transcripts (Aohsp70 and Aohsp90) from the grass thrip, Anaphothrips obscurus (Müller) (Thysanoptera: Thripidae), which is a polymorphic winged pest of corn and wheat. The cDNA sequences of Aohsp70 and Aohsp90 are 2382 and 2504 bp long, and encode proteins with calculated molecular weights of 70.02 kDa and 83.40 kDa, respectively. Aohsp90 was highly expressed in adults of both brachypters and macropters. Aohsp70 had different expression patterns in brachypters and macropters and was also highly expressed in the pupae of macropters. After adults were exposed to an ascending series of heat shocks, the expression of both Aohsp70 and Aohsp90 were up-regulated. In macropters and brachypters, the maximum induced levels of Aohsp70 (approximately 90-fold and 280-fold, respectively) were higher than Aohsp90 (approximately 2.4-fold and 1.8-fold, respectively). In addition, the up-regulation of Aohsp70 was significantly higher in brachypters than in macropters. Brachypters had a significantly higher Ltem50 (43.2°C) than macropters (42.5°C), which implied that brachypters are more tolerant to thermal stress than macropters. This study has shown that the expression patterns of Aohsp70 and Aohsp90 are variable among different life stages and thermal stress induced different levels of expressions in macropterous and brachypterous adults.
Keywords: heat shock protein, wing polymorphism, thermal stress, thrip
Insects are small poikilotherms whose development, survival, range, and abundance are directly affected by environmental temperatures (Stevnbak et al. 2009). Temperature outside the optimal temperature range can influence the physiological features and seasonal activity of insects (Bale et al. 2002, Hoffmann et al. 2003, Duan et al. 2014). Previous studies have shown that thermal stress may decrease insects water content (Prange 1996), modulate the property and fluidity of the cell membranes (Neven 2000; Rensing and Ruoff 2002), and affect the activity of enzymes (Greenspan et al. 1980). Insects have evolved various strategies to deal with these impairments caused by temperature stress.
Heat shock proteins (Hsps) are ubiquitous and evolutionarily conserved proteins found in all living organisms and are essential for environmental adaptation (King and MacRae 2015). The Hsps are highly associated with a wide range of physiological and biochemical processes. They not only function as molecular chaperones promoting the correct refolding of proteins and preventing aggregation of denatured proteins during environmental stresses, but also are involved in diverse specific cellular processes including immune defense reactions, metabolic detoxification, signal transduction, and DNA replication (Richter et al. 2010, Shu et al. 2011, Tungjitwitayakul et al. 2016). In addition, Hsps play a vital role in insects’ responses to extreme temperatures (Rinehart et al. 2007, King and MacRae 2015, Xu et al. 2015). Many experiments in insects such as Drosophila have confirmed that insect thermotolerances are highly associated with the expression of Hsps (Rutherford and Lindquist 1998).
In insects, Hsps form super-families and are classified into four groups on the basis of similarities in molecular weight and amino acid sequences, including the Hsp90s, Hsp70s, Hsp60s, and small Hsps with molecular masses ranging 12–43 kDa (Shen et al. 2015, Tungjitwitayakul et al. 2015). Hsp70 and Hsp90 are highly conserved in all eukaryotes and prokaryotes, and have been extensively studied. Hsp70s and Hsp90s both consist of two highly conserved domains: an N-terminal ATP-binding domain and a C-terminal substrate-binding domain (Lin et al. 2001; Taipale et al. 2010). These proteins generally serve in regulating the adaptions of animals to unconformable changes in the environment and serve as a predominant self-protection mechanism (Chen et al. 2015). Up-regulation of Hsp70s and Hsp90s mRNA levels in response to cold and heat shock, density, starvation, poison, ultraviolet-C, and diapause have been reported in several insects (Sosalegowda et al. 2010, Tedeschiab et al. 2015, Tedeschi et al. 2016, Zhang et al. 2015).
The grass thrip, Anaphothrips obscurus (Müller) (Thysanoptera: Thripidae), is a cosmopolitan insect pest that is distributed throughout the temperate regions of the world (Köppä 1970). The feeding damage from A. obscurus causes linear rust-like markings on the leaves of crops, such as timothy, corn, and wheat (Mound and Masumoto 2009). A. obscurus adults have three wing morphs: brachypters, macropters, and an intermediate phenotype (Kamm 1972). Changes in wing phenotypes can be induced by environmental conditions such as photoperiod, host plant quality, and population density (Kamm 1972, Reisig et al. 2010). Brachypters and macropters differ greatly in morphology, reproductive biology, behavior, and life history traits (Jiang et al. 2015, Jiang et al. 2016). Brachypterous A. obscurus populations can live in wider temperature ranges than macropters (Bailey 1948). However, the molecular aspects of A. obscurus thermo-tolerance have not been previously studied.
In this study, the cDNA transcripts of two Hsps (Hsp70 and Hsp90) were cloned from A. obscurus and their expression patterns in brachypters and macropters were determined by utilizing quantitative real-time PCR (RT-qPCR).The temperature required to kill 50% of exposed individuals (Ltem50) was also studied.
Materials and Methods
Insects
A. obscurus was originally collected from corn leaves (Zea mays L. var. Zhengdan 958) in Yangling, Shaanxi, China (34° 16′N, 108° 4′E) in June of 2011. The laboratory cohorts were maintained in an environmental chamber (25 ± 1°C, 60 ± 10 RH, with a photoperiod of 16:8 (L:D) h), and they were continuously reared on wheat (Triticumaestivum L. var. Xinong 979) for several generations. The samples used in this experiment contained brachypterous and macropterous adults according to Nakao’s definition (Nakao 1996). Brachypterous nymphs were reared on host plants grown in substrate within Plexiglas cages with a mesh cover (15 cm diameter, 50 cm high). Macropterous nymphs were reared on pieces of host plants and maintained in glass bottles (2 cm diameter, 3.5 cm high). The second instar nymphs, pupae, and adults (0–24 h after emergence) of brachypters and macropters were collected for our experiment. One hundred twenty samples from different development stages were collected to assess the expression patterns of the Hsp genes. Three independent biological replications were performed for each treatment.
Heat Shock Treatment of Adults
Groups of 40 brachypterous or macropterous adults were collected in 1.5-ml tubes and immersed in a water bath (KE WEI, Beijing, China) at 40, 41, 42, 43, and 44°C for 2 h. Treatments at 25°C were used as a control. After the heat treatments, survival was recorded. Individuals showing no response after prodding with a brush were recorded as dead. Living samples were frozen in liquid-nitrogen and then stored at −80°C until RNA isolation. Three independent biological replications were performed.
RNA Isolation and cDNA Synthesis
The total RNA of different development stages (second instar nymphs, pupae, and adults) and the heat-treated samples were extracted using RNAisoPlus Kit (Takara, Dalian, China) according to the manufacturer’s instructions. RNA purity and concentration were determined with Infinite 200 Pro NanoQuant (Tecan, Shandong, China). First-strand cDNA was synthesized from 2 μg of total RNA using a PrimerScript RT reagent Kit with a gDNA Eraser (Takara, Dalian, China), and then stored at −20°C until used.
