ABSTRACT
Background
Small, dense low-density lipoprotein (sd-LDL) and glycated LDL (g-LDL) have been associated with cardiovascular disease (CVD) in chronic kidney disease (CKD) in patients >60 years of age. Since young adult and paediatric patients have shorter exposure to Framingham-type risk factors, our study aims to determine whether younger CKD patients exhibit the same sd-LDL and g-LDL pattern.
Methods
After ethics board approval, this cross-sectional study was conducted at two universities with 44 patients (mean ± standard deviation age 12.6 ± 4.9, range 2–24 years) with CKD stage of 1–5. Laboratory parameters studied were Cystatin C (CysC), CysC estimated glomerular filtration rate (eGFR) (calculated from the Filler formula), sd-LDL, g-LDL and albumin. Lipid samples were measured for sd-LDL and g-LDL using ELISA. Non-linear correlation analysis was performed to determine the relationship between g-LDL, sd-LDL and eGFR. Clinical Trials Registration is at clinicaltrials.gov, NCT02126293, https://clinicaltrials.gov/ct2/show/NCT02126293.
Results
Triglycerides, but not total cholesterol and calculated LDL, were associated with CKD stages (ANOVA P = 0.0091). As in adults, sd-LDL was significantly associated with CKD stages (ANOVA P = 0.0133), CysC eGFR (r = −0.6495, P < 0.00001), and body mass index (r = −0.3895, P = 0.0189), but not with age. By contrast, there was no significant correlation between g-LDL and CKD stages or CysC eGFR (P = 0.9678).
Conclusions
Our study demonstrates that only triglycerides and sd-LDL were associated with CKD stages in this young cohort without confounding Framingham-type CVD risk factors. While larger studies are needed, this study suggests that lowering sd-LDL levels may be a potential target to ameliorate the long-term CVD risks in paediatric CKD patients.
Keywords: cardiovascular risk, chronic kidney disease, disordered lipids, Framingham-type risk factors, small dense LDL, triglycerides
Introduction
Lipoprotein transfer proteins are complex particles composed of 80–100 proteins per particle [organized by a single apolipoprotein B for low-density lipoprotein (LDL) and larger particles] that increase the availability of fats and allow them to be taken up by the cells in the body via receptor-mediated endocytosis [1, 2]. A single LDL particle is about 220–275 angstroms in diameter and typically transports 3000 to 6000 fat molecules/particle, which vary in size depending on the number and mix of fat molecules contained within [3]. The lipids that are transported by LDL particles include all fat molecules with cholesterol, phospholipids and triglycerides. The proportions of these fats vary considerably. LDL particles pose a risk for cardiovascular disease (CVD) when they invade the endothelium and become oxidized, since the oxidized forms are more easily retained by the proteoglycans [1, 3]. Small, dense (sd-LDL) and glycated (g-LDL) LDL have been associated with CVD risk [4].
It has long been known that patients with chronic kidney disease (CKD) usually have disordered lipid profiles and tend to succumb to cardiovascular events [5, 6]. The impaired lipid metabolism seen in patients with CKD impacts the cardiovascular system in several ways, namely by preventing the formation of high-density lipoprotein (HDL), impairing reverse cholesterol transport, intensifying the prevailing systemic oxidative stress and inflammation, and increasing the risk of atherosclerotic cardiovascular disease and CKD progression [7]. Cyclically, this HDL deficiency and dysfunction contributes to CKD progression by promoting glomerulosclerosis and tubular damage and dysfunction [8, 9]. Such patients also tend to have an increase in triglycerides [10]. Although cardiovascular morbidity has been linked to LDL, some LDL is favourable as it serves as the main carrier for the transport of cholesterol to the cells, and LDL levels may be normal in patients with CVD [11] and may even be reduced in CKD patients [10]. LDL consists of several subclasses with distinct sizes, densities and physicochemical compositions [11]. sd-LDL [11] and g-LDL [4] have specifically been associated with cardiovascular disease. More recently, a specific pattern of elevated oxidized LDL [10] and sd-LDL was described with worsening CKD [12], and sd-LDL levels were associated with mortality [13]. Notably, these studies were performed in typical adult CKD patients over 60 years of age, where typical Framingham-type risk factors may confound CKD-specific changes. Framingham risk factors include age, gender, total and HDL cholesterol, smoking, diabetes, systolic blood pressure and treatment for high blood pressure [14]. The similarities and differences between the lipid profiles of patients with metabolic syndrome and patients with CKD have been previously highlighted [15]. We were interested in seeing whether children would also exhibit the sd-LDL elevation found with a worsening estimated glomerular filtration rate (eGFR) where Framingham-type risk factors are typically absent. We measured sd-LDL and g-LDL in children and adolescents with CKD Stages 1–5 who were ‘not on dialysis’. We hypothesized that the concentration of sd-LDL would increase with worsening kidney function while g-LDL would remain unaffected because children and young adults with CKD typically do not have diabetes or other more conventional cardiovascular risk factors.
