Abstract
Schizophrenia (SCZ) is associated with differences in subcortical brain volumes and intracranial volume (ICV). However, little is known about the underlying etiology of these brain alterations. Here, we explored whether brain structure volumes and SCZ share genetic risk factors. Using conditional false discovery rate (FDR) analysis, we integrated genome-wide association study (GWAS) data on SCZ (n = 82315) and GWAS data on 7 subcortical brain volumes and ICV (n = 11840). By conditioning the FDR on overlapping associations, this statistical approach increases power to discover genetic loci. To assess the credibility of our approach, we studied the identified loci in larger GWAS samples on ICV (n = 26577) and hippocampal volume (n = 26814). We observed polygenic overlap between SCZ and volumes of hippocampus, putamen, and ICV. Based on conjunctional FDR < 0.05, we identified 2 loci shared between SCZ and ICV implicating genes FOXO3 (rs10457180) and ITIH4 (rs4687658), 2 loci shared between SCZ and hippocampal volume implicating SLC4A10 (rs4664442) and SPATS2L (rs1653290), and 2 loci shared between SCZ and volume of putamen implicating DCC (rs4632195) and DLG2 (rs11233632). The loci shared between SCZ and hippocampal volume or ICV had not reached significance in the primary GWAS on brain phenotypes. Proving our point of increased power, 2 loci did reach genome-wide significance with ICV (rs10457180) and hippocampal volume (rs4664442) in the larger GWAS. Three of the 6 identified loci are novel for SCZ. Altogether, the findings provide new insights into the relationship between SCZ and brain structure volumes, suggesting that their genetic architectures are not independent.
Keywords: pleiotropy, genome-wide association study, conditional false discovery rate, subcortical brain structures, common genetic variants
Introduction
Schizophrenia (SCZ) is a severe mental disorder with a complex and heterogeneous pathophysiology. Although differences in subcortical brain volumes and intracranial volume (ICV) have consistently been reported in SCZ,1–3 the molecular mechanisms underlying these brain abnormalities are poorly understood. Both SCZ and brain structure volumes have high heritability estimates (60%–80%4 and 40%–90%,5 respectively), and brain volume alterations are also present in unaffected relatives of patients with SCZ,6,7 suggesting that genetic risk underlying SCZ may also influence brain structure volumes. In the current study, we aimed to determine whether common genetic variants are shared between SCZ and subcortical brain volumes and ICV.
Subcortical brain structures regulate a variety of functions including emotion,8 movement,9 cognition,9,10 and reward-seeking behavior11 in concert with other brain areas. Recent neuroimaging meta-analyses report consistent, albeit subtle, differences in volumes of subcortical brain regions and ICV in SCZ.1–3 Compared with healthy controls, patients with SCZ show decreased volumes of hippocampus, amygdala, thalamus, nucleus accumbens, and ICV, and larger volumes of pallidum and lateral ventricles.1–3 One meta-analysis also found larger volumes of putamen and caudate nucleus in SCZ,2 but this was not evident in the other meta-analyses.1,3 Some brain volume alterations have been linked to functional disturbances in SCZ. For example, larger volumes of putamen were related to worse verbal learning, executive functioning, and working memory performance among patients with SCZ,12 while global reductions in brain volume are consistently associated with decreased intelligence.13 Subcortical brain regions are also important for the working mechanisms of antipsychotics. The majority of dopamine D2-receptors, which all effective antipsychotics act upon, are located in basal ganglia structures putamen, caudate nucleus, and nucleus accumbens,14 and some studies found that treatment with antipsychotics may alter the volumes of basal ganglia15 and the hippocampus.1
Genome-wide association studies (GWASs) have identified over 100 single nucleotide polymorphisms (SNPs) associated with SCZ,16 10 variants with hippocampal volume,5,17–20 8 variants with ICV,5,17,21,22 4 variants with putamen volume,5 and 1 variant with caudate volume.5 However, the variants identified at the genome-wide significance threshold explain only a small fraction of the heritability, and most of the genetic variants influencing these phenotypes remain to be uncovered.5,16–22 To increase discovery of small genetic effects in polygenic phenotypes, we have developed a conditional false discovery rate (condFDR) method, which builds on an empirical Bayesian statistical framework.23–25 Compared to the conventional (frequentist) GWAS statistical approach, the condFDR method improves statistical power by leveraging overlapping associations in independent GWAS to re-rank test statistics.23–25 Moreover, the condFDR method improves discovery of genetic variants shared between phenotypes regardless of the direction of allelic effects.24,25 Using this approach, we have identified shared variants between many neuropsychiatric disorders and related phenotypes,26–32 and substantially increased discovery of risk variants.23–25
Recently, Franke et al33 applied a battery of statistical tools to examine genetic overlap between subcortical brain volumes and SCZ. They reported no evidence of genetic overlap at the genome-wide level or at the level of individual risk variants.33 To estimate genetic overlap at the genome-wide level, they applied widely used methods such as polygenic risk scores34 and linkage disequilibrium (LD) score regression.33,35 However, these tools are unable to pinpoint individual shared variants or uncover mixed effect directions among shared genetic effects.29,30 Moreover, lack of genome-wide correlation does not imply statistical independence of individual variants.24 To identify individual shared variants, combined GWASs meta-analysis may increase statistical power, but yield test statistics skewed toward the most strongly powered GWAS, and cannot identify variants with opposite effect directions.33 Alternatively, the conjunction analysis, which determines whether a particular SNP is linked to 2 phenotypes,33 can detect opposite effect directions, but does not capitalize on the inherent power in leveraging overlapping GWAS associations to increase statistical power.24,25,33 In the present study, we applied the condFDR approach to investigate genetic overlap between SCZ and subcortical brain volumes and ICV, and analyzed large GWASs of brain phenotypes from the ENIGMA Consortium (Enhancing NeuroImaging Genetics through Meta-Analysis)5 and of SCZ from PGC (Psychiatric Genomics Consortium).16
Methods
Ethics Statement
All GWASs performed and investigated in the current study were approved by the local ethic committees and informed consent was obtained from all participants. Further, the Norwegian IRB for the South-East region has evaluated the current protocol and found that no additional IRB approval was needed, because no individual data was used.