Cloning of Hsps cDNA
Based on the conserved amino acid sequences of other insect Hsps (GenBank accession: ADO14473, AFJ20626, AGE92595, AGE15504, ADO14474, ADE34169, AFN65689, ACH85202), degenerate primers were designed for Aohsp70 and Aohsp90 amplification using Primer Premier5 software (Premier, CA) (Table 1). PCR reactions were conducted as follows: 94°C for 3 min, 30 cycles of 95°C for 30 s, 56°C (hsp70)/55°C (hsp90) for 90 s, 72°C for 2 min, with a final extension at 72°C for 10 min. Specific primers for 3′-/5′-RACE were designed according to the methods described for the 3′-full RACE kit and 5′-full RACE core set ver. 2.0 (Takara, Dalian, China), respectively. PCR reactions for 3′-RACE: 94°C for 3 min, 30 cycles of 95°C for 30 s, 66°C (hsp70)/65°C (hsp90) for 90 s, 72°C for 3 min, with a final extension at 72°C for 10 min. PCR reactions for 5′-RACE: 94°C for 3 min, 30 cycles of 95°C for 30 s, 65°C (hsp70)/66°C (hsp90) for 90 s, and a final extension at 72°C for 3 min. The full open reading frame (ORF) sequences of hsp70 and hsp90 were verified by PCR. PCR products were purified using a gel purification kit (BioTeKe, Beijing, China) and cloned into a PMD-19T Easy Vector System (Takara, Dalian, China) for sequencing (Sangon Biotech, Shanghai, China).
Table 1.
Primers used in this study
| Primer name | Primer sequence from 5′ to 3′ | Use |
|---|---|---|
| Hsp70-F | ATYATCGCCAAYGAYCARGG | Degenerate PCR |
| Hsp70-R | ATGACRCCACCRGCMGTYTC | Degenerate PCR |
| Hsp90-F | HTBGGCCGNGGHACCAAGAT | Degenerate PCR |
| Hsp90-R | GCYTGSGCYTTCATKATRCG | Degenerate PCR |
| Action-F | RTHGCCCCHGARGARCAYCC | Degenerate PCR |
| Action-R | CCWCCGATCCAKACRGAGTA | Degenerate PCR |
| UPM-Long | CTAATACGACTCACTATAGGGC AAGCAGTGGTATCAACGCAGAGT | RACE |
| UPM-short | CTAATACGACTCACTATAGGGC | RACE |
| NUP | AAGCAGTGGTATCAACGCAGAGT | RACE |
| AP-C | AAGCAGTGGTATCAACGCAGAG TACGCGGGGGGGGGG | RACE |
| Hsp70-3-inner | CTACAAGGAGGAGGACGACAAGCAGC | 3′RACE |
| Hsp70-3-outer | TCGACTTCTACACCAAGGTGTCCCG | 3′RACE |
| Hsp90-3-inner | GGGTCTTGAGCTTCCTGAAGATGAAG | 3′RACE |
| Hsp90-3-outer | GACTGAGCCCATTGACGAGTATGTTG | 3′RACE |
| Hsp70-5-inner | CCTGCCTCTGGCTGTTGAAGTAG | 5′RACE |
| Hsp70-5-outer | ATCTTGGTCAGGACCATGGAGCTGAT | 5′RACE |
| Hsp90-5-inner | GGTCCGCAACCAGGTAGGCTGAGTAG | 5′RACE |
| Hsp90-5-outer | TCTCCACCAGCAGCTTGATGGGATAT | 5′RACE |
| qHsp70-F | TCTTCAACGGCAAGTCGCTC | q PCR |
| qHsp70-R | AACTTGGTCATCACGCCACC | q PCR |
| qHsp90-F | ACGCGAGGAAGATAAGGCCA | q PCR |
| qHsp90-R | ACGTTCCATGTTGGCAGTCC | q PCR |
| qAction-F | TGCTGCATCATCAAGCTCCC | q PCR |
| qAction-R | GTCTCGTGGATACCGCAAGC | q PCR |
| fHsp70-F | CGTGAACGAATCTAAGTGACAATGCC | Full-length validation |
| fHsp70-R | TTAGTCGACTTCTTCAACTGAGGGGC | Full-length validation |
| fHsp90-F | CTTTCTTCGTTCCTTGTGCTCCGTT | Full-length validation |
| fHsp90-R | TGTGTCTTTGGCGATGATGAGATGA | Full-length validation |
Bioinformatics Analysis
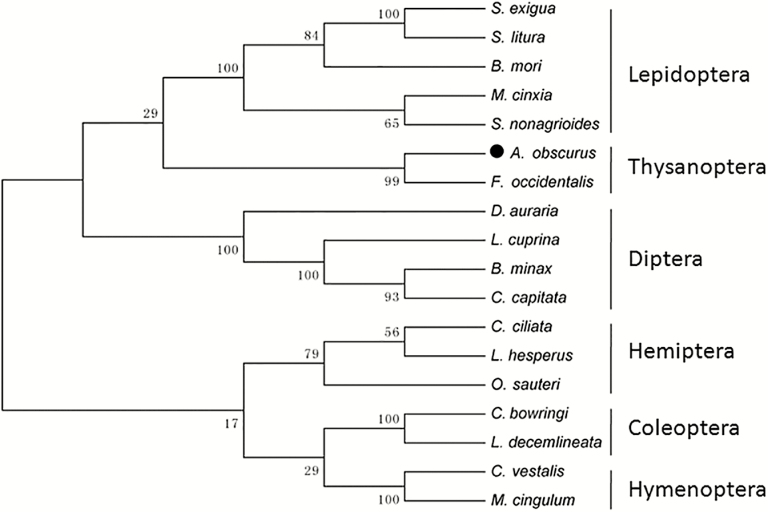
The BLAST program (http://www.ncbi.ncbi.nlm.nih.gov/BLAST) was applied to compare the similarity of Aohsps with other insect Hsps. The molecular weight and the theoretical isoelectric points were analyzed with the Expert Protein Analysis System (http://genedoc.software.informer.com). Multiple sequences alignments were identified using the ClustalX1.83 software. The phylogenetic tree was constructed by the Neighbor-Joining algorithm with bootstrapping (1,000 replicates) using MEGA 5.1 software. A total of 18 Hsp70s protein sequences were used for phylogenetic analyses. The accession numbers of these protein sequences are shown in Fig. 5.
Fig. 5.
Phylogenetic tree of Hsp70s. The percentage bootstrap values obtained from 1000 replicates were shown in the cladogram. The species and their Hsp70 accession numbers were as follows: Anaphothrips obscurus (MF773976), Frankliniella occidentalis (AFX84617), Bombyx mori (BAF69068), Colaphellus bowringi (AHF52926), Corythucha ciliate (AGT99186), Cotesia vestalis (AGF34717), Leptinotarsa decemlineata (AHA36970), Lygus hesperus (AFX84560), Melitaea cinxia (AGR84224), Sesamia nonagrioides (ABZ10939), Spodoptera exigua (ACN78407), Spodoptera litura (ADV03160), Macrocentrus cingulum (ACD84944), Orius sauteri (AIK01869), Lucilia cuprina (AEF38376), Bactrocera minax (AIA62361), Drosophila auraria (CAA04699), Ceratitis capitata (CAA70153).
