Abstract
Introduction:
Dementia encompasses diseases of progressive memory loss and neurological alterations, including Alzheimer disease. Hypertension is one risk factor proposed for development of Alzheimer disease. The objective is to evaluate the current literature for use of diuretics in the prevention of dementia.
Methods:
Literature was not considered if published before January 1, 2000, or after May 31, 2015. PubMed was used to locate sources. Four search terms were used to find data: Alzheimer disease, antihypertensive agents, diuretics, and dementia.
Results:
Four studies of efficacy of diuretic usage in the prevention against dementia met criteria. Potassium-sparing diuretics displayed risk reduction of Alzheimer disease and maintenance of cognitive function. Risk reduction was demonstrated when used alone (adjusted hazard ratio [aHR] 0.09, 95% confidence interval [CI] 0.01-0.41) as compared to use of other antihypertensives without potassium-sparing diuretics (aHR 0.76, 95% CI 0.49-1.15). Other antihypertensive drug classes did show some benefit, however. Diuretic and angiotensin receptor blocker users had a lower Alzheimer disease risk versus those with no antihypertensive use (hazard ratio 0.40, 95% CI 0.26-0.61) and (hazard ratio 0.37, 95% CI 0.19-0.72), respectively. Additionally, thiazide diuretics were also shown to reduce Alzheimer risk. Thiazide and potassium-sparing combination significantly reduced risk versus non-antihypertensive users (aHR 0.63, 95% CI 0.42-0.94).
Discussion:
Available research demonstrates an inverse association between diuretic use and the incident rate of dementia. Specifically, this has been found with thiazide and potassium-sparing diuretics when used alone or in combination. This review suggests that patients receiving diuretics for hypertension may receive an added neuroprotective effect.
Keywords: diuretic, potassium-sparing, dementia, Alzheimer
Introduction
Dementia is an umbrella term that encompasses diseases characterized by the progressive loss of memory and alterations in the brain.1 Epidemiologic studies suggest that 10% of people over age 70 and about 40% of people over age 85 have significant memory loss.2 Half of these cases are attributable to Alzheimer disease. In 2010, approximately 4.7 million Americans were living with Alzheimer disease.3 By 2050, roughly 13.8 million Americans will be living with Alzheimer disease, placing a substantial burden on our health care system.3
The most prominent symptom of Alzheimer disease is memory impairment; however, nonmemory symptoms include poor judgment and insight, difficulty with organization or navigation, and progressive motor and language deficits.1,2
The exact cause of dementia remains an enigma; therefore, it is difficult to prevent. Although advanced age seems to be the only definite risk factor for development of dementia, other possible risk factors include family history, mild cognitive impairment, cardiovascular disease, social engagement, diet, and head trauma.1,2,4-7 Women are more likely than men to develop Alzheimer disease.4,6 Although it is unknown why there is a higher incidence in women, women tend to live longer than men, and the risk of dementia progresses with age.6 Additionally, research indicates a strong genetic component in the development of Alzheimer disease.1,2,4,7 Apolipoprotein E (APOE), a lipoprotein of the brain, is becoming a significant indicator for Alzheimer disease. The presence of just 1 APOE-ε4 allele has been associated with a 2- to 3-fold increased risk of developing Alzheimer disease.2 Similarly, the presence of 2 of these alleles are associated with a 16-fold higher risk of developing Alzheimer disease.