Participant Samples
We obtained GWAS results in the form of summary statistics (P-values and z-scores). GWAS data on SCZ were acquired from PGC (n = 82315; http://pgc.unc.edu/),16 and GWAS data on MRI volumetric measures on amygdala, caudate nucleus, hippocampus, nucleus accumbens, pallidum, putamen, and thalamus and ICV from ENIGMA (n = 11,840; http://enigma.ini.usc.edu/).5 To limit sample overlap, 1848 individuals (978 SCZ cases, 870 controls) from the ENIGMA cohort present in the PGC cohort were removed. For details of the inclusion criteria, genotyping and phenotype characteristics, see supplementary methods or the original publications.5,16 We corrected all P-values for inflation using a genomic inflation control procedure.26,28,36,37
Statistical Analyses
To visualize pleiotropic enrichment, we constructed conditional Q-Q plots where we display the distribution of P-values for the primary phenotype conditional on significance levels in a secondary phenotype. Associations in the primary phenotype (eg, SCZ) were conditioned on a P-value threshold in the secondary phenotype (eg, ICV), that is, P < 0.1, P < 0.01, and P < 0.001. If statistical enrichment of the primary phenotype exists, there should be successive leftward deflections as levels of association with the secondary phenotype increase.23,25–28,31,36 The enrichment seen can be directly interpreted in terms of true discovery rate (1−FDR)38 (see supplementary methods for details). To control for spurious enrichment, conditional Q-Q plots were constructed after random pruning averaged over 100 iterations. At each iteration, one SNP in every LD block (defined by LD r2 > .1) was randomly selected and the empirical cumulative distribution functions were computed using the corresponding P-values. Given the long range LD within the extended major histocompatibility complex (MHC) and its strong association with SCZ,16 we excluded SNPs in this region (genome build 19 location 25652429–33368333) and SNPs in LD (r2 > .1) with such SNPs before fitting the condFDR model to avoid any potential bias for FDR estimation due to the complex LD structure in this region.39
To identify shared variants between SCZ and brain volumes, we used the condFDR statistical framework and analyzed brain phenotypes that showed genetic enrichment based on association with SCZ, which implies improved statistical power for SNP discovery.23,26–31,36 The standard FDR framework derives from a model that assumes the distribution of test statistics in a GWAS to be a mixture of null and non-null effects, with true associations (non-null effects) having more extreme test statistics than false associations (null effects). The condFDR is an extension of the standard FDR, and incorporates information from GWAS summary statistics of a secondary phenotype to re-rank test statistics and estimate per-SNP condFDR values of the primary phenotype. After repeating the condFDR procedure for both phenotypes, shared genetic variants were identified at conjunctional FDR (conjFDR) <0.05, which is given by the maximum between the condFDRs for both phenotypes.40 Hence, a low conjFDR is only possible if there is an association with the 2 phenotypes of interest jointly. On the basis of 1KGP LD structure, the significant SNPs identified were clustered into LD blocks at the LD r2 >.1 level. Any block may contain more than one SNP. We investigated the direction of allelic effects in the conjunctional SNPs by comparing the SCZ z-scores against brain volume z-scores. Two SNPs showed ambiguous effect directions due to T–A and C–G polymorphisms with unknown strand affiliations for these effects. To provide a measure of the effect directions in SCZ and brain volumes for these variants, we identified SNPs in strong LD with these SNPs (ie, within the same risk locus) and compared their SCZ z-scores against brain volume z-scores. To assess the credibility of our approach, we obtained the P-values of the identified variants in larger GWAS on ICV22 (n = 26577) and hippocampal volume20 (n = 26814) from combined ENIGMA2 + CHARGE meta-analyses. Additionally, we estimated the variance of SCZ risk and brain volumes explained by each conjunctional variant. For details, see supplementary methods.
Brain Gene Expression
We determined the overall messenger RNA (mRNA) expression of genes jointly implicated in SCZ and brain volumes. Using the publicly available dataset provided by the Human Brain Transcriptome project (http://hbatlas.org), we assessed mRNA expression trajectories in 6 regions of the developing and adult human brain.41 Spanning periods from embryonic development to late adulthood, this dataset provides genome-wide, exon-level transcriptome data generated using the Affymetrix GeneChip Human Exon 1.0 ST Arrays from over 1340 tissue samples sampled from both hemispheres of postmortem human brains (n = 57).41 Additionally, we determined whether the conjunctional variants may regulate gene expression (ie, expression quantitative trait loci [eQTL] functionality) using 2 independent datasets from GTEx42 and the UK Brain Expression Consortium (UKBEC).43 For details, see supplementary methods.