Quantitative Real-Time PCR
Based on the sequences of ORFs, primers for RT-qPCR were designed using Primer-Blast (www.ncbi.nlm.nih.gov/tools/primer-blast) (Table 1). A. obscurus β-actin was used as a reference gene. The RT-qPCR was carried out using a Cycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules) in a final volume of 20 μl reaction mixture containing 10 μl of UltraSYBR Mixture (CWBIO, Beijing, China), 0.5 μl of each primer, 1 μl of cDNA template, and 8 μl of ddH2O. The reactions were conducted as follows: 95°C for 10 min, 40 cycles of 15 s at 95°C, 1 min at 60°C. Each RT-qPCR reaction was performed in three biological replicates and each sample was technically repeated three times. The relative expression levels of Hsps were calculated by the 2−∆∆Ct method (Livak and Schmittgen 2001).
Statistical Analysis
Data was analyzed with SPSS statistics 20 (IBM, USA). Differences in expression levels of Hsp70 and Hsp90 among different heat stress temperatures and developmental stages were analyzed with a one-way ANOVA analysis and Tukey’s HSD multiple tests. Statistically significant differences between brachypters and macropters were analyzed by a t-test. Probit regression was used to estimate the Ltem50 (Li et al. 2011).
Results
Characterization of Aohsp70 and Aohsp90
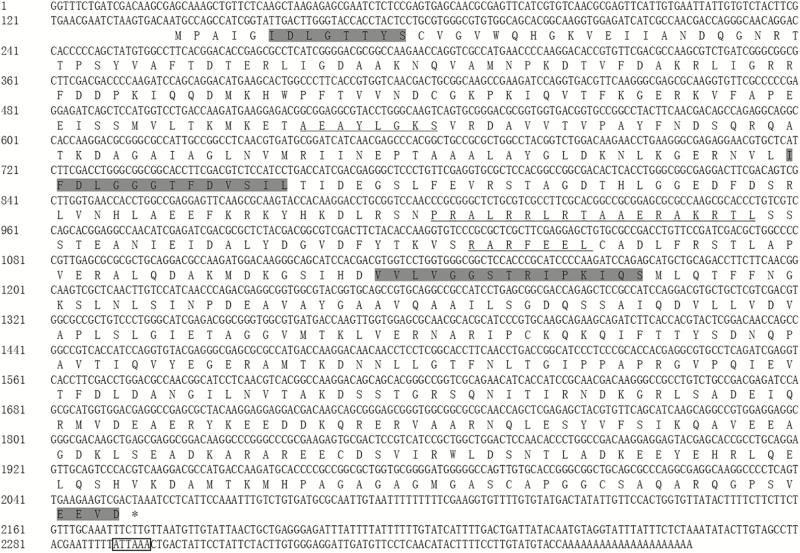
The Hsp transcripts obtained from A. obscurus were named Aohsp70 and Aohsp90 and deposited in the GenBank with accession numbers MF773976 (Aohsp70) and MF773977 (Aohsp90). The cDNA sequence of Aohsp70 had 2382 bp containing a 138 bp 5′ untranslated region (UTR), a 327 bp 3′ UTR and a 1917 bp ORF (Fig.1). The ORF encoded a protein with 638 amino acid residues. The theoretical isoelectric point was 5.50 and the predicted molecular weight was 70.02 kDa. Signature sequences of the Hsp70 family, ‘LDLGTTVS’, ‘IFDLGGGTFDVSIL’, and ‘VVLVGGSTRIPKIQS’ were found in Aohsp70. Aohsp70 also contained an ATP-GTP binding site ‘AEAYLGKS’, a bipartite nuclear localization signal ‘PRALRRLRTAAERAKRTL’ and a non-organellar consensus motif ‘RARFEEL’ (Sonoda et al. 2006b, Daugaard et al. 2007,Tungjitwitayakul et al. 2008).
Fig. 1.
Nucleotide and deduced amino acid sequences of Aohsp70 in A. obscurus. Three signature sequences of Hsp70 family and a C-terminal four amino acids ‘EEVD’ were shaded. The putative ATP-GTP binding site (AEAYLGQP), the deduced bipartite nuclear localization signals (PRALRRLRTAAERAKRTL) and the non-organellar consensus motifs (RARFEEL) were underlined. The putative polyadenylation signal ‘ATTAA’ was indicated with a box.
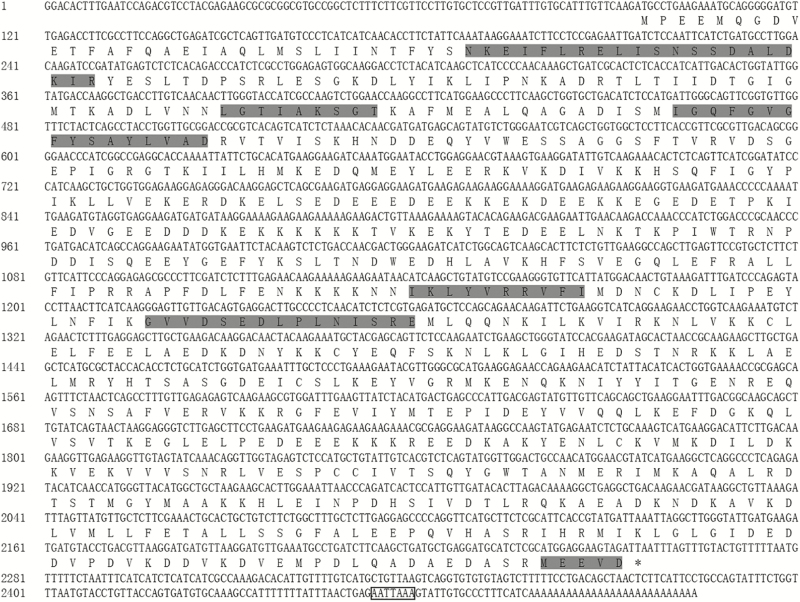
The cDNA sequence of Aohsp90 was 2504 bp with a 94 bp 5′ UTR, a 247 bp 3′ UTR and a 2163 bp ORF encoding 720 amino acid residues (Fig. 2). The theoretical isoelectric point was 5.01 and the predicted molecular weight was 83.40 kDa. Aohsp90 contained five signatures of the Hsp90 family, including: ‘NKEIFLRELISNSSDALDKIR’, ‘LGTIAKSGT’, ‘IGQFGVGFYSAYLVAD’, ‘IKLYVRRVFI’, and ‘GVVDSEDLPLNISRE’ (Gupta 1995). A highly conserved ‘MEEVD’ motif was found at the C-terminus (Pearl and Prodromou 2006).
Fig. 2.
