Abstract
Background and Aims
The relationship between plant carbon economy and drought responses of co-occurring woody species can be assessed by comparing carbohydrate (C) dynamics following drought and rain periods, relating these dynamics to species’ functional traits. We studied nine woody species coexisting in a continental Mediterranean shrubland that experienced severe drought effects followed by rain.
Methods
We measured total non-structural carbohydrates (NSC) and soluble sugars (SS) in roots and stems during drought and after an autumn rain pulse in plants exhibiting leaf loss and in undefoliated ones. We explored whether their dynamics were related to foliage recovery and functional traits (height [H], specific leaf area [SLA], wood density [WD]).
Key Results
During drought, NSC concentrations were overall lower in stems and roots of plants experiencing leaf loss, while SS decreases were smaller. Roots had higher NSC concentrations than stems. After the rain, NSC concentrations continued to decrease, while SS increased. Green foliage recovered after rain, particularly in plants previously experiencing higher leaf loss, independently of NSC concentrations during drought. Species with lower WD tended to have more SS during drought and lower SS increases after rain. In low-WD species, plants with severe leaf loss had lower NSC relative to undefoliated ones. No significant relationship was found between H or SLA and C content or dynamics.
Conclusions
Our community-level study reveals that, while responses were species-specific, C stocks overall diminished in plants affected by prolonged drought and did not increase after a pulse of seasonal rain. Dynamics were faster for SS than NSC. We found limited depletion of SS, consistent with their role in basal metabolic, transport and signalling functions. In a scenario of increased drought under climate change, NSC stocks in woody plants are expected to decrease differentially in coexisting species, with potential implications for their adaptive abilities and community dynamics.
Keywords: Climate change, canopy dieback, die-off, drought, Mediterranean, non-structural carbohydrates, shrubland, plant functional traits, soluble sugars, wood density
INTRODUCTION
Climate change is increasing aridity in many parts of the world (Dai, 2012) and this trend is predicted to continue in the near future, particularly in the Mediterranean Basin (Gao et al., 2006; Giannakopoulos et al., 2009). As a result, Mediterranean plant communities are experiencing longer periods of drought, punctuated by precipitation events that usually follow a seasonal pattern (Sarris et al., 2007; Briffa et al., 2009). The role of these pulses is extremely relevant since they could determine the resilience of plant communities under climate change (Lloret et al., 2012). Although drought events are known to lead to vegetation die-off and canopy dieback in many regions of the world (Allen et al., 2010; Greenwood et al., 2017), most studies have dealt with acute episodes of heatwaves and/or water deficit (e.g. Breshears et al., 2005; Galiano et al., 2011; Lloret et al., 2016; Venturas et al., 2016) or prolonged drought periods (e.g. Sapes et al., 2017), and the more common scenario of drought periods punctuated by pulses of rain has not been explored in any depth.
Although most of our knowledge on vegetation die-off has focused on forests, the phenomenon also occurs in shrublands (e.g. Palacio et al., 2007; Lloret et al., 2016; Sapes et al., 2017), a type of ecosystem of great importance in terms of global extension, diversity and ecosystem services (McKell, 1975; van Wilgen et al., 1996; Petri et al., 2015). The diversity of the woody species coexisting in many shrubland communities provides an opportunity to study the general processes involved in canopy dieback and the subsequent recovery across species, as well as relating the range of responses (Breshears et al., 2005; Sapes et al., 2017) to species’ traits, life history strategies (Lloret et al., 2016), drought tolerance (Rosas et al., 2013) and carbon economy strategies (Klein et al., 2014; Galiano et al., 2017).