Results
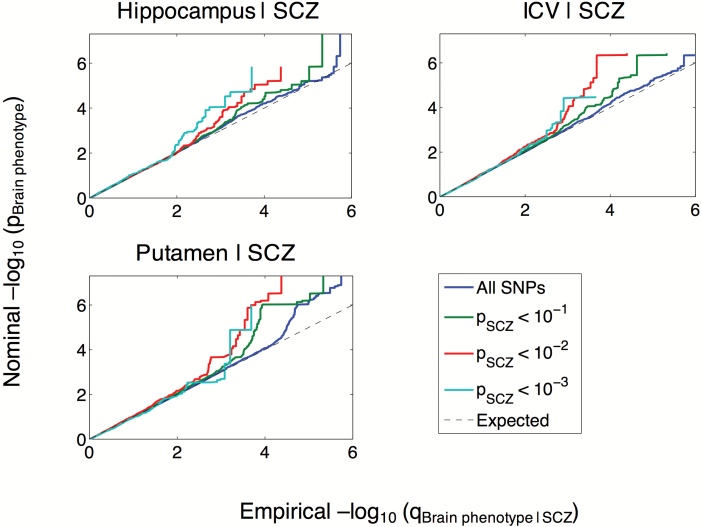
In the conditional Q-Q plots, we observed SNP enrichment for volumes of hippocampus, putamen, and ICV with SCZ (figure 1), indicating increased power for SNP discovery in these phenotypes gained by conditioning on association with SCZ.23–25 For none of the remaining brain volumes (caudate, pallidum, thalamus, accumbens, and amygdala) did we observe enrichment of associations conditional on SCZ (supplementary figure 1). We also present the reverse conditional Q-Q plots, which suggest enrichment of associations with SCZ as a function of association with volumes of hippocampus and amygdala (supplementary figure 2).
Fig. 1.
Conditional Q-Q plots of nominal vs empirical −log10P-values (corrected for inflation) in volumes of hippocampus, putamen, and intracranial volume (ICV) below the standard GWAS threshold of P < 5 × 10−8 as a function of significance of association with schizophrenia (SCZ) at the level of −log10(P) ≥ 1, −log10(P) ≥ 2, −log10(P) ≥ 3 corresponding to P ≤ .1, P ≤ .01, P ≤ .001, respectively. The blue lines indicate all SNPs. The dashed lines indicate the null hypothesis.
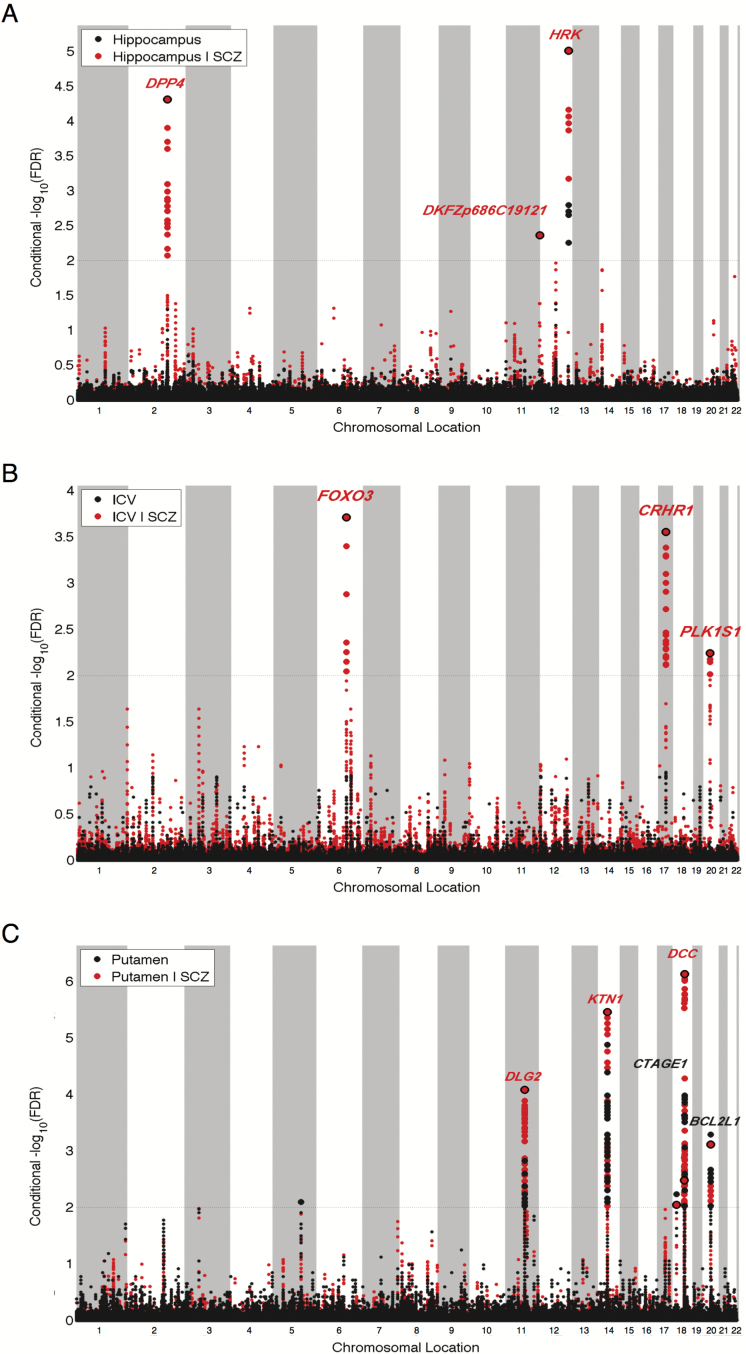
Given the indication of genetic enrichment in volumes of hippocampus, putamen, and ICV based on SNP association with SCZ, we followed up with condFDR analyses to increase discovery of SNPs associated with these brain volume phenotypes. Based on condFDR <0.01, we identified 3 loci associated with hippocampal volume, 6 loci associated with volume of putamen, and 3 loci associated with ICV (supplementary table 1). In figure 2, we present condFDR Manhattan plots for volumes of hippocampus, putamen, and ICV, showing all SNPs with a condFDR <0.01 within an LD block in relation to their chromosomal position. These figures demonstrate the increased statistical power for identifying loci associated with these brain volumes by conditioning on association with SCZ.
Fig. 2.