Nucleotide and deduced amino acid sequences of Aohsp90 in A. obscurus. Five signature sequences of Hsp90 family and a C-terminal five amino acids (MEEVD) were shaded. The putative polyadenylation signal ‘ATTAAA’ was indicated with a box.
Sequence and Phylogenetic Analysis
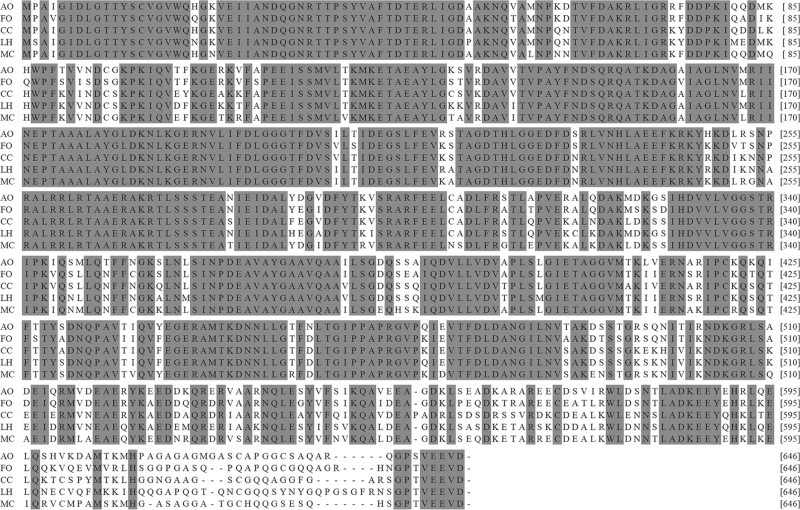
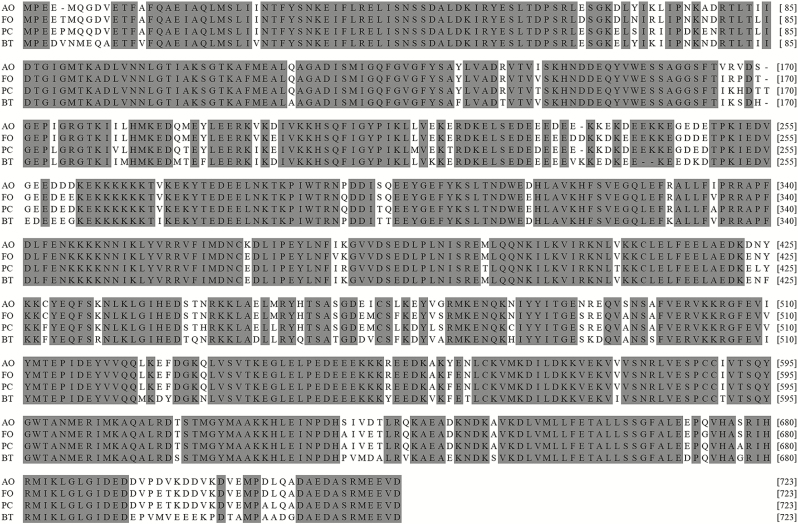
The amino acid sequences of Aohsp70/90 shared a close relationship with the Hsp70/90s of other insects (Figs. 3 and 4). The identity of Aohsp70 with the Hsp70s of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) (AFX84617), Melitaea cinxia L. (Lepidoptera: Nymphalidae) (AGR84229), Lygus hesperus Knight (Hemiptera: Miridae) (AFX84560), and Corythucha ciliate (Say) (Hemiptera: Tingidae) (ARW29611) were 87, 84, 83, and 82%, respectively. Aohsp90 shared identities of 94, 86, and 85% with Hsp90s in F. occidentalis (AFQ23182), Empoasca onukii Matsuda (Hemiptera :Cicadellidae) (AIM18803), and Nilaparvata lugens (Stål) (Homoptera: Delphacidae) (XP022202749), respectively.
Fig. 3.
Alignments of Aohsp70 with Hsp70s from other insects. Identical or similar amino acids were grey. The species names and Hsp70 accession numbers were as follows: AO, Anaphothrips obscurus (MF773976); FO, Frankliniella occidentalis (AFX84617); CC, Corythucha Ciliate (ARW29611); LH, Lygus hesperus (AFX84560); MC, Melitaea cinxia (AGR84229).
Fig. 4.
Alignments of Aohsp90 with Hsp90s from other insects. Identical or similar amino acids were grey. The species names and accession numbers were as follows: AO, Anaphothrips obscurus (MF773977); FO, Frankliniella occidentalis (AFQ23182); PC, Panony chuscitri (ADG20111); BT, Bemisia tabaci (ADG03466).
In the phylogenetic tree, 18 insect Hsp70s from Lepidoptera, Hemiptera, Thysanoptera, Hymenoptera and Coleoptera and Diptera were clustered into branches by the phylogeny of the insect orders (Fig. 5). Aohsp70 was assigned to the branch of Thysanoptera and neighbored the clade of F. occidentalis Hsp70s, with a bootstrap value of 99% in 1,000 replicates. This relationship correlates well with the traditional taxonomic status of this insect.
Developmental Expression Patterns of Aohsps
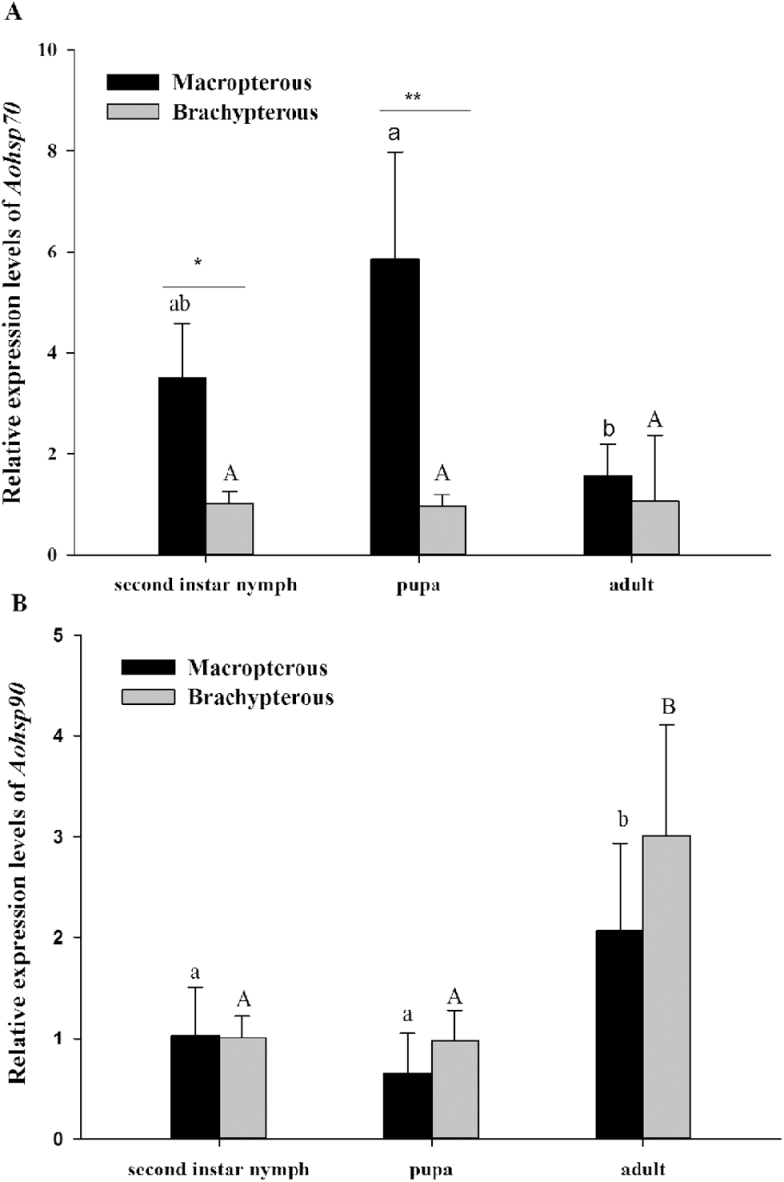
A RT-qPCR was conducted to determine the expression patterns of Aohsp70 and Aohsp90 during different developmental stages. The expression levels of Aohsp70 in macropters reached a peak level in pupae and then decreased in adults, while the expression levels in brachypters were relatively constant during tested life stages (Fig. 6A macropters: F2, 26 = 8.49, P < 0.001; brachypters: F2, 26 = 2.72, P > 0.05). Moreover, the expression levels of Aohsp70 in second instar nymphs (t = 4.71, P = 0.001) and pupae (t = 6.84, P < 0.001) were significantly higher in macropters than brachypters.