Materials and methods
Methods
The study adhered to the Declaration of Helsinki. The Research Ethics Boards of Western and McMaster Universities approved the study as part of a cross-sectional study of biomarkers of CVD in children and young adults with CKD (Western Ontario: REB#16962E, McMaster University: REB#12-537). The goal of the original study was to describe FGF23 and other biomarkers in relationship to renal function in children and young adults with CKD and comprised 157 patients from London and Hamilton. As an ancillary study, we conducted this cross-sectional sub-study of 44 children, adolescents and young adults, who were not on dialysis, and were recruited between 17 March 2010 and 28 October 2013 with CKD from Stages 1 to 5 (CKD was diagnosed according to the 2012 KDIGO clinical practice guideline [16]). They had blood work performed as part of their regular assessment and had abundant serum. The original inclusion criteria for the study period that determined the sample size were patients from 1.5 years to 50 years with CKD Stages 1–5 who require regular assessments to measure their renal function and bone status. However, for the purposes of this study, we excluded patients >25 years of age. Additional exclusion criteria included patients who were younger than 1.5 years of age because of the developmental changes of GFR. Clinical Trials Registration is at clinicaltrials.gov, NCT02126293, https://clinicaltrials.gov/ct2/show/NCT02126293.
Materials
The samples for the lipid study were selected at random based on the abundant samples available in the repository at the Translational Research Centre at the London Health Sciences Centre [17] and in an effort to have an even distribution of patients with CKD Stages 1–5. Other laboratory parameters that were necessary for our analysis were taken from our database (Microsoft Excel for Mac v.14.6.8) or from our centre’s electronic medical chart database. Laboratory parameters that were used for this study included Cystatin C (CysC), CysC eGFR, sd-LDL, g-LDL and albumin. We calculated CysC eGFR using the Filler formula [18] and body mass index (BMI) from the patients’ measured height and weight. Even though BMI is age-dependent in children, BMI values were not converted to z-scores because there are no z-scores available for young adults. sd-LDL and g-LDL were measured using commercially available ELISA kits by MyBioSource, Inc., San Diego, CA , USA. The sd-LDL was measured with the qualitative sandwich ELISA ‘Human small dense low density lipoprotein (sLDL) ELISA Kit (Cat.No: MBS700740)’ using undiluted original serum samples, with a detection range between 0.312 and 20 nmol/mL. The g-LDL was measured with the qualitative sandwich ELISA ‘GLDL ELISA KIT (Cat.No: MBS020040)’ using undiluted original serum samples. The detection range is 0.25 –8 nmol/mL. Standards were used as per the manufacturer’s instructions. As there were some inconsistencies with the expected concentrations and those of the standards, we report the ELISA readings as relative units (rU).
Statistical analysis
Contiguous data were analysed for normal distribution using the Kolmogorov Smirnov test. For normally distributed parameters, parametric and otherwise non-parametric statistical comparator tests were applied based on the distribution of the data using GraphPad Prism v.5.0f and Microsoft Excel version 15.34 for Mac. Data are expressed as average ± 1 standard deviation (SD) for normally distributed parameters, and median (25th, 75th percentile) otherwise. As appropriate for the distribution, linear or non-linear correlation analysis was performed to assess the relationship between age, eGFR, sd-LDL and g-LDL. A P-value of <0.05 was considered significant for all analyses.