“Conditional FDR (condFDR) Manhattan plots” of conditional −log10 (FDR) values for (A) volume of hippocampus, (B) intracranial volume (ICV), and (C) volume of putamen conditioned on schizophrenia (SCZ). Unconditioned FDR values are shown in black, condFDR values in red. SNPs with conditional −log10 FDR > 2.0 (ie, condFDR < 0.01) are shown with large points. A black line around the large points indicates the most significant SNP in each LD block. This SNP is annotated with the closest gene.
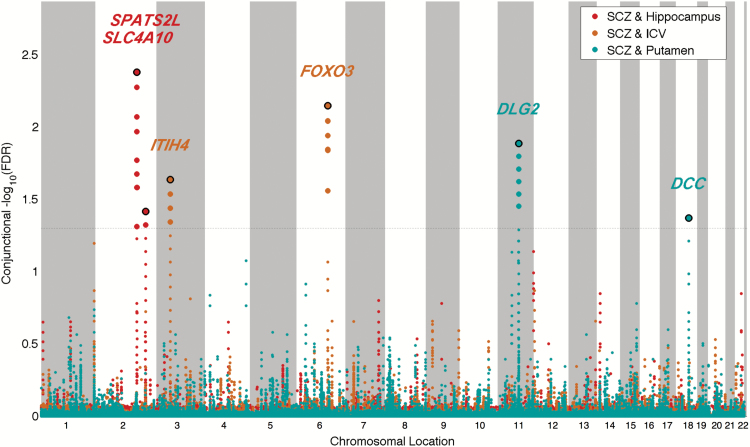
To identify shared loci between SCZ and volumes of hippocampus, putamen, and ICV we used conjFDR analysis. At conjFDR <0.05, 2 loci were shared between hippocampal volume and SCZ: rs4664442 (chromosome 2q24.2; intronic variant within SLC4A10) and rs1653290 (chromosome 2q33.1; nearest gene SPATS2L, intergenic variant). Two loci were shared between ICV and SCZ: rs4687658 (chromosome 3p21.1; intronic variant within ITIH4) and rs10457180 (chromosome 6q21; intronic variant within FOXO3). Finally, 2 loci were shared between putamen volume and SCZ: rs11233632 (chromosome 11q14.1; intronic variant within DLG2) and rs4632195 (chromosome 18q21.2; intronic variant within DCC). Despite the strong association of the MHC region with SCZ,16 we did not detect any significant genetic effects overlapping between SCZ and brain structure volumes located to this region. The strongest shared signal within the MHC region was detected at ZNF391 (rs12663650; conjFDR = 0.12) and was shared between SCZ and volume of putamen (supplementary table 2). This subthreshold finding must be interpreted cautiously given the complex LD in this region. To visualize the shared loci, we constructed a conjFDR Manhattan plot (figure 3). All SNPs without pruning are shown, and the strongest signal in each LD block is encircled in black. The enlarged data points represent the SNPs at conjFDR<0.05, whereas the small points represent other SNPs. As expected, the variance explained by each conjunctional variant for SCZ risk and brain volumes was low (table 1).
Fig. 3.
“ConjFDR Manhattan plot” of conjunctional −log10 (FDR) values for schizophrenia (SCZ) and hippocampal volume, intracranial volume (ICV), and volume of putamen; the conjunctions are denoted as SCZ & hippocampus, SCZ & ICV, and SCZ & putamen, respectively. SNPs with conjFDR < 0.05 (ie, conjunctional −log10(FDR) > 1.3) are shown with enlarged data points. A black circle around the enlarged data points indicates the most significant SNP in each LD block and this SNP was annotated with the closest gene which is listed above the symbols in each locus. The figure shows the localization of the “conjunctional loci,” and further details are provided in table 1.
Table 1.
Genetic Loci With conjFDR < 0.05 Shared Between SCZ and Brain Structure Volumes
| Locus | Marker | Nearest Gene (Annotation) | Chr | A1/A2 | Z-Score SCZ | Z-Score Brain Volume | ConjFDR | P-Value SCZ16 | P-Value Brain Volume5 | Variance Explained SCZ (%) | Variance Explained Brain Volume (%) | Other Genes in Genomic Region Defined by LD | ENIGMA2 and CHARGE Combined GWAS20,22 (n ≥ 26577) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCZ (n = 82315) and hippocampal volume (n = 11621) | |||||||||||||
| 1 | rs4664442a | SLC4A10 (intron) | 2q24.2 | G/A | −4.32 | −4.82 | 4.16e-03 | 1.56e-05 | 1.41e-06 | 0.17 | 0.20 | DPP4 | 2.53E-10 |
| 2 | rs1653290a | SPATS2L (intergenic) | 2q33.1 | A/T | NAb | NA | 3.83e-02 | 5.44e-07 | 1.82e-04 | 0.21 | 0.12 | — | 1.46E-01 |
| SCZ (n = 82315) and ICV (n = 9826) | |||||||||||||
| 3 | rs4687658a | ITIH4 (intron) | 3p21.1 | C/G | NAb | NA | 2.31e-02 | 1.49e-05 | 1.09e-04 | 0.18 | 0.15 | TMEM110, MUSTN1, SFMBT1 | 3.50E-04 |
| 4 | rs10457180 | FOXO3 (intron) | 6q21 | G/A | 4.20 | −4.14 | 7.11e-03 | 2.72e-05 | 3.43e-05 | 0.16 | 0.18 | — | 4.12E-11 |
| SCZ (n = 82315) and volume of putamen (n = 11598) | |||||||||||||
| 5 | rs11233632c | DLG2 (intron) | 11q14.1 | C/T | 3.98 | 4.14 | 1.30e-02 | 6.89e-05 | 3.41e-05 | 0.13 | 0.15 | — | — |
| 6 | rs4632195c | DCC (intron) | 18q21.2 | C/T | −3.66 | −4.10 | 4.26e-02 | 2.49e-04 | 4.11e-05 | 0.12 | 0.15 | — | — |
Independent complex or single gene loci (r2 < .1) with single nucleotide polymorphisms with a conjFDR < 0.05 shared between SCZ and volumes of hippocampus, putamen, and ICV. The most significant SNPs in each LD block are listed. Chromosomal position, closest gene, z-scores and P-values in the phenotypes, the percentage variance in SCZ and brain volumes explained by each variant, and other genes in genomic region defined by LD > 0.6 are also listed. The effect sizes are given with reference to allele 1 (A1). All data were first corrected for genomic inflation, and the MHC-region was excluded from the FDR computation. Chr, chromosome; A1, allele number 1; A2, allele number 2; NA, not available z-score signs due to A–T and C–G polymorphisms; conjFDR, conjunctional false discovery rate; GWAS, genome-wide association study; ICV, intracranial volume; LD, linkage disequilibrium; MAF, minor allele frequency; SCZ, schizophrenia.