Hypertension is also emerging as a possible factor in the development of dementia.1,2,4,8-10 It has been shown to contribute to alterations in brain structure.8-10 One of the most important factors in macrovascular cerebral complications, such as stroke and, consequently, vascular dementia, is hypertension. However, it may also contribute to dementia due to microvascular changes, in particular, white matter lesions (WML). Hypertensive patients with WML performed significantly worse on neuropsychological testing compared to those without WML, demonstrating an association between WML and hypertension as well as an association between WML and cognitive function.8 Also, patients with poorly controlled hypertension versus those with adequately controlled hypertension scored significantly lower on cognitive assessment tests as well as on attention function and executive function tests.9
Serum potassium levels in midlife (ages 46-60) are also thought to be associated with Alzheimer disease.11 One of the hallmark features of Alzheimer disease is the neuritic plaques that deposit into the brain tissue.1,2,11 These plaques are, in part, composed of the amino acid amyloid beta. Particularly in Alzheimer disease, it is the isoform of 42 amino acids that seems to predominantly accumulate in the neuritic plaques, hence Aβ42. One study followed women for 24 years to determine any connection between low serum potassium and a biological marker for Alzheimer, specifically Aβ42. The results revealed that reduced serum potassium in midlife (age 40-60) is associated with reduced cerebrospinal fluid Aβ42. Low cerebrospinal fluid Aβ42 levels are thought to be associated with increased deposition of Aβ42 into the brain.1,11
There may be other mechanisms by which potassium impacts the nervous system. McCabe and colleagues12 demonstrated that potassium inhibits the formational rate of reactive oxygen species in endothelial cells. Cisternas and colleagues13 found that increasing dietary potassium produced better cognitive performance in mice. Their data also suggested that dietary increases in potassium at least partially prevented oxidative stress.
According to the 20-year Atherosclerosis Risk in Communities (ARIC) neurocognitive study,10 hypertension seems to cause progressive neurological damage over time rather than acutely. This study assessed cognitive change over 20 years in hypertensive patients. Cognitive decline did not differ significantly between patients with prehypertension and those without hypertension. Patients with hypertension experienced even greater decline during the study.10