Results
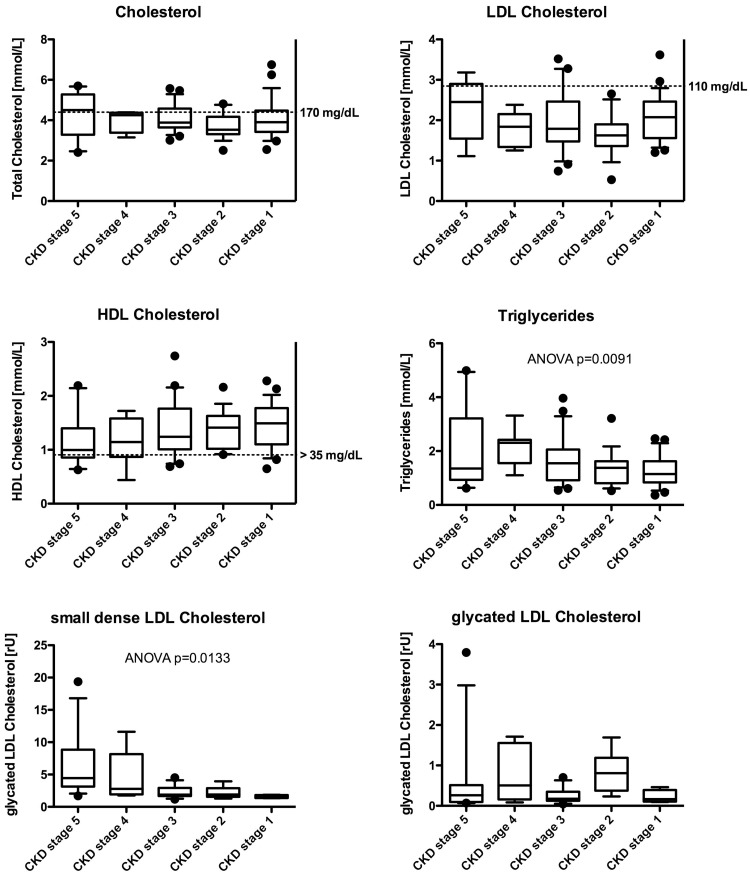
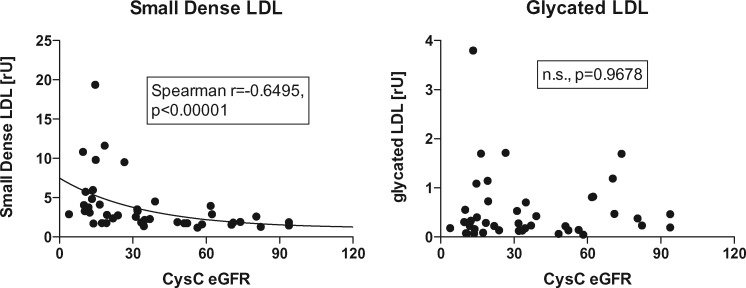
The primary diagnoses of our study cohort are given in Table 1. Patients ranged in age from 2 to 24 years, with a mean age of 12.6 ± 4.9 years; 40 patients were ≤18 years old, and 4 were between the ages of 19 and 24. Of these patients, 23 were male and 21 were female. CKD stage distribution was as follows: Stage 1 = 4, Stage 2 = 7, Stage 3 = 13, Stage 4 = 8, Stage 5 = 12. Average age (± 1 SD) was 14.1 ± 2.5 years for CKD Stage 1, 11.1 ± 3.6 years for CKD Stage 2, 12.3 ± 4.3 years for CKD Stage 3, 20.4 ± 6.5 for CKD Stage 4 and 13.8 ± 5.9 years for CKD Stage 5. Median BMI and laboratory values are given in Table 2. Neither sd-LDL nor eGFR correlated with age. Total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides are summarized in Figure 1. We also provide the sd-LDL and g-LDL measurements in Figure 1. As seen, only very few patients had elevated total cholesterol or LDL cholesterol levels, or decreased HDL cholesterol levels. Only triglycerides were associated with the stage of CKD (ANOVA P = 0.0091). Figure 2 shows the relationship of sd-LDL and g-LDL with CysC eGFR. Interestingly, sd-LDL cholesterol, but not g-LDL cholesterol, was associated with both the CysC eGFR (Spearman r = −0.6495, P < 0.00001) and the stage of CKD (ANOVA P = 0.0133) (Figures 1 and 2). The patients’ BMI also correlated with sd-LDL (Spearman r = −0.3895, P = 0.0189). There was no significant correlation between CysC eGFR and g-LDL (Figure 2, right).
Table 1.
Patients breakdown by CKD aetiology
| CKD aetiology | |||
|---|---|---|---|
| Hereditary | Acquired | ||
| Renal dysplasia | 5 | Renal transplant | 13 |
| Genetic conditionsa | 12 | Haemolytic uraemic syndrome | 4 |
| Obstructive uropathy | 3 | Spina bifida and neurogenic bladder | 2 |
| Idiopathic Fanconi syndrome | 1 | Glomerulonephritis, FSGS | 3 |
| Ischaemic acute kidney injury | 1 | ||
Such as autosomal recessive polycystic kidney disease, nephronopthisis and syndromes associated with renal dysplasia. FSGS, focal segmental glomerulosclerosis.