aLoci identified by the primary SCZ GWAS.16
bSNPs in strong LD with rs1653290 show the same effect directions in hippocampal volume and SCZ, and SNPs in strong LD with rs4687658 showed the same effect directions in ICV and SCZ (supplementary table 3).
cLoci identified by the primary brain structure volume GWAS.5
Next, we evaluated the directionality of allelic effects in SNPs associated with SCZ risk and brain structure volumes (table 1). For rs4664442 (SLC4A10), we found the same direction of effect between hippocampal volume and SCZ risk. For rs10457180 (FOXO3), we found an opposite direction of effect between ICV and SCZ risk. For rs11233632 (DLG2) and rs4632195 (DCC) variants, we found the same direction of effect between putamen volume and SCZ risk. Due to T–A and C–G polymorphisms, the directions of effect in SCZ and brain volumes could not be determined for rs1653290 (SPATS2L) and rs4687658 (ITIH4), respectively. Hence, we identified SNPs in strong LD with rs1653290 and rs4687658 and compared their SCZ z-scores to their brain volume z-scores (supplementary table 3). SNPs in LD with rs1653290 showed consistently concordant associations for hippocampal volume and SCZ, while SNPs in LD with rs4687658 showed consistently concordant associations for ICV and SCZ.
Further, we looked up the identified SNPs in the combined ENIGMA2 + CHARGE GWASs for ICV22 and hippocampal volume20 (which both include the ENIGMA2 data used above). In the combined GWAS, 2 SNPs were now significant at the GWAS threshold (rs4664442 for hippocampal volume and rs10457180 for ICV), rs1653290 showed suggestive association with ICV, while rs1653290 was not significantly associated with hippocampal volume (table 1). Four of 6 conditional loci associated with hippocampal volume and ICV reached genome-wide significance in the combined ENIGMA2 + CHARGE GWAS, while 2 loci showed suggestive associations (supplementary table 1).
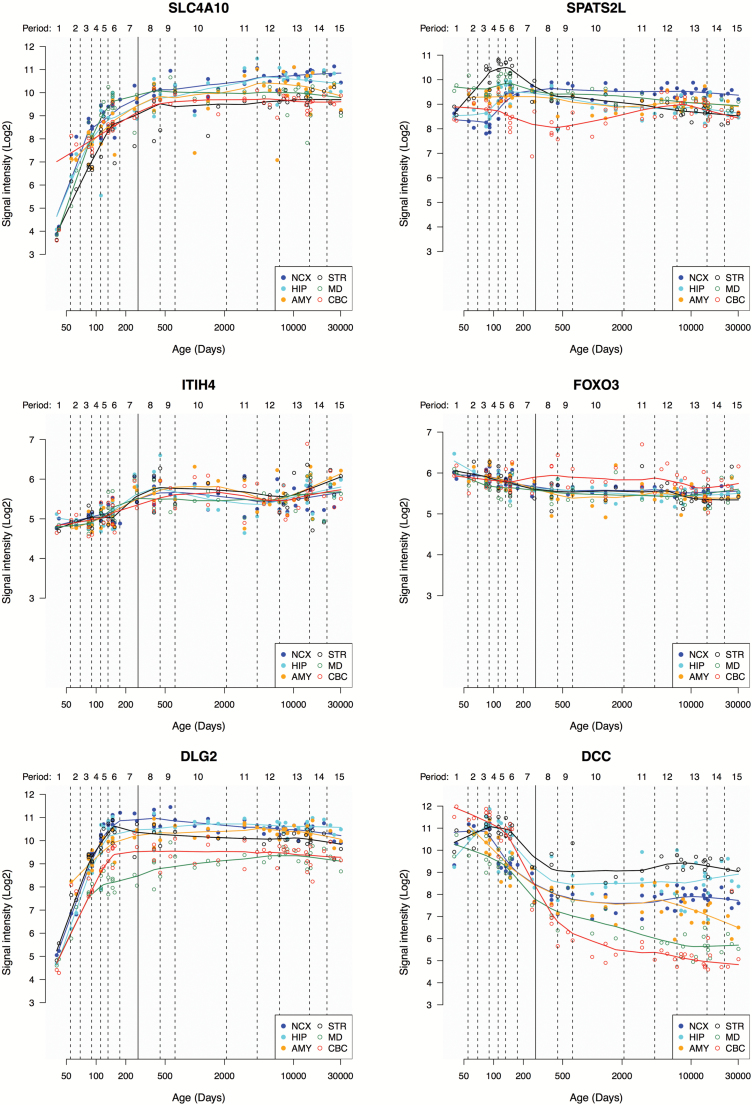
Using data from the Human Brain Transcriptome Project, we determined the distribution of mRNA expression for SLC4A10, SPATS2L, ITIH4, FOXO3, DLG2, and DCC and show that the genes are globally expressed in the developing and adult human brain (figure 4). One mechanism whereby SNPs may affect phenotype variation is through altered gene expression, that is, eQTL functionality. Using GTEx42 data, we found that 2 SNPs were significantly associated with altered gene expression in brain tissue (supplementary table 4): rs1653290 for FTCDNL1 in the hypothalamus, and rs4687658 for ITIH4 in several brain regions (supplementary figure 3). We assessed these associations in the independent UKBEC43 dataset (supplementary table 5), and found replicable eQTL functionality of rs4687658 for ITIH4 in several brain regions (supplementary figure 4), but no replicable association for rs1653290.