The Atherosclerosis Risk in Communities study10 supports the notion that controlling blood pressure in midlife may protect against the development of dementia later in life. Furthermore, the serum potassium study also suggests that maintenance of serum potassium in midlife may also impact the development of dementia. The purpose of this review was to evaluate the current literature for associations between diuretic use and incidence of dementia.
Methods
Literature Search
A literature search was conducted using the PubMed database. Literature published between January 1, 2000, and May 31, 2015, was included. The search for eligible literature was conducted during the month of May 2015. Four search terms were used: Alzheimer disease, antihypertensive agents, diuretics, and dementia. Free searches were performed as well as using these tags as MeSH (Medical Subject Headings) terms. Literature was selected to provide results for the overall decline in cognition over time as well as the ancillary development of dementia or Alzheimer disease. Literature was also gathered using bibliographies of pertinent studies. The data for this review were gathered in a 2-step approach. First, the use of antihypertensive drugs and their effect on cognition and incidence of Alzheimer disease was determined. Next, these effects were assessed to determine if drug type had any bearing on outcome.
Literature Review Process
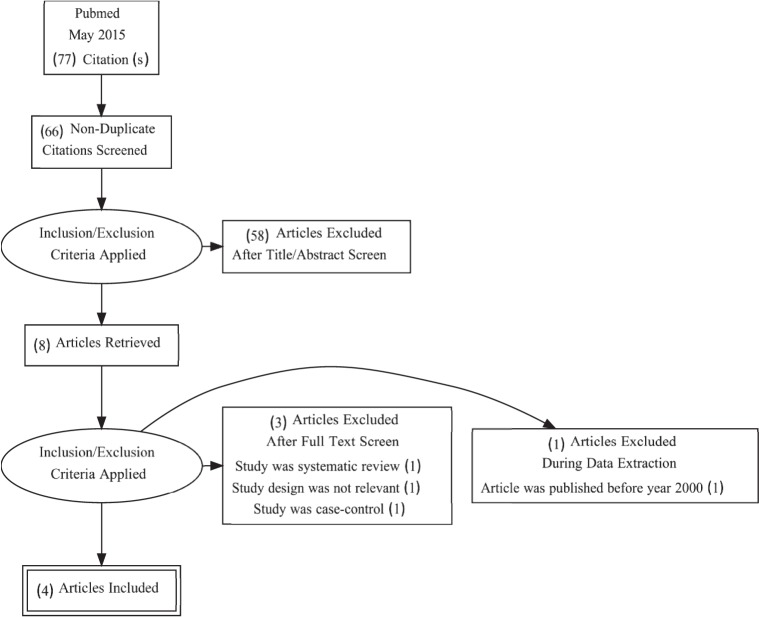
The literature attained and used for this review was chosen by a set of criteria (Figure). Studies had to exclude dementia patients or differentiate them from participants without dementia to separately assess them. Participants had to be elderly, hypertensive, currently taking antihypertensives, and at risk for dementia. Periodic cognitive assessments of participants for incident dementia were needed for inclusion in this review. Literature was selected if the population was large enough to allow adjusting for confounding factors, such as age, sex, and education; if antihypertensive use versus dementia risk or cognitive function use was directly observed; or if antihypertensives used were divided into subclasses. Literature was excluded if participants were treated with cholinesterase inhibitors, tricyclic antidepressants, antipsychotics, anti-Parkinson medications, or other medications with significant psychotropic or central anticholinergic effects. Literature was also excluded if participants had a history of Parkinson disease, had been hospitalized within the past 10 years for depression, or had received electroconvulsive therapy within the last 10 years. Types of literature excluded for this review were case control, case series, meta-analysis, and other literature review studies; other types of literature (ie, prospective cohort studies) were considered for inclusion.
FIGURE.
Selection of included articles for review
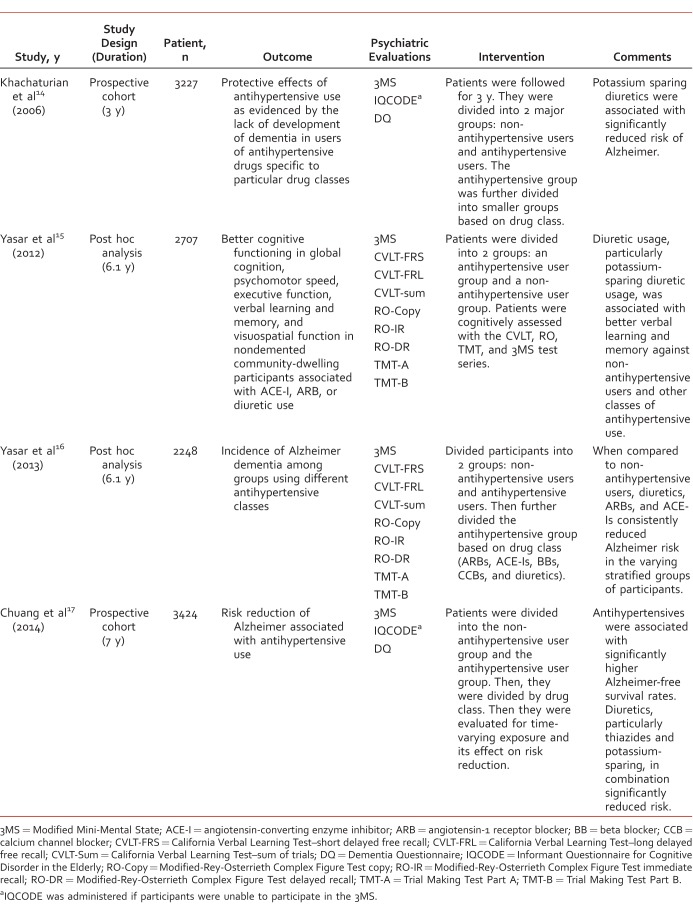
The Table provides a summary of design, methods, and findings for each of the studies reviewed.
TABLE.