The mechanistic basis underlying drought-induced canopy dieback and eventual mortality has still not been completely identified, but there is agreement that it involves not only the impairment of the long-distance transport connecting the supply and demand of water and carbon but also alterations in the carbon balance (McDowell et al., 2008, 2011, 2013; Sala et al., 2010; Adams et al., 2017). In terms of the carbon economy, plants distribute recent carbon assimilated by leaves (sources) into various carbon sinks such as growth, metabolic maintenance, storage, defence, export and reproduction (Chapin et al., 1990; Körner, 2003). It has been suggested that stored non-structural carbohydrates (NSC) are never fully depleted under average conditions because a certain concentration of soluble sugars (SS) is required to sustain immediate plant functions such as osmoregulation, transport and signalling (Sala et al., 2010; Hartmann and Trumbore, 2016; Martínez-Vilalta et al., 2016). The storage component is of major importance for plant function and survival under stressful conditions (O’Brien et al., 2014; Sala and Mencuccini, 2014). More particularly, stored NSC such as soluble sugars and starch provide a buffer against periods of water limitation in which stomatal closure prevents photosynthetic carbon uptake (McDowell et al., 2008, 2011) as they provide the carbon that maintains basic metabolism and defence during drought stress (McDowell and Sevanto, 2010; Sala et al., 2012). However, the numerous observational and experimental studies undertaken to date on NSC dynamics in response to drought have yielded mixed results, from decreases (Galiano et al., 2011; Mitchell et al., 2013; Sevanto et al., 2014; Aguadé et al., 2015) to increases in NSC concentrations, or no change at all (Sala and Hoch, 2009; Anderegg et al., 2012; Gruber et al., 2012; Hartmann et al., 2013). There are also some contrasting results with respect to stems and roots (Klein et al., 2014). Because photosynthesis is less sensitive to water stress than growth, increases in NSC are often observed during the early stages of drought (McDowell et al., 2011; Mitchell et al., 2013; Hagedorn et al., 2016). Nevertheless, modelling and experimental research indicate that NSC reserves should eventually decline if drought lasts for sufficiently long periods (cf. above; McDowell et al., 2013).
A species’ carbon economy can also be related to growth and survival strategies associated with particular combinations of traits. Species’ traits are in turn crucial to understanding how environmental filters such as water availability determine the assemblage of species in a local community (Shipley, 2010). A general, two-dimensional global spectrum of plant form and function has been identified, with the two main axes corresponding to leaf economics and plant size (Díaz et al., 2016). The leaf economics spectrum highlights the trade-off between carbon and nutrient investments in leaf construction and the duration of returns on those investments. The spectrum runs from species with conservative leaf traits (i.e. long leaf lifespan, low specific leaf area [SLA], expensive construction and slow returns on investments of C and nutrients) to species with acquisitive leaf traits (i.e. short lifespan, high SLA values, cheap construction and fast returns on investment) (Wright et al., 2004; Shipley et al., 2006; Westoby et al., 2013). Resource-conservative species are often considered to be more resistant to carbon loss and drought stress (Saura-Mas and Lloret, 2007), although a species’ response often involves many interconnected traits (McDowell et al., 2011; Anderegg et al., 2012). More conservative species, for example, tend to have higher wood density and low growth rates (Chave et al., 2009). Wood density is also a moderately good predictor of resistance to drought-induced embolism (Hacke et al., 2001). The second main axis, plant size or maximum height, is related to life-history features and the ability to use resources over a continuum of colonization–exploitation (Díaz et al., 2016). Accordingly, specific functional traits have been correlated with different responses to extreme drought episodes in coexisting species (e.g. Skelton et al., 2015; Pivovaroff et al., 2016). In a recent global synthesis, Greenwood et al. (2017) reported that species with higher SLA or lower wood density were more susceptible to drought-induced mortality. In Mediterranean shrublands in south-west Spain, Lloret et al. (2016) found that species’ resistance and resilience to drought was explained by water economy and recruitment-related traits, while another study in Californian chaparral highlighted the relevance of plant size (Venturas et al., 2016), probably associated with deeper roots. However, the link between functional traits, plant C economy and drought-induced die-off merits further exploration. To our knowledge, no studies have addressed carbon dynamics over a sequence of drought and subsequent rain at the plant community level, where different species with contrasting functional traits coexist.
Here, our main aim was to assess NSC dynamics in the aerial (stems) and underground (roots) organs of woody species coexisting in a Mediterranean semi-arid shrubland during a drought-induced dieback event and subsequent recovery after a rain episode. Canopy dieback was estimated via the loss of leaves because leaf-shedding often occurs during drought episodes as an avoidance mechanism that maintains a favourable water status by reducing the transpiring leaf area (Dobbertin and Brang, 2001; Poyatos et al., 2013; Jump et al., 2017). We hypothesized that: (1) within species, plants with leaf loss exhibit lower NSC concentrations compared with undefoliated plants due to their reduced capacity to assimilate C over the duration of the drought episode; (2) SS are less variable than total NSC over time when plants with different levels of canopy dieback (leaf loss) are compared within species, reflecting the need to maintain some minimum concentrations of SS to sustain metabolism and osmoregulation; (3) an increase in green foliage following a post-drought rainy period is associated with a reduction in overall NSC concentrations because the C supply from the recovered canopy is insufficient to compensate for investment in new tissues or replenish stored reserves in a context of long-term drought; and (4) species characterized by high growth potential (low wood density), less longevity and ability to compete (small size) or acquisitive leaf traits (high SLA) have lower and more variable NSC concentrations, reflecting a faster response to environmental variability (Trumbore et al., 2015).