Fig. 6.
Relative expression levels of Aohsp70 (A) and Aohsp90 (B) at different developmental stages between macropters and brachypters. The different letters on the error bars indicated a significant difference at the 0.05 level (Tukey’s test). The capital letters represented macropters and the lower-case letters represented brachypters. The asterisks above the bars indicated a significant difference between the two morphs (t-test, *0.001 < P < 0.01 and **P < 0.001). Data are presented as mean ± SE.
Aohsp90 had similar expression patterns in macropters and brachypters and presented higher expression levels in adults than in immature stages of both brachypters and macropters (Fig. 6B; F2, 26 = 10.44, P < 0.001; brachypters: F2, 26 = 6.07, P < 0.001).
Mortality of Adults After Heat Shock
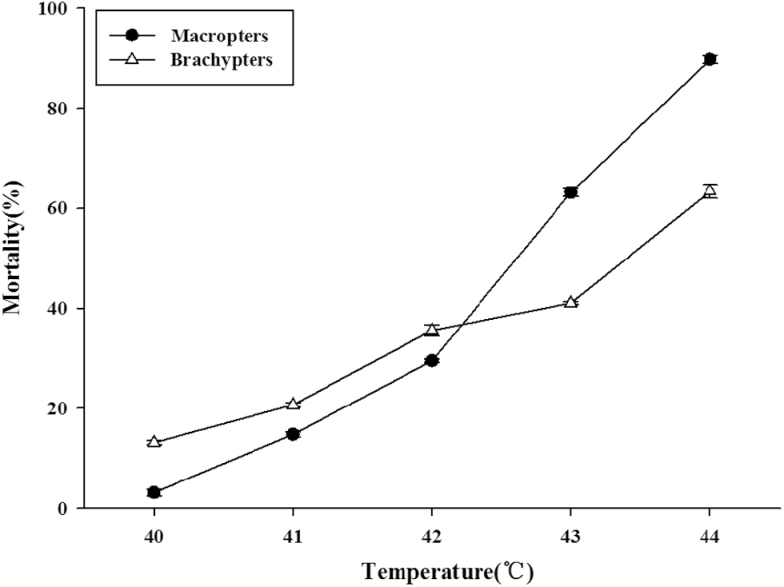
After heat shock treatment with an ascending series of temperature, the mortality of macropterous and brachypterous adults increased with the increasing of temperature (Fig. 7). Brachypters had a significantly higher Ltem50 (43.2°C) than macropters (42.5°C) t = 5.42, P = 0.006 (Table 2), which show that brachypters are more heat tolerant than macropters.
Fig. 7.
Effect of heat shock on the mortality of A. obscurus adults after 2 h heat shock treatment. Data are presented as mean ± SE.
Table 2.
Higher lethal temperature values (Ltem50) for adults
| n | Slope + SE | Ltem50 (°C) | 95% CL | Chi-square | P | |
|---|---|---|---|---|---|---|
| Macropters | 200 | 76.01 + 4.42 | 42.5 | 42.36–42.63 | 6.331 | 0.995 |
| Brachypters | 200 | 34.47 + 3.08 | 43.2 | 42.96–43.60 | 5.024 | 0.999 |
Expression Patterns of Aohsps After Heat Shock
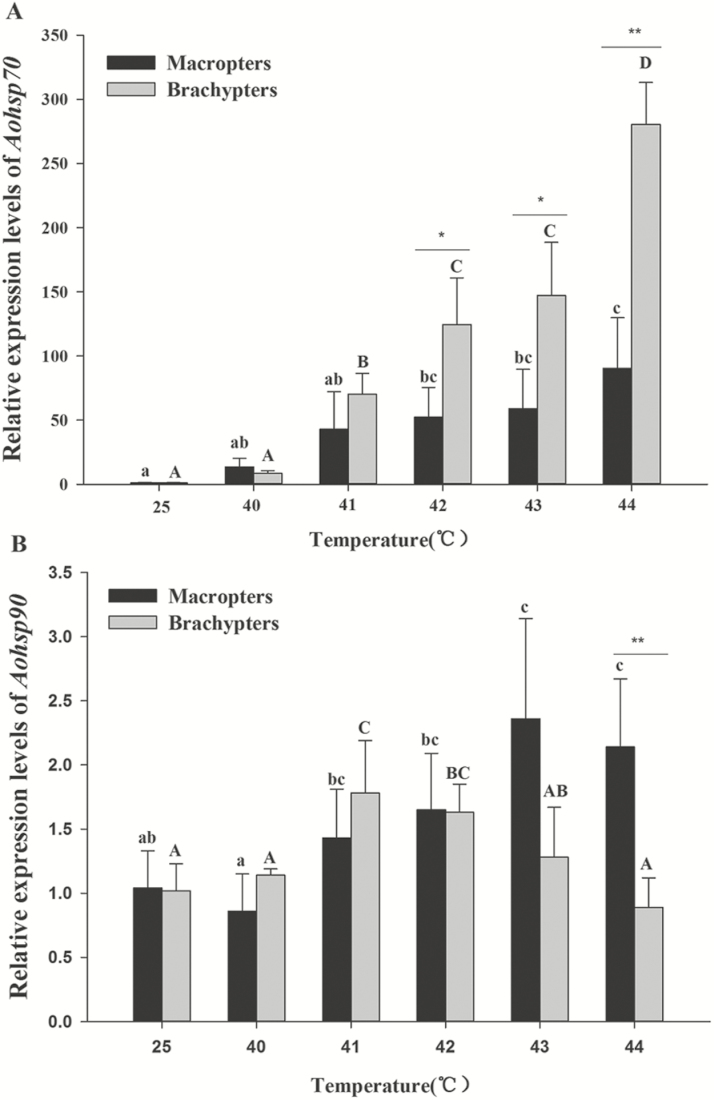
The two adult morphs had different expression patterns of Aohsp70 and Aohsp90 in response to thermal stress. The expression levels of Aohsp70 in macropters and brachypters increased with higher heat shock temperatures and reached their peak at 44°C in both macropters (approximately 90-fold) and brachypters (approximately 280-fold).Compared with the control groups (25°C), Aohsp70 was significantly induced by the temperatures exceeding 42°C in macropters and 41°C in brachypters (Fig. 8A; macropters: F5, 53 = 4.764, P < 0.001; brachypters: F5, 53 = 96.60, P < 0.001). Furthermore, the expression levels were significantly higher in brachypters than in macropters at 42°C (t = 4.25, P = 0.001), 43°C (t = 4.20, P = 0.002) and 44°C (t = 10.29, P < 0.001), respectively.