Summary of design, methods, and findings for studies reviewed
Results
Antihypertensive Medication Use and Incident Alzheimer Disease: The Cache County Study14
Khachaturian and colleagues14 investigated any protective effect associated with antihypertensive use and the development of Alzheimer disease. The study involved 2 waves of data collection from participants, the first of which took place 3 years prior to the second. There were 3227 participants included for this analysis, 10 of which had no information on antihypertensive use during Wave 1. This population consisted of highly educated, elderly men and women. They were divided into 2 groups: antihypertensive users and those not taking antihypertensives. There were no significant differences in baseline demographics between the 2 groups. The antihypertensive group was further divided based on the class of antihypertensive medication being used: angiotensin-converting enzyme (ACE) inhibitors, beta blockers, calcium channel blockers, and diuretics. Adjusted hazard ratios were used and included those for age; sex; education; number of APOE ε4 alleles; and history of stroke, diabetes, myocardial infarction, or hypercholesterolemia.
These results demonstrated that antihypertensive use was associated with a significantly reduced Alzheimer disease risk (adjusted hazard ratio [aHR] 0.64, 95% confidence interval [CI] 0.41-0.98). They also showed that beta blockers trended toward having protective effects against development of Alzheimer disease (aHR 0.53, 95% CI 0.22-1.09).14 Dihydropyridine (DHP) calcium channel blockers trended toward more risk reduction (aHR 0.53, 95% CI 0.16-1.34) than non-DHP calcium channel blockers (aHR 1.16, 95% CI 0.55-2.20). Interestingly, the results indicated that diuretics were associated with a statistically significant reduction of Alzheimer risk (aHR 0.61, 95% CI 0.37-0.98). The reduction of risk by diuretics was attributed to potassium-sparing diuretics (aHR 0.26, 95% CI 0.08-0.64). Approximately half of potassium-sparing diuretic use was combined with another antihypertensive agent. Potassium-sparing diuretics demonstrated significant risk reduction when used alone (aHR 0.09, 95% CI 0.01-0.41) as compared to other antihypertensives used without potassium-sparing diuretics (aHR 0.76, 95% CI 0.49-1.15). Results including only participants receiving antihypertensives, potassium-sparing diuretics still produced a statistically significant reduction in Alzheimer risk (aHR 0.24, 95% CI 0.06-0.68).
Diuretic Use Is Associated With Better Learning and Memory in Older Adults in the GEM (Ginkgo Evaluation of Memory) Study15
Yasar and colleagues15 performed a post hoc analysis of cognitive functioning and the learning capacity in community-dwelling, nondemented people aged 75 years or older. Different domains of cognitive function were measured against diuretics, ACE inhibitors, or angiotensin-II receptor blocker (ARB) usage. There were 2707 participants with a majority who were white and highly educated. Of these, 53% used antihypertensives. Results indicated that diuretic use corresponded to improved verbal learning and memory compared to the nondrug group. Investigators used the verbal learning and memory tests, California Verbal Learning Test (CVLT)–short delayed free recall (FRS), CVLT–long delayed free recall (FRL), and CVLT–sum of trials (Sum), for which diuretic users performed significantly better than nondrug users on the CVLT-Sum (P ≤ .02). Likewise, diuretics users had better verbal learning and memory than users of the other antihypertensive groups on the CVLT-FRL (P = .02) and CVLT-Sum (P < .001).
Potassium-sparing diuretics particularly were associated with better performance in the memory and verbal learning portion of the cognitive assessment.15 Potassium-sparing diuretic users had better scores on the CVLT-FRS (P = .01), CVLT-FRL (P < .001), and CVLT-Sum (P = .01) than the nondrug group. When analyzed against other antihypertensive use, potassium-sparing diuretic users were still found to have better verbal learning and memory performance (P = .03, P < .001, and P < .001, respectively). For participants receiving either ACE inhibitors or ARBs, no difference was observed between these 2 groups and the nondrug group or any other antihypertensive group in the 6 cognitive domains assessed.