Table 2.
Median laboratory values
| Blood parameter | Median (Q1, Q3) | Unit |
|---|---|---|
| BMI | 19.02±4.35 | kg/m2 |
| CysC | 2.28 (1.25–4.61) | mg/L |
| CysC eGFR | 36.5 (16.75–71.25) | mL/min/1.73 m2 |
| Albumin | 43.5 (40.8–46.0) | g/L |
| g-LDL | 0.23 (0.14–0.59) | rU |
| sd-LDL | 2.47 (1.74–3.98) | rU |
Fig. 1.
Lipid parameters by CKD stage.
Fig. 2.
sd- and g-LDL versus CysC eGFR using the Filler formula [18]. Average age (± 1 SD) was 14.1 ± 2.5 years for CKD Stage 1; 11.1 ± 3.6 years for CKD Stage 2; 12.3 ± 4.3 years for CKD Stage 3; 20.4 ± 6.5 for CKD Stage 4; and 13.8 ± 5.9 years for CKD Stage 5.
Median microalbumin to creatinine ratio was 19.7 g/mol (range 0.2–44.6, interquartile range 2.35–46.5 g/mol). Data were not normally distributed. Interestingly, microalbumin/creatinine ratio correlated significantly with sd-LDL (P = 0.0033) and with triglycerides (P = 0.0441). Using multivariate analysis and adjusting for microalbumin, CysC eGFR remained significant (P = 0.0135). We also adjusted for BMI and for age. Both of these were not significant in the multivariate analysis. Therefore, only CysC eGFR and microalbuminuria affected sd-LDL.
Discussion
In an effort to unravel the cardiovascular aetiology and outcomes that pose the greatest threat to patients with CKD, previous studies in adults have examined the use of lipid biomarkers to predict and monitor patient CVD outcomes, since traditional risk factors do not fully explain the high incidence of CVD in CKD, and traditional lipid measures do not sufficiently predict these outcomes in patients with CKD [19–22]. This effort, however, may be confounded by the Framingham factors seen in adults that are absent in children.
As predicted, the lack of correlation between CysC eGFR and g-LDL seen in our study suggests that traditional Framingham-like cardiovascular risk factors such as glycosylation may not be important factors in the cardiovascular morbidity and mortality of non-diabetic CKD patients. The sd-LDL, however, was associated with CysC eGFR, stage of CKD and BMI, thereby confirming adult data [23] and solidifying the notion that a CKD patient’s sd-LDL level may be a very important atherogenic risk factor, and can be used as a predictor for CVD morbidity and mortality [12, 19, 24–26]. The results of this study also conformed to the significantly higher levels of sd-LDL compared with LDL seen in patients with coronary artery disease, and this relationship’s association with the incidence of cardiovascular events independently of LDL itself [27, 28]. Similar to past studies [29], our study also suggests that sd-LDL may be a more suitable marker than LDL for measuring CVD, which is particularly important considering the most recent paediatric/adolescent guidelines that only use LDL as a marker for dyslipidaemia and atherosclerosis [30, 31].
Our study has several limitations, including its retrospective design, the wide age range of our patients and the modest sample size. Absolute BMI was used, and it is possible that the correlation between BMI and sd-LDL was therefore confounded by age, although age was not correlated with sd-LDL. A strength of our study is the use of CysC eGFR rather than Schwartz eGFR; the former is a more accurate biomarker for kidney function, and by the virtual elimination of Framingham factor confounders, a trait that cannot be replicated in adult studies. The formula used for calculating the eGFR is the only formula that has been validated in both children and adults [32].