Fig. 4.
Expression trajectories of SLC4A10, SPATS2L, ITIH4, FOXO3, DLG2, and DCC in the developing and adult human brain, provided by the Human Brain Transcriptome project.41 Line plots show the log2-transformed gene exon array signal intensity from the early fetal period to late adulthood in 6 brain regions. The solid line between periods 7 and 8 (approximately post-conception day 280) separates prenatal from postnatal periods. NCX, neocortex; HIP, hippocampus; AMY, amygdala; STR, striatum; MD, mediodorsal nucleus of the thalamus; CBC, cerebellar cortex.
Discussion
In the present article, we analyzed GWASs data using the condFDR approach to address the question of genetic overlap between SCZ and brain structure volumes. We found that genetic variants associated with SCZ are also associated with volumes of hippocampus, putamen, and ICV, indicating that common molecular genetic mechanisms influence these phenotypes. Using conjFDR analysis, we identified 6 genetic loci shared between SCZ and volumes of hippocampus, putamen, and ICV (figure 3). Among the identified loci, 3 are novel risk loci for SCZ. None of the loci found here to be associated with hippocampal volume or ICV had been singled out in the primary GWAS on brain structure volumes.5 In the recently published larger GWAS on ICV and hippocampal volume,20,22 however, 2 loci did reach the genome-wide significance level for association with ICV (rs10457180) and hippocampal volume (rs4664442) (table 1), demonstrating the increased power and credibility of the condFDR approach.
We determined the mRNA expression in the human brain of the genes implicated by the conjFDR analysis (figure 4). The genes are globally expressed in the developing and adult human brain, indicating that they may influence brain function across the life span. There were some expression differences across brain regions, the most notable being the postnatal enrichment of DCC in the striatum. Further, we evaluated the eQTL functionality of the conjunctional variants using the independent GTEx42 and UKBEC43 datasets, which revealed replicable eQTL associations of rs4687658 for ITIH4 in several brain regions (supplementary figures 3 and 4). Other eQTL-associations did not replicate, which could result from differences in methodological techniques or sample configuration between the eQTL databases, or reflect the relatively small sample sizes. The eQTL results should be re-assessed when larger brain-eQTL databases are available.
We identified 2 loci shared between SCZ and ICV. Given that ICV is driven by brain growth, and that the maximum size of ICV is reached in early adolescence,44 the observed decrease in ICV among patients with SCZ suggests that neurodevelopment is disrupted in this group.1–3 We identified an intronic variant within FOXO3 (rs10457180), where the SCZ risk allele G was associated with smaller ICV. This locus was not discovered in the primary GWAS on SCZ16 or ICV,5 but reached genome-wide significance in the larger GWAS on ICV.22 Interestingly, genome-wide significant associations for the same FOXO3 locus was reported in GWASs on longevity45 and intelligence.46 FOXO3 belongs to the FOXO transcription family, which regulates a variety of cellular processes including oxidative stress resistance and adult stem cell homeostasis.47 An influence of FOXO3 on ICV is consistent with 2 studies reporting that different genetic manipulations of FOXO3 in mice resulted in increased48 or decreased49 brain weight, probably reflecting disturbed regulation of the neural stem cell pool. We also identified an intronic variant within ITIH4 (rs4687658) shared between ICV and SCZ with unknown directionality of allelic effects due to a G-C polymorphism. However, SNPs in LD with rs4687658 showed the same effect directions in ICV and SCZ (ie, the SCZ risk alleles are associated with larger ICV; supplementary table 3). Rs4687658 was identified in the original SCZ GWAS,16 while another ITIH4 variant (rs2239547, in LD (r2 = .801) with rs4687658) was identified in a SCZ GWAS in people of Han Chinese ancestry.50 ITIH4 is considered an acute-phase protein involved in various inflammatory responses.51 Recently, another ITIH4 variant (rs4687657, in complete LD with rs2239547) was shown to regulate ITIH4 expression in the prefrontal cortex.52 This finding is in compliance with our eQTL investigation which revealed that rs4687658 may regulate ITIH4 expression in several brain regions (supplementary figures 3 and 4).
We found 2 loci shared between hippocampal volume and SCZ. Patients with SCZ typically display smaller hippocampal volumes compared with controls.1–3 However, for the identified shared intronic variant within SLC4A10 (rs4664442) on chromosome 2q24.2, the SCZ risk allele A was associated with larger hippocampal volume. The shared 2q24.2 locus contains 2 genes, SLC4A10 and DPP4. SLC4A10 encodes a bicarbonate transporter abundant in mouse hippocampal interneurons and CA3 pyramidal cells which regulates neuronal pH and excitability.53DPP4 encodes dipeptidyl peptidase 4, a widely expressed enzyme associated with immune regulation, glucose homeostasis, and signal transduction.54 Interestingly, another SLC4A10 variant (rs2909457, in LD [r2 = .976] with rs4664442) was previously identified as a joint variant for hippocampal volume and SCZ after tests of GWAS-significant SCZ hits for association with brain volumes.5,33 Moreover, rs4664442 was not identified in the primary GWAS on hippocampal volume,5 but reached genome-wide significance in the larger GWAS on hippocampal volume.20 We also detected an intergenic variant close to SPATS2L (rs1653290) shared between SCZ and hippocampal volume with unknown effect directionality due to an A–T polymorphism. However, SNPs in LD with rs1653290 show the same effect directions in hippocampal volume and SCZ (ie, the SCZ risk alleles are associated with larger hippocampal volume; supplementary table 3). This locus was genome-wide significant in the primary SCZ GWAS.16 However, the weak P-value in the larger GWAS on hippocampal volume20 (table 1), may suggest that it is a spurious finding for hippocampal volume.