Antihypertensive Drugs Decrease Risk of Alzheimer Disease: Ginkgo Evaluation of Memory Study16
Yasar and colleagues16 determined whether the use of antihypertensives was associated with a decreased risk of developing Alzheimer disease in participants with little to no cognitive impairment. There were 2248 participants chosen for this study, which was a post hoc analysis of the GEMS trial. Comparisons were made with patients taking antihypertensives and not taking antihypertensives as well as comparisons between antihypertensive user groups. Diuretics, ARBs, ACE inhibitors, and beta blockers were associated with a reduced rate of Alzheimer (hazard ratio [HR] 0.46, 95% CI 0.32-0.68, P < .001; HR 0.35, 95% CI 0.19-0.65, P = .001; HR 0.56, 95% CI 0.37-0.85, P = .001; and HR 0.64, 95% CI 0.44-0.72, P = .01, respectively) compared with no antihypertensive use. Calcium channel blocker usage was not associated with reduction of the incidence of Alzheimer disease (HR 0.67, 95% CI 0.93-1.04, P = .07).
Comparable reductions were observed with diuretic, ARB, and ACE inhibitor use in participants with normal baseline cognition (HR 0.51, 95% CI 0.31-0.82, P = .006; HR 0.31, 95% CI 0.14-0.68, P = .003; and HR 0.50, 95% CI 0.29-0.83, P = .008, respectively) versus those not taking antihypertensives.16 Diuretics were the only class associated with a significant risk reduction in participants with baseline mild cognitive impairment (HR 0.38, 95% CI 0.20-0.73, P = .004). Participants reporting use of diuretics and ARBs had a lower Alzheimer disease risk versus those with no antihypertensive use (HR 0.40, 95% CI 0.26-0.61, P < .0001 and HR 0.37, 95% CI 0.19-0.72, P < .004, respectively).
Use of Diuretics Is Associated With Reduced Risk of Alzheimer Disease: The Cache County Study17
Chuang and colleagues17 sought to determine an association between the use of antihypertensives and the risk of developing Alzheimer disease. There were 3424 participants, mostly white and highly educated, and the study was conducted in 4 waves spanning 12 years. Data were utilized from all 4 waves of this study if participants completed at least 1 follow-up assessment. Seven had missing data on antihypertensive use, leaving 3417 participants. Adjusted hazard ratios were used and included those for age; sex; education; number of APOE ε4 alleles; history of stroke, diabetes, myocardial infarction, coronary artery bypass graft, or hypercholesterolemia; and smoking and drinking habits.
Antihypertensive users had a significantly higher rate of Alzheimer disease-free survival than nonusers.17 When comparing each class of medication to other antihypertensive medication users and nonusers, diuretics were associated with a statistically significant reduced risk of Alzheimer disease (aHR 0.72, 95% CI 0.56-0.93). Other antihypertensives did not demonstrate a significant association.
When the subclasses of diuretics were analyzed, thiazide diuretics and potassium-sparing diuretics demonstrated reduced Alzheimer disease risk (aHR 0.71, 95% CI 0.53-0.94 and aHR 0.70, 95% CI 0.48-1.00, respectively) compared with loop diuretics (aHR 0.98, 95% CI 0.67-1.43).17 To further investigate this, the combination effects of thiazide and potassium-sparing diuretics were compared to nonusers. The use of both diuretics combined was associated with a significantly decreased risk of Alzheimer disease (aHR 0.63, 95% CI 0.42-0.94). The use of either category alone correlated to a decreased Alzheimer risk but was not statistically significant (aHR 0.69, 95% CI 0.47-1.02 and aHR 0.56, 95% CI 0.18-1.76, respectively). This trend was also seen when the data were compared to antihypertensive users. However, the thiazide and potassium-sparing combination (aHR 0.69, 95% CI 0.45-1.06), thiazide-only treatment (aHR 0.78, 95% CI 0.51-1.17), and potassium-sparing–only treatment (aHR 0.64, 95% CI 0.20-2.02) risk reduction of Alzheimer was not statistically significant.
Discussion
Select antihypertensive medications are associated with a decrease in the incident risk of developing dementia, particularly Alzheimer dementia.14-17 Because the tests used in these studies assess both the risk of developing dementia and cognitive skills, one could determine that antihypertensives may not only reduce dementia risk, but also may promote improved learning and memory in patients receiving this treatment.