Fig. 8.
Relative expression levels of Aohsp70 (A) and Aohsp90 (B) in adults exposed to each test temperature for 2 h (40–44°C). Expression levels were first normalized to the aboundance of the reference gene, ß-actin, and then to the 25°C control. The different letters on the error bars indicated significant difference at the 0.05 levels (Tukey’s test). The capital letters represented macropters and the lower-case letters represented brachypters. The asterisks above the bars indicated a significant differences between the two morphs (t-test, *0.001 < P < 0.01 and **P < 0.001). Data were presented as mean ± SE.
The expression of Aohsp90 did not show a continued increase with rising temperatures and the peak expression level was found at 43°C in macropters (approximately 2.4-fold) and at 41°C in brachypters (approximately 1.8-fold) (Fig. 8B; macropters: F5, 53 = 4.76, P < 0.001; brachypters: F5, 53 = 12.22, P < 0.001).
Discussion
In China, A. obscurus develops throughout the entire year. Molecular factors, such as Hsps, may play important roles in A. obscurus survival in response to temperature changes. In this study, we cloned two Hsp transcripts (Aohsp70 and Aohsp90) from A. obscurus for the first time and determined their expression in the two morphs in response to short-term heat stress treatments. Aohsp70 and Aohsp90 contained the conserved sequences and characteristic motifs of the Hsp70 and Hsp90 families, respectively. The conserved EEVD tetra-peptide was found at the C-termini of Aohsp70 and Aohsp90 (Fuertes et al. 2004). Homology alignments showed that the predicted amino acid sequences of Aohsp70 and Aohsp90 share close relationships with Hsps in other insect species (Pearl and Prodromou 2006). Phylogenetic analysis indicated that Hsp70 is a candidate marker for taxonomic classification at the order level (Zhang and Denlinger 2010).
The expression of insect Hsps are highly variable among different developmental stages, depending upon the species (Okada et al. 2014). In Plutella xylostella L. (Lepidoptera: Plutellidae), southern blot analysis showed that the detectable expression of hsp19.5 is observed at the pupa stage while expression of hsp90 and hsc70 are detected at both pupa and adult stages (Sonoda et al. 2006a). In Hermetia illucens L. (Diptera: Stratiomyidae), Hihsp70 was over expressed in second instar larvae while HiHsp90 was highly expressed in the fifth larval stages (Giannetto et al. 2017). In our study, the expression of Aohsp70 reached a peak level in pupae, and the next highest levels were found in second instar nymphs in A. obscurus macropters. However, Aohsp70 was consistently expressed among all tested developmental stages in brachypters. Aohsp90 had a different expression pattern compared to Aohsp70. The expression levels of Aohsp90 were significantly higher in adults than other tested stages and this expression pattern was found in both brachypters and macropters. Two Aohsps had different expression patterns showed their transcript levels were regulated during A. obscurus development.
Temperatures stimulating induced Hsps are closely associated with insect temperature-tolerance and the induction varies between sexes and wing morphs of insects (Lu et al. 2016a). In N. lugens adults, Nlhsp90 was highly induced by heat shock and played an important role in thermo-tolerance. Nlhsp90 was significantly induced at a lower temperature in macropters than in brachypters and the expression levels of Nlhsp90 in macropters were higher than those in brachypters at the same temperature stress levels (Lu et al. 2016b). In Rhopalosiphum padi (L.) (Hemiptera: Aphididae), the mRNA levels of Hsps increased under thermal stress and reached maximal induction at a lower temperature in the alate morph (36°C) than in the apterous morph (37°C) (Li et al. 2017). The heat-induced Hsps in insects such as R. padi and N. lugens might be associated with thermotolerance. In our study, Aohsp70 and Aohsp90 were induced by heat stress at a lower temperature in brachypters than in macropters, and the relative expressions of Aohsp70 were significantly higher in brachypters than in macropters to the same thermal stress. Evidence in the literature has shown that the insect Hsp70 is an important stress chaperone in insects and appears to be a more prominent contributor to thermotolerance than the other Hsps (Delinger and Yocum 1998). In Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), Bthsp70 is an important thermal stress protein. Females with Bthsp70 knockdown had a significantly decreased in thermo-tolerance compared to females with Bthsp90 knockdown and the control (Lü and Wan 2011). Hsp70 in Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) (Zhang and Denlinger 2010) and F. occidentalis (Wang et al. 2014) were also found to be more thermally sensitive than those with Hsp90. The results observed for expression patterns of the Aohsp genes in different wing morphs were similar to those observed in other insect species. In A. obscurus, the relative expression of Aohsp70 was highly up-regulated by heat shock, while the relative expression of Aohsp90 appeared to be less induced. The maximum expression of Aohsp70 was a 280-fold increase after the heat shock, but for Aohsp90 only two-fold. These results suggest that Aohsp70 play an important role in the thermo-tolerance of A. obscurus.
Temperature is a critical factor in determining the geographic distribution, population abundance, and even the survival of insects (Shipp et al. 1996). As a result of global warming, insects are likely to encounter higher temperatures in the future (Piyaphongkul et al. 2012). These increasing temperatures may elicit the adaptive induction of Hsps in insects and up-regulate their response to higher temperature (Alto and Bettinardi 2013, Sivan et al. 2017). In A. obscurus, brachypters had a significantly higher Ltem50 than macropters, which shows that brachypters are more heat tolerant. The trend was consistent with the expression patterns of Aohsp70. Bailey (1948) also demonstrated that A. obscurus brachypterous populations can live in wider temperature ranges than macropters. Normally in summer, the populations of macropters were larger than those of brachypters (Kamm 1972). Macropters can fly, which allows them to avoid severe heat by moving to shadier, cooler habitats. Li and Gong (2017) inferred that insects possess an efficient thermal sensory system and appropriate behavior such as staying within their favorite temperature range to avoid extreme thermal stress. The study about thermal avoidance behavior in Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) also showed that the wild T. castaneum spent more time in the arean of 30°C than that in 42°C (Kim et al. 2015). In contrast, brachypters are flightless and not very mobile. Physiological ecologists suggested that thermoregulatory behavior will evolve to optimize physiological performance (Haupt et al. 2017). Therefore, brachypters are more likely to be exposed to high temperatures that activate the higher expression of Hsps such as Aohsp70. We suggested that the Aohsp70, which varies in different wing morphs induced by heat shock, play a role in the thermal resistance of A. obscurus.