Finally, we uncovered 2 loci shared between volume of putamen and SCZ. Although some neuroimaging studies have found larger volumes of putamen in SCZ,2 other studies have not reported any significant association.1,3 We identified an intronic variant within DCC (rs4632195), where the SCZ risk allele T was associated with larger putamen volume. In the primary ENIGMA GWAS, another DCC variant (rs62097986, in LD [r2 = .627] with rs4632195) was identified for putamen volume.5DCC encodes a netrin receptor highly expressed in dopaminergic neurons which facilitates growth and migration of axons and dendrites.55 Intriguingly, DCC-netrin interactions mediate the proper development of dopaminergic projections to prefrontal cortex and striatum in mice.55,56 This is of particular relevance to SCZ, as all effective antipsychotic drugs target the dopaminergic system, which is strongly implicated in the pathophysiology of SCZ.57 Notably, a small case–control study has previously identified DCC as a candidate gene in SCZ.58 We also detected an intronic variant within DLG2 (rs11233632), where the SCZ risk allele C was associated with larger putamen volume. Another DLG2 variant (rs683250, in LD [r2 = .710] with rs11233632) was identified for putamen volume in the original ENIGMA GWAS,5 while copy-number variants in DLG2 have been associated with SCZ.59DLG2 encodes the postsynaptic density protein 93 which scaffolds neurotransmitter receptors and enzymes into signaling complexes at the postsynaptic terminal and may contribute to cognitive flexibility and learning.60
The number of shared loci identified here is consistent with previous condFDR analyses, which have elucidated genetic overlap between several polygenic phenotypes including SCZ,26–28,32 cognitive traits,30 personality traits,29 and immune-related diseases.23,27,31,36 Several conjunctional and conditional loci discovered here were not identified in the primary GWASs, and only locus rs4664442 was implicated as a joint variant for SCZ and brain structure phenotypes using conventional statistical tools,5,33 demonstrating the increased power of the condFDR approach. As indicated by the P-values obtained from the larger GWAS on brain structures,20,22 it is likely that the loci discovered here would have been identified in the original GWAS if the samples had been larger. The fact that rs1653290 was not significantly associated with hippocampal volume in the larger meta-analysis,20 may suggest that this association was an artefact, or reflect methodological or cohort differences between the CHARGE and ENIGMA GWASs. Altogether, the present study demonstrates how combining GWAS summary statistics using condFDR analysis provides a useful alternative to other statistical tools evaluating genetic overlap, which did not report any shared genetic influences between SCZ and brain structure volumes.33 The power of the condFDR approach depends on the sample size of the investigated GWASs and the extent of genetic overlap between the traits. More shared loci between SCZ and subcortical brain volumes and ICV are expected to be uncovered when larger GWAS samples are available.
In conclusion, the study provides new insights into the relationship between SCZ and brain structure formation by identifying 6 genetic loci jointly influencing SCZ and volumes of hippocampus, putamen, and ICV, suggesting a shared genetic basis between these polygenic phenotypes. As indicated by the low phenotypic variance explained by each conjunctional variant (table 1), the identified variants are not informative clinically. Careful efforts are required to determine the true underlying causal variants responsible for the shared associations and to clarify the specific molecular genetic mechanisms involved.61 By indicating that genetic risk underlying SCZ may contribute to brain volume alterations, the findings should motivate further efforts to elucidate genetic influences shared between neuropsychiatric diseases and key brain structure phenotypes. Important challenges ahead will be to determine the spatiotemporal influences of the identified loci on brain development and function, and clarify their interactions with other genetic and environmental risk factors.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Supplementary Material
Acknowledgments
This work was supported by NIH (NS057198 and EB00790), NIMH (R01MH100351), the Research Council of Norway (229129, 213837, 223273, and 251134), the South-East Norway Regional Health Authority (2013–123, 2016–064, and 2017-004), and KG Jebsen Foundation (SKGJ-2011–36). We thank the research participants and researchers of the ENIGMA and the PGC consortia for making this work possible. Dr Andreassen has received a speaker’s honorarium from Lundbeck. The other authors have no conflicts of interests to declare.