Both potassium-sparing and thiazide diuretics were associated with reduced cognitive decline and lower incident rates of dementia.14-17 As stated earlier, potassium inhibits the formational rate of reactive oxygen species, and a high dietary potassium intake could improve cognitive function in mice.12,13 These findings both lend credibility to the present finding that potassium-sparing diuretics are associated with lower rates of dementia.
Although the theoretical benefit of potassium-sparing diuretics is maintaining adequate potassium serum concentrations, thiazide diuretics also demonstrated a beneficial association despite their side effect of hypokalemia.17 The contrary nature of this finding can be seen in the mechanisms by which these two drug classes function.18 Potassium-sparing diuretics act primarily in the late distal tubules and collecting ducts of the kidney on sodium channels, preventing its reabsorption and consequently preventing potassium excretion. However, thiazides chiefly act in the distal tubules of the kidney on the Na-Cl symporter. This causes the excretion of sodium and chloride and, as a result, potassium also. The discrepancy here begets yet another question as to why 2 very different effects cause similar outcomes.
A potential explanation for this might lie in the adverse effect profile of thiazide diuretics because their chronic use results in decreased excretion of uric acid. In turn, this activity elevates uric acid levels in the body. Uric acid itself is an antioxidant.19,20 It has also been shown to be neuroprotective against the development of neurodegenerative diseases due to age.21 Although hyperuricemia is correlated with gout, nephropathy, and hypertension, inadequate levels of uric acid have been associated with neurological diseases, such as Parkinson, multiple sclerosis, and dementias. This is thought to result in increasing superoxide dismutase activity by uric acid in the CNS, preventing toxic buildup of nitric oxide (NO). Nitric oxide is a beneficial compound, but in high concentrations, it becomes neurotoxic and may mediate glutamate neurotoxicity.20,22 The neurotoxicity associated with NO begins with excitotoxicity initiated by glutamate activity at the N-methyl-D-aspartate receptor.23 This results in increased NO formation and ultimately in neurotoxicity and neuronal death.24
Other antihypertensives were also shown to be beneficial. Angiotensin-converting enzyme inhibitors and ARBs were associated with a reduced risk of Alzheimer dementia.16 The benefits of ACE inhibitors and ARBs support our findings that potassium plays a role in neurocognitive health due to the fact that both of these antihypertensive drug classes also increase serum potassium levels. However, ACE inhibitors and ARBs may have a protective mechanism unique to renin-angiotensin-aldosterone system inhibition. Beta blockers had benefit as well that may be due simply to their ability to decrease blood pressure, providing protection against vascular damage in the brain. DHP calcium channel blockers were associated with more protective effects than non-DHP blockers. However, it is also unknown whether these drugs have a unique mechanism for protecting neurocognitive health aside from their antihypertensive activity.
The Eighth Joint National Committee guidelines25 on hypertension do not address patients at risk for the development of dementia. This review may guide research that could be included in future guidelines. This review suggests that drug class has a significant impact on cognitive outcome. Therefore, it would seem that potassium-sparing diuretics, thiazide diuretics, ACE inhibitors, or ARBs would be reasonable choices for hypertensive populations with at least 1 copy of the APOE-e4 allele or a history of mild cognitive impairment with the first choice being potassium-sparing diuretics use in combination with a thiazide diuretic. The literature in this review would suggest that elderly patients (65 years of age or older) would benefit from this treatment option as the average age of participants in this review was 76 years.
Limitations of this review include the use of observational literature, which is subject to the possibility of confounding.14-17 The researchers adjusted for the effects of age, sex, race, socioeconomic status, education, and lifestyle habits, such as smoking. However, there were potential confounders that could not be accounted for, such as the indicated use for antihypertensives. Of the literature used in the review, only 1 study17 was able to control for indicated use of antihypertensives. Another limitation was the potential for mortality bias.14-17 Some participants may have died before dementia could be detected. The studies evaluated had 1 psychiatric evaluation in common (Modified Mini-Mental State); however, a variety of other tools were used in each of the studies. This can make compilation of the data difficult. The final limitation is that its generalizability is limited because participants were mostly white and highly educated.