Acknowledgments
We thank Dr. William Harvey Reissig (Cornell University) and Dr. John Richard Schrock (Emporia State University) for language correction of the manuscript. We also thank Xing-long Huang and Shuo-ying Ning, (Northwest Agriculture and Forestry University) for valuable suggestion in data analysis. This project was supported by the National Natural Science Foundation of China (3127344).
References Cited
- Alto B. W., and Bettinardi D.. 2013. Temperature and dengue virus infection in mosquitoes: independent effects on the immature and adult stages. Am. J. Trop. Med. Hyg. 88: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S. F. 1948. Grain and grass-infesting thrips. J. Econ. Entomol. 41: 701–706. [Google Scholar]
- Bale J. S., Masters G. J., Hodkinson I. D., Awmack C., Bezemer T. M., Brown V. K., Butterfield J., Buse A., Coulson J. C., Farrar J.,. et al. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biol. 8: 1–16. [Google Scholar]
- Chen W., D. Li M. Zhang Y. Zhao W. Wu, and Zhang G.. 2015. Cloning and differential expression of five heat shock protein genes associated with thermal stress and development in the polyphagous predatory mite Neoseiulus cucumeris (Acari: Phytoseiidae). Exp. Appl. Acarol. 67: 65–85. [DOI] [PubMed] [Google Scholar]
- Daugaard M., M. Rohde, and Jäättelä M.. 2007. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 581: 3702–3710. [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., and Yocum G. D.. 1998. Physiology of heat sensitivity, pp. 6–53. In G. J. Hallman and D. L. Denlinger (eds.), Temperature sensitivity in insects and application in integrated pest management. Westview Press, Boulder, Colorado. [Google Scholar]
- Duan J. J., Jennings D. E., Williams D. C., and Larson K. M.. 2014. Patterns of parasitoid host utilization and development across a range of temperatures: implications for biological control of an invasive forest pest. BioControl. 59: 659–669. [Google Scholar]
- Fuertes M. A., J. M. Pérez M. Soto M. Menéndez, and Alonso C.. 2004. Thermodynamic stability of the C-terminal domain of the human inducible heat shock protein 70. Biochim. Biophys. Acta. 1699: 45–56. [DOI] [PubMed] [Google Scholar]
- Giannetto A., S. Oliva L. Mazza G. Mondello D. Savastano A. Mauceri, and Fasulo S.. 2017. Molecular characterization and expression analysis of heat shock protein 70 and 90 from Hermetia illucens reared in a food waste bioconversion pilot plant. Gene. 627: 15–25. [DOI] [PubMed] [Google Scholar]
- Greenspan R. J., J. A. Finn Jr, and Hall J. C.. 1980. Acetylcholinesterase mutants in Drosophila and their effects on the structure and function of the central nervous system. J. Comp. Neurol. 189: 741–774. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. 1995. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol. 12: 1063–1073. [DOI] [PubMed] [Google Scholar]
- Haupt T. M., B. J. Sinclair, and Chown S. L.. 2017. Thermal preference and performance in a sub-Antarctic caterpillar: a test of the coadaptation hypothesis and its alternatives. J. Insect Physiol. 98: 108–116. [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Sørensen J. G., and Loeschcke V.. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28: 175–216. [Google Scholar]
- Jiang H.-X., Niu S.-H., Li X.-W., Zhang X.-C., and Feng J.-N.. 2015. Comparison of developmental and reproductive biology in wing diphenic Anaphothrips obscurus (Thysanoptera: Thripidae). J. Asia-Pac. Entomol. 18: 735–739. [Google Scholar]
- Jiang H.-X., Zhang X.-C., Niu S.-H., and Feng J.-N.. 2016. Effects of temperature on the development, reproduction and population growth of Anaphothrips obscurus (Thysanoptera: Thripidae). J. Asia-Pac. Entomol. 19: 1175–1181. [Google Scholar]
- Kamm J. A. 1972. Environmental influence on reproduction, diapause, and morph determination of Anaphothrips obscurus (Thysanoptera: Thripidae). Environ. Entomol. 1: 16–19. [Google Scholar]
- Köppä P. 1970. Studies on the thrips (Thysanoptera) species most commonly occurring on cereals in Finland. Ann.Agr.Fenn. 9: 191–265. [Google Scholar]
- Kim H. G., D. Margolies, and Park Y.. 2015. The roles of thermal transient receptor potential channels in thermotactic behavior and in thermal acclimation in the red flour beetle, Tribolium castaneum. J. Insect Physiol. 76: 47–55. [DOI] [PubMed] [Google Scholar]
- King A. M. and MacRae T. H.. 2015. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 60: 59–75. [DOI] [PubMed] [Google Scholar]
- Li K. and Gong Z.. 2017. Feeling hot and cold: thermal sensation in Drosophila. Neurosci. Bull. 33: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. B., L. Shi, M. X. Lu, J. J. Wang, and Y. Z. Du. 2011. Thermal tolerance of Frankliniella occidentalis: effects of temperature, exposure time, and gender. J. Therm. Biol. 36: 437–442.