References
- 1. van Erp TG, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. Apr 2016;21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Okada N, Fukunaga M, Yamashita F, et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. Oct 2016;21(10):1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hibar DP, Stein JL, Renteria ME, et al. Common genetic variants influence human subcortical brain structures. Nature. Apr 09 2015;520(7546):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moran ME, Hulshoff Pol H, Gogtay N. A family affair: brain abnormalities in siblings of patients with schizophrenia. Brain. 2013;136:3215–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. [DOI] [PubMed] [Google Scholar]
- 8. Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. [DOI] [PubMed] [Google Scholar]
- 9. Leisman G, Braun-Benjamin O, Melillo R. Cognitive-motor interactions of the basal ganglia in development. Front Syst Neurosci. 2014;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poldrack RA, Clark J, Paré-Blagoev EJ, et al. Interactive memory systems in the human brain. Nature. 2001;414:546–550. [DOI] [PubMed] [Google Scholar]
- 11. Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartberg CB, Sundet K, Rimol LM, et al. Subcortical brain volumes relate to neurocognition in schizophrenia and bipolar disorder and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1122–1130. [DOI] [PubMed] [Google Scholar]
- 13. Kubota M, van Haren NE, Haijma SV, et al. Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry. 2015;72:803–812. [DOI] [PubMed] [Google Scholar]
- 14. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. [DOI] [PubMed] [Google Scholar]
- 15. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stein JL, Medland SE, Vasquez AA, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bis JC, DeCarli C, Smith AV, et al. Common variants at 12q14 and 12q24 are associated with hippocampal volume. Nat Genet. 2012;44:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Melville SA, Buros J, Parrado AR, et al. ; Alzheimer’s Disease Neuroimaging Initiative Multiple loci influencing hippocampal degeneration identified by genome scan. Ann Neurol. 2012;72:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hibar DP, Adams HH, Jahanshad N, et al. Novel genetic loci associated with hippocampal volume. Nat Commun. 2017;8:13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikram MA, Fornage M, Smith AV, et al. Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat Genet. 2012;44:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams HH, Hibar DP, Chouraki V, et al. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat Neurosci. Dec 2016;19(12):1569–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu JZ, Hov JR, Folseraas T, et al. ; UK-PSCSC Consortium; International PSC Study Group; International IBD Genetics Consortium Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schork AJ, Wang Y, Thompson WK, Dale AM, Andreassen OA. New statistical approaches exploit the polygenic architecture of schizophrenia–implications for the underlying neurobiology. Curr Opin Neurobiol. 2016;36:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andreassen OA, Thompson WK, Dale AM. Boosting the power of schizophrenia genetics by leveraging new statistical tools. Schizophr Bull. 2014;40:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andreassen OA, Thompson WK, Schork AJ, et al. ; Psychiatric Genomics Consortium (PGC); Bipolar Disorder and Schizophrenia Working Groups Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9:e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andreassen OA, Harbo HF, Wang Y, et al. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune related gene loci. Mol Psychiatry. Feb 2015;20(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andreassen OA, Djurovic S, Thompson WK, et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am J Hum Genet. 2013;92:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smeland OB, Wang Y, Lo MT, et al. Identification of genetic loci shared between schizophrenia and the big five personality traits. Sci Rep. 2017;7:2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smeland OB, Frei O, Kauppi K, et al. Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal-numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry. July 26 2017. doi:10.1001/jamapsychiatry.2017.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desikan RS, Schork AJ, Wang Y, et al. Polygenic overlap between c-reactive protein, plasma lipids and alzheimer’s disease. Circulation. Jun 09 2015;131(23):2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Hellard S, Wang Y, Witoelar A, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium Identification of gene loci that overlap between schizophrenia and educational attainment. Schizophr Bull. 2017;43:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franke B, Stein JL, Ripke S, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium; ENIGMA Consortium Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci. 2016;19:420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bulik-Sullivan BK, Loh PR, Finucane HK, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andreassen OA, Desikan RS, Wang Y, et al. Abundant genetic overlap between blood lipids and immune-mediated diseases indicates shared molecular genetic mechanisms. PLoS One. 2015;10:e0123057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schork AJ, Thompson WK, Pham P, et al. All SNPs are not created equal: genome-wide association studies reveal a consistent pattern of enrichment among functionally annotated SNPs. PLoS Genet. 2013;9:e1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 39. Schwartzman A, Lin X. The effect of correlation in false discovery rate estimation. Biometrika. 2011;98:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. [DOI] [PubMed] [Google Scholar]
- 41. Kang HJ, Kawasawa YI, Cheng F, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramasamy A, Trabzuni D, Guelfi S, et al. ; UK Brain Expression Consortium; North American Brain Expression Consortium Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014;17:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. [DOI] [PubMed] [Google Scholar]
- 45. Broer L, Buchman AS, Deelen J, et al. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J Gerontol A Biol Sci Med Sci. 2015;70:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sniekers S, Stringer S, Watanabe K, et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet. Jul 2017;49(7):1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. [DOI] [PubMed] [Google Scholar]
- 48. Renault VM, Rafalski VA, Morgan AA, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmidt-Strassburger U, Schips TG, Maier HJ, et al. Expression of constitutively active FoxO3 in murine forebrain leads to a loss of neural progenitors. FASEB J. 2012;26:4990–5001. [DOI] [PubMed] [Google Scholar]
- 50. Li Z, Xiang Y, Chen J, et al. Loci with genome-wide associations with schizophrenia in the Han Chinese population. Br J Psychiatry. 2015;207:490–494. [DOI] [PubMed] [Google Scholar]
- 51. Song J, Patel M, Rosenzweig CN, et al. Quantification of fragments of human serum inter-alpha-trypsin inhibitor heavy chain 4 by a surface-enhanced laser desorption/ionization-based immunoassay. Clin Chem. 2006;52:1045–1053. [DOI] [PubMed] [Google Scholar]
- 52. Ohi K, Shimada T, Nitta Y, et al. Schizophrenia risk variants in ITIH4 and CALN1 regulate gene expression in the dorsolateral prefrontal cortex. Psychiatr Genet. 2016;26:142–143. [DOI] [PubMed] [Google Scholar]
- 53. Jacobs S, Ruusuvuori E, Sipilä ST, et al. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci U S A. 2008;105:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. [DOI] [PubMed] [Google Scholar]
- 55. Van den Heuvel DM, Pasterkamp RJ. Getting connected in the dopamine system. Prog Neurobiol. 2008;85:75–93. [DOI] [PubMed] [Google Scholar]
- 56. Manitt C, Mimee A, Eng C, et al. The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J Neurosci. 2011;31:8381–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 58. Grant A, Fathalli F, Rouleau G, Joober R, Flores C. Association between schizophrenia and genetic variation in DCC: a case-control study. Schizophr Res. 2012;137:26–31. [DOI] [PubMed] [Google Scholar]
- 59. Kirov G, Pocklington AJ, Holmans P, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nithianantharajah J, Komiyama NH, McKechanie A, et al. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat Neurosci. 2013;16:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet. 2013;14:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.