This review has several strengths. The sample sizes were large enough to observe effects of different subclasses of antihypertensives. The patient population was also large enough to control confounders, such as age, sex, education, and APOE genotype. Because the population was large enough to control for these factors, the hazard ratios provide an accurate representation of the data.
Conclusion
In this review, an inverse relationship was found between the use of diuretics and the incident rate of dementia. This benefit was found to be statistically significant when thiazide and potassium-sparing diuretics were used in combination. These findings suggest that diuretics might have a particular niche in hypertension management in patients at high risk of developing dementia, particularly those that have hypertension and are at least 65 years old; have at least one copy of the APOE-e4 allele; or have a history of mild cognitive impairment. However, long-term studies are needed to determine the antihypertensive regimen that provides the most benefit in an aging society.
Footnotes
Disclosures: Neither author has any disclosures of interest.
References
- 1. APA Work Group on Alzheimer's Disease and other Dementias, Rabins PV, Blacker D, Rovner BW, Rummans T Schneider LS, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Second edition. Am J Psychiatry. 2007; 164 12 Suppl: 5- 56. PubMed PMID: 18340692. [PubMed] [Google Scholar]
- 2. Seeley WW, Miller BL. . Dementia. : Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson J, Loscalzo J, et al. Harrison's principles of internal medicine. 19 ed. New York: McGraw-Hill; 2014. [Google Scholar]
- 3. Hebert LE, Weuve J, Scherr PA, Evans DA. . Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013; 80 19: 1778- 83. DOI: 10.1212/WNL.0b013e31828726f5. PubMed PMID: 23390181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alzheimer's Association. 2015. Alzheimer's disease facts and figures. Alzheimers Dement. 2015; 3 11: 332- 84. PubMed PMID: 25984581. [DOI] [PubMed] [Google Scholar]
- 5. Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999; 14 6: 481- 93. PubMed PMID: 10398359. [PubMed] [Google Scholar]
- 6. Erol R, Dawn Brooker R, Peel E, Brooker D. . Women and dementia: A global research review; 2015. June Available from: http://www.researchgate.net/publication/277865960_Women_and_Dementia_A_global_research_review [Google Scholar]
- 7. Michaelson DM. . APOE ε4: the most prevalent yet understudied risk factor for Alzheimer's disease. Alzheimers Dement. 2014; 10 6: 861- 8. DOI: 10.1016/j.jalz.2014.06.015. PubMed PMID: 25217293. [DOI] [PubMed] [Google Scholar]
- 8. Sierra C, De La Sierra A, Salamero M, Sobrino J, Gómez-Angelats E, Coca A. . Silent cerebral white matter lesions and cognitive function in middle-aged essential hypertensive patients. Am J Hypertens. 2004; 17 6: 529- 34. DOI: 10.1016/j.amjhyper.2004.02.014. PubMed PMID: 15177527. [DOI] [PubMed] [Google Scholar]
- 9. Spinelli C, De Caro MF, Schirosi G, Mezzapesa D, De Benedittis L, Chiapparino C, et al. Impaired cognitive executive dysfunction in adult treated hypertensives with a confirmed diagnosis of poorly controlled blood pressure. Int J Med Sci. 2014; 11 8: 771- 8. DOI: 10.7150/ijms.8147. PubMed PMID: 24936139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014; 17 10: 1218- 27. DOI: 10.1001/jamaneurol.2014.1646. PubMed PMID: 25090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mielke MM, Zandi PP, Blennow K, Gustafson D, Sjögren M, Rosengren L, et al. Low serum potassium in mid life associated with decreased cerebrospinal fluid Abeta42 in late life. Alzheimer Dis Assoc Disord. 2006; 20 1: 30- 6. DOI: 10.1097/01.wad.0000201848.67954.7d. PubMed PMID: 16493233. [DOI] [PubMed] [Google Scholar]