Without the aforementioned confounding Framingham risk factors seen in adults, these results bring us closer to determining a mechanism for the elevated sd-LDL levels seen in patients with CKD and to addressing their role in CVD as the leading cause of death in patients with CKD. It also contributes to scepticism regarding our historically clear-cut view that grouped cholesterol is either ‘good’ or ‘bad’; its use as a biomarker may in fact be measuring more fundamental variables [12], and our focus may have to narrow to a greater understanding of sub-fractions of lipoprotein transfer lipids. Furthermore, our findings in children, echoed by studies in adults, also strongly indicate that there is a need for a faster, less expensive, less laborious and more efficient way of measuring the different classes of LDL, particularly sd-LDL. Comparable to strategies employed within the SHARP trial [33], our results suggest that targeting sd-LDL may be a viable option for reducing long-term cardiovascular risks in paediatric CKD patients, particularly for those who are in the later stages of CKD. The mechanism is not well understood. Upregulation of GPIHBP1 in a model of 5/6 nephrectomy in rats and subsequent lipoprotein lipase deficiency which interferes with normal metabolism of VLDL and chylomicrons may play a role [7]; however, a recent in-depth review did not offer a clear explanation [34]. The question is how to lower sd-LDL levels? Possible options are fibrates and LDL apheresis, however, fibrates are often not well tolerated and LDL apheresis is invasive and access to this treatment modality is limited [35]. Newer fibrates such as fenofibrate in a simvastatin combination have recently demonstrated good safety profiles in adults and even reduced proteinuria [36].
In summary, this study confirms the specific pattern of sd-LDL being associated with worsening eGFR in the absence of any association with g-LDL. The results strongly suggest that this pattern is CKD specific and not confounded by Framingham-type risk factors.
Acknowledgements
We would like to thank the Translational Research Centre under the leadership of Dr Douglas Fraser and the expert sample handling by Carolina Gillio-Meina for handling and processing our samples in the repository. We also thank Ms Marta Kobrzynski for her expert editing. Finally, we thank Mr Connor Smith, chemistry student at Western University, for his assistance with the ELISA measurements.
Authors’ contributions
Conception or design: G.Filler, C.F., C.M., S.T.; providing intellectual content of critical importance to work described: G.Filler, C.F., C.M., S.T.; performed the experiments: G.Filler, C.S.; analysis and interpretation of data, or both: G.Filler, G.Fusch; drafting the article or revising it: G.Filler, L.S.; final approval of version to be published: G.Filler, S.T., C.M., C.S., L.S., G.Fusch, C.F.
Conflicts of interest statement
None declared.
References
- 1. Dashti M, Kulik W, Hoek F. et al. A phospholipidomic analysis of all defined human plasma lipoproteins. Sci Rep 2011; 1: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dashty M, Motazacker MM, Levels J. et al. Proteome of human plasma very low-density lipoprotein and low-density lipoprotein exhibits a link with coagulation and lipid metabolism. Thromb Haemost 2014; 111: 518–530 [DOI] [PubMed] [Google Scholar]
- 3. Segrest JP, Jones MK, De Loof H. et al. Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res 2001; 42:1346–1367 [PubMed] [Google Scholar]
- 4. Soran H, Durrington PN.. Susceptibility of LDL and its subfractions to glycation. Curr Opin Lipidol 2011; 22: 254–261 [DOI] [PubMed] [Google Scholar]
- 5. Filler G. Challenges in pediatric transplantation: the impact of chronic kidney disease and cardiovascular risk factors on long-term outcomes and recommended management strategies. Pediatr Transplant 2011; 15: 25–31 [DOI] [PubMed] [Google Scholar]
- 6. Kaysen GA. Lipid and lipoprotein metabolism in chronic kidney disease. J Ren Nutr 2009; 19: 73–77 [DOI] [PubMed] [Google Scholar]
- 7. Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol 2016; 12: 37–47 [DOI] [PubMed] [Google Scholar]
- 8. Zhao YY, Wang HL, Cheng XL. et al. Metabolomics analysis reveals the association between lipid abnormalities and oxidative stress, inflammation, fibrosis, and Nrf2 dysfunction in aristolochic acid-induced nephropathy. Sci Rep 2015; 5: 12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao YY, Vaziri ND, Lin RC.. Lipidomics: new insight into kidney disease. Adv Clin Chem 2015; 68: 153–175 [DOI] [PubMed] [Google Scholar]
- 10. Kaysen GA. New insights into lipid metabolism in chronic kidney disease. J Ren Nutr 2011; 21: 120–123 [DOI] [PubMed] [Google Scholar]