- Li Y., Zhao Q., Duan X., Song C., and Chen M.. 2017. Transcription of four Rhopalosiphumpadi (L.) heat shock protein genes and their responses to heat stress and insecticide exposure. Comp. Biochem. Physiol. A. 205: 48–57. [DOI] [PubMed] [Google Scholar]
- Lin B. L., J. S. Wang H. C. Liu R. W. Chen Y. Meyer A. Barakat, and Delseny M.. 2001. Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana. Cell Stress Chaperones. 6: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lü Z. C. and Wan F. H.. 2011. Using double-stranded RNA to explore the role of heat shock protein genes in heat tolerance in Bemisia tabaci (Gennadius). J. Exp. Biol. 214: 764–769. [DOI] [PubMed] [Google Scholar]
- Lu K., X. Chen W. Liu, and Zhou Q.. 2016a. Characterization of heat shock cognate protein 70 gene and its differential expression in response to thermal stress between two wing morphs of Nilaparvata lugens (Stål). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 199: 47–53. [DOI] [PubMed] [Google Scholar]
- Lu K., X. Chen W. Liu, and Zhou Q.. 2016b. Identification of a heat shock protein 90 gene involved in resistance to temperature stress in two wing-morphs of Nilaparvata lugens (Stål). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 197: 1–8. [DOI] [PubMed] [Google Scholar]
- Mound L., and Masumoto M.. 2009. Australian Thripinae of the Anaphothrips genus-group (Thysanoptera), with three new genera and thirty-three new species. Zootaxa. 1: 1–76. [Google Scholar]
- Nakao S. 1996. Wing length variation and seasonal changes in wing form composition of two Anaphothrips species (Thysanoptera: Thripidae). Jpn. J. Appl.Entomol. Zool. 40: 15–24. [Google Scholar]
- Neven L. G. 2000. Physiological responses of insects to heat. Postharvest Biol. Technol. 21: 103–111. [Google Scholar]
- Okada Y., Teramura K., and Takahashi K. H.. 2014. Heat shock proteins mediate trade-offs between early-life reproduction and late survival in Drosophila melanogaster. Physiol. Entomol. 39: 304–312. [Google Scholar]
- Pearl L. H. and Prodromou C.. 2006. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 75: 271–294. [DOI] [PubMed] [Google Scholar]
- Piyaphongkul J., J. Pritchard, and Bale J.. 2012. Can tropical insects stand the heat? A case study with the brown planthopper Nilaparvata lugens (Stål). Plos One. 7: e29409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange H. D. 1996. Evaporative cooling in insects. J. Insect Physiol. 42: 493–499. [Google Scholar]
- Reisig D. D., L. D. Godfrey, and Marcum D. B.. 2010. Grass thrips (Anaphothrips obscurus) (Thysanoptera: Thripidae) population dynamics and sampling method comparison in Timothy. Environ. Entomol. 39: 1617–1625. [DOI] [PubMed] [Google Scholar]
- Rensing L. and Ruoff P.. 2002. Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol. Int. 19: 807–864. [DOI] [PubMed] [Google Scholar]
- Richter K., M. Haslbeck, and Buchner J.. 2010. The heat shock response: life on the verge of death. Mol. Cell. 40: 253–266. [DOI] [PubMed] [Google Scholar]
- Rinehart J. P., A. Li G. D. Yocum R. M. Robich S. A. Hayward, and Denlinger D. L.. 2007. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 104: 11130–11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S. L. and Lindquist S.. 1998. Hsp90 as a capacitor for morphological evolution. Nature. 396: 336–342. [DOI] [PubMed] [Google Scholar]
- Shen Q., Zhao L., Xie G., Wei P., Yang M., Wang S., Zhang F., and Tang B.. 2015. Cloning three Harmonia axyridis (Coleoptera: Coccinellidae) heat shock protein 70 family genes: regulatory function related to heat and starvation stress. J. Entomol. Sci. 50: 168–185. [Google Scholar]
- Shipp J. L., Ward K. I., and Gillespie T. J.. 1996. Influence of temperature and vapor pressure deficit on the rate of predation by the predatory mite, Amblyseiuscuc umeris, on Frankliniella occidentalis. Entomol. Exp. Appl. 78: 31–38. [Google Scholar]
- Shu Y., Y. Du, and Wang J.. 2011. Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 158: 102–110. [DOI] [PubMed] [Google Scholar]
- Sivan A., A. N. Shriram N. Muruganandam, and Thamizhmani R.. 2017. Expression of heat shock proteins (HSPs) in Aedes aegypti (L) and Aedes albopictus (Skuse) (Diptera: Culicidae) larvae in response to thermal stress. Acta Trop. 167: 121–127. [DOI] [PubMed] [Google Scholar]
- Sonoda S., M. Ashfaq, and Tsumuki H.. 2006a. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch. Insect Biochem. Physiol. 62: 80–90. [DOI] [PubMed] [Google Scholar]
- Sonoda S., K. Fukumoto Y. Izumi H. Yoshida, and Tsumuki H.. 2006b. Cloning of heat shock protein genes (hsp90 and hsc70) and their expression during larval diapause and cold tolerance acquisition in the rice stem borer, Chilo suppressalis Walker. Arch. Insect Biochem. Physiol. 63: 36–47. [DOI] [PubMed] [Google Scholar]
- Sosalegowda A. H., R. R. Kundapur, and Boregowda M. H.. 2010. Molecular characterization of heat shock proteins 90 (HSP83?) and 70 in tropical strains of Bombyx mori. Proteomics. 10: 2734–2745. [DOI] [PubMed] [Google Scholar]
- Stevnbak K., Scherber C., Gladbach D., and Christensen S.. 2009. Climate change strongly affects interaction between herbivorous insects, plants, and rhizosphere biota. IOPConf. Ser. Earth Environ. Sci. 6: 042014. [Google Scholar]
- Taipale M., D. F. Jarosz, and Lindquist S.. 2010. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11: 515–528. [DOI] [PubMed] [Google Scholar]
- Tedeschi J. N., Kennington W. J., Tomkins J. L., Berry O., Whiting S., Meekan M. G., and Mitchell N. J.. 2016. Heritable variation in heat shock gene expression: a potential mechanism for adaptation to thermal stress in embryos of sea turtles. Proc. Biol. Sci. 283: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschiab J. N., Kenningtona W. J., Berryc O., Whitingd S., Meekanbe M., and Mitchellab N. J.. 2015. Increased expression of Hsp70 and Hsp90 mRNA as biomarkers of thermal stress in loggerhead turtle embryos (Carettacaretta). J. Therm. Biol. 47: 42–50. [DOI] [PubMed] [Google Scholar]
- Tungjitwitayakul J., N. Tatun T. Singtripop, and Sakurai S.. 2008. Characteristic expression of three heat shock-responsive genes during larval diapause in the bamboo borer Omphisa fuscidentalis. Zoolog. Sci. 25: 321–333. [DOI] [PubMed] [Google Scholar]
- Tungjitwitayakul J., N. Tatun B. Vajarasathira, and Sakurai S.. 2015. Expression of heat shock protein genes in different developmental stages and after temperature stress in the maize weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 108: 1313–1323. [DOI] [PubMed] [Google Scholar]
- Tungjitwitayakul J., Tatun N., Vajarasathira B., and Sakurai S.. 2016. Effects of ultraviolet-C and microwave irradiation on the expression of heat shock protein genes in the maize weevil (Coleoptera: Curculionidae). Eur. J. Entomol. 113: 135–142. [DOI] [PubMed] [Google Scholar]
- Wang H.-H., Reitz S. R., Wang L.-X., Wang S.-Y., Li X., and Lei Z.-R.. 2014. The mRNA expression profiles of five heat shock protein genes from Frankliniella occidentalis at different stages and their responses to temperatures and insecticides. J. Integr. Agr. 13: 2196–2210. [Google Scholar]
- Xu H. J., J., Xue B., Lu X. C., Zhang J. C., Zhuo S. F., He X. F., Ma Y. Q., Jiang H. W., Fan J. Y., Xu et al. 2015. Two insulin receptors determine alternative wing morphs in planthoppers. Nature. 519: 464–467. [DOI] [PubMed] [Google Scholar]
- Zhang Q. and Denlinger D. L.. 2010. Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J. Insect Physiol. 56: 138–150. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu Y., Zhang J., Guo Y., and Ma E.. 2015. Molecular cloning and mRNA expression of heat shock protein genes and their response to cadmium stress in the grasshopper Oxyachinensis. Plos One. 7: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]