- 12. McCabe RD, Bakarich MA, Srivastava K, Young DB. . Potassium inhibits free radical formation. Hypertension. 1994; 24 1: 77- 82. DOI: 10.1161/01.HYP.24.1.77. PubMed PMID: 8021011. [DOI] [PubMed] [Google Scholar]
- 13. Cisternas P, Lindsay CB, Salazar P, Silva-Alvarez C, Retamales RM, Serrano FG, et al. The increased potassium intake improves cognitive performance and attenuates histopathological markers in a model of Alzheimer's disease. Biochim Biophys Acta. 2015; 1852 12: 2630- 44. DOI: 10.1016/j.bbadis.2015.09.009. PubMed PMID: 26391254. [DOI] [PubMed] [Google Scholar]
- 14. Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, et al. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol. 2006; 63 5: 686- 92. DOI: 10.1001/archneur.63.5.noc60013. PubMed PMID: 16533956. [DOI] [PubMed] [Google Scholar]
- 15. Yasar S, Lin F-M, Fried LP, Kawas CH, Sink KM, DeKosky ST, et al. Diuretic use is associated with better learning and memory in older adults in the Ginkgo Evaluation of Memory Study. Alzheimers Dement. 2012; 8 3: 188- 95. DOI: 10.1016/j.jalz.2011.03.010. PubMed PMID: 22465175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yasar S, Xia J, Yao W, Furberg CD, Xue Q-L, Mercado CI, et al. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology. 2013; 81 10: 896- 903. DOI: 10.1212/WNL.0b013e3182a35228. PubMed PMID: 23911756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chuang Y-F, Breitner JCS, Chiu Y-L, Khachaturian A, Hayden K, Corcoran C, et al. Use of diuretics is associated with reduced risk of Alzheimer's disease: the Cache County Study. Neurobiol Aging. 2014; 35 11: 2429- 35. DOI: 10.1016/j.neurobiolaging.2014.05.002. PubMed PMID: 24910391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reilly RF, Jackson EK. . Regulation of renal function and vascular volume. In: Brunton LL, Chabner BA, Knollmann BC, editors Goodman & Gilman's the pharmacological basis of therapeutics. 12 ed. New York: McGraw-Hill; 2011.
- 19. Bender DA, . Free radicals & antioxidant nutrients. : Rodwell VW, Bender DA, Botham KM, Kennelly PJ, Weil P, Victor W, et al. Harper's illustrated biochemistry. 30 ed. New York: McGraw-Hill; 2015. [Google Scholar]
- 20. Dawson VL, Dawson TM. . Nitric oxide neurotoxicity. J Chem Neuroanat. 1996; 10 3-4: 179- 90. DOI: 10.1016/0891-0618(96)00148-2. PubMed PMID: 8811421. [DOI] [PubMed] [Google Scholar]
- 21. Cutler RG, Camandola S, Malott KF, Edelhauser MA, Mattson MP. . The role of uric acid and methyl derivatives in the prevention of age-related neurodegenerative disorders. Curr Top Med Chem. 2015; 15 21: 2233- 8. DOI: 10.2174/1568026615666150610143234. PubMed PMID: 26059354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montoliu C, Llansola M, Kosenko E, Corbalán R, Felipo V. . Role of cyclic GMP in glutamate neurotoxicity in primary cultures of cerebellar neurons. Neuropharmacology. 1999; 38 12: 1883- 91. DOI: 10.1016/S0028-3908(99)00071-4. PubMed PMID: 10608283. [DOI] [PubMed] [Google Scholar]
- 23. Choi DW, Maulucci-Gedde M, Kriegstein AR. . Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987; 7 2: 357- 68. PubMed PMID: 2880937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. . Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991; 88 14: 6368- 71. PubMed PMID: 1648740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311 5: 507- 20. DOI: 10.1001/jama.2013.284427. PubMed PMID: 24352797. [DOI] [PubMed] [Google Scholar]