- 11. Hirayama S, Miida T. Small dense LDL: An emerging risk factor for cardiovascular disease. Clin Chim Acta 2012; 414: 215–224 [DOI] [PubMed] [Google Scholar]
- 12. Chu M, Wang AY, Chan IH. et al. Serum small-dense LDL abnormalities in chronic renal disease patients. Br J Biomed Sci 2012; 69: 99–102 [PubMed] [Google Scholar]
- 13. Shen H, Xu Y, Lu J. et al. Small dense low-density lipoprotein cholesterol was associated with future cardiovascular events in chronic kidney disease patients. BMC Nephrol 2016; 17: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kannel WB, Dawber TR, Kagan A. et al. Factors of risk in the development of coronary heart disease–six year follow-up experience. The Framingham Study . Ann Intern Med 1961; 55: 33–50 [DOI] [PubMed] [Google Scholar]
- 15. Kaysen GA. Metabolic syndrome and renal failure: similarities and differences. Panminerva Med 2006; 48: 151–164 [PubMed] [Google Scholar]
- 16. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013; 3: 19–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gillio-Meina C, Zielke HR, Fraser DD.. Translational research in pediatrics IV: solid tissue collection and processing. Pediatrics 2016; 137 [DOI] [PubMed] [Google Scholar]
- 18. Filler G, Lepage N.. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol 2003; 18: 981–985 [DOI] [PubMed] [Google Scholar]
- 19. Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med 2010; 268: 456–467 [DOI] [PubMed] [Google Scholar]
- 20. Rubin C, Nolin TD, Himmelfarb J.. Are biomarkers useful for assessing cardiovascular risk in patients with chronic kidney disease? Curr Opin Nephrol Hypertens 2007; 16: 506–511 [DOI] [PubMed] [Google Scholar]
- 21. Kanbay M, Siriopol D, Saglam M. et al. Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients. J Clin Endocrinol Metab 2014; 99: E1854–E1861 [DOI] [PubMed] [Google Scholar]
- 22. Tonelli M, Muntner P, Lloyd A. et al. Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol 2013; 24: 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savic J, Zeljkovic A, Bogavac-Stanojevic N. et al. Association of small, dense low-density lipoprotein cholesterol and galectin-3 in patients with chronic kidney disease. Scand J Clin Lab Invest 2014; 74: 637–643 [DOI] [PubMed] [Google Scholar]
- 24. Campos H, Genest JJ Jr, Blijlevens E. et al. Low density lipoprotein particle size and coronary artery disease. Arterioscler Thromb 1992; 12: 187–195 [DOI] [PubMed] [Google Scholar]
- 25. Coresh J, Kwiterovich PO Jr, Smith HH. et al. Association of plasma triglyceride concentration and LDL particle diameter, density, and chemical composition with premature coronary artery disease in men and women. J Lipid Res 1993; 34: 1687–1697 [PubMed] [Google Scholar]
- 26. Austin MA, Breslow JL, Hennekens CH. et al. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988; 260: 1917–1921 [PubMed] [Google Scholar]
- 27. Ai M, Otokozawa S, Asztalos BF. et al. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem 2010; 56: 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arai H, Kokubo Y, Watanabe M. et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb 2013; 20: 195–203 [DOI] [PubMed] [Google Scholar]
- 29. Koba S, Yokota Y, Hirano T. et al. Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb 2008; 15: 250–260 [DOI] [PubMed] [Google Scholar]
- 30. Gooding HC, Rodday AM, Wong JB. et al. Application of Pediatric and Adult Guidelines for treatment of lipid levels among US adolescents transitioning to young adulthood. JAMA Pediatr 2015; 169: 569–574 [DOI] [PubMed] [Google Scholar]
- 31. Lozano P, Henrikson NB, Morrison CC. et al. Lipid screening in childhood and adolescence for detection of multifactorial dyslipidemia: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016; 316: 634–644 [DOI] [PubMed] [Google Scholar]
- 32. White CA, Akbari A, Doucette S. et al. Effect of clinical variables and immunosuppression on serum cystatin C and beta-trace protein in kidney transplant recipients. Am J Kidney Dis 2009; 54: 922–930 [DOI] [PubMed] [Google Scholar]
- 33. Baigent C, Landray MJ, Reith C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377: 2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mikolasevic I, Zutelija M, Mavrinac V. et al. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovasc Dis 2017; 10: 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Filler G, Lee M, Hegele RA.. Barriers to the implementation of lipoprotein apheresis in Canada. Can J Cardiol 2017; 33: 409–411 [DOI] [PubMed] [Google Scholar]
- 36. Filippatos TD, Elisaf MS.. Safety considerations with fenofibrate/simvastatin combination. Expert Opin Drug Saf 2015; 14: 1481–1493 [DOI] [PubMed] [Google Scholar]