MATERIALS AND METHODS
Site description
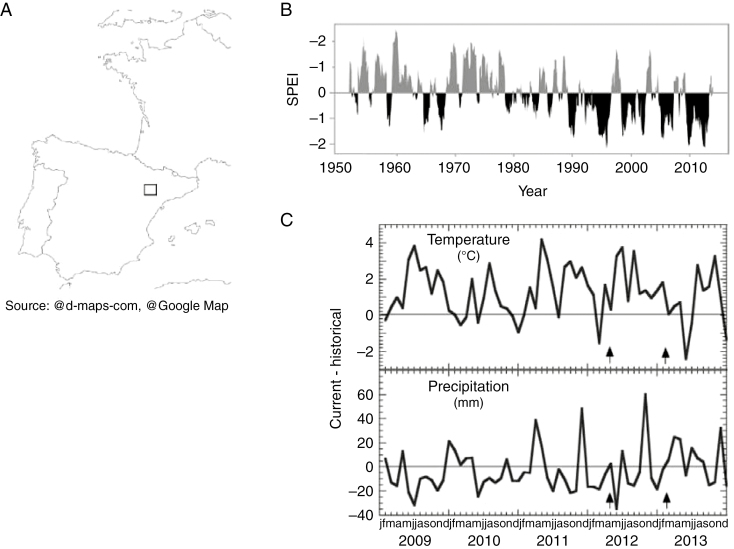
The study took place in the Barranco de Valcuerna (Monegros, central Ebro valley, Spain, 41°25′ N, 0°4′ E; Fig. 1), at ~280 m above sea level (Fig. 1A). The climate is continental Mediterranean with a mean annual temperature of 14.8 °C and mean annual precipitation of 390 mm; there are strong contrasts between the seasons, particularly with respect to temperatures, which range from mean values of 6.1 °C in January to 23.8 °C in July. In recent decades, the combination of higher temperatures and low precipitation has increased aridity in the region (Fig. 1B), resulting in a loss of vegetation cover (Vicente-Serrano et al., 2012; Sapes et al., 2017). However, the general trend towards increasing aridity is periodically interrupted by rainfall episodes that exceed the historical average precipitation, particularly in spring and autumn (Fig. 1C).
Fig. 1.
(A) Location of the study site. (B) Standardized precipitation–evapotranspiration index (SPEI) for years 1951–2013, calculated using a time scale of 12 months. Negative values (black) indicate water deficit (i.e. drought periods) while positive ones (grey) indicate water surplus relative to reference climatic conditions. Each SPEI value is calculated from the data of the last 12 months. (C) Difference between historical (1951–2000) and current (2009–13) mean monthly temperature and precipitation. Arrows indicate the two sampling dates (drought in April 2012 and post-rain in February 2013). Climatic data correspond to Zaragoza Airport, 90 km west of the Valcuerna study site. Source: Agencia Española de Meteorología (AEMET), Ministerio de Agricultura, Alimentación y Medio Ambiente, Gobierno de España.
The vegetation in this area is a mosaic of grassland, shrubland and Pinus halepensis open forest (Braun-Blanquet and Bolós, 1957; Terradas, 1986). We focused our study on an open continental–Mediterranean shrubland dominated by suffruticose shrubs (Thymus vulgaris, Lithospermum fruticosum, Thymelaea tinctoria, Helianthemum sp. pl.), small shrubs (Rosmarinus officinalis, Genista scorpius, Globularia alypum, Cistus libanotis, Helianthemum syriacum) and large shrubs (Quercus coccifera, Rhamnus lycioides, Pistacia lentiscus), with the occasional presence of short (Juniperus phoenicea) and taller (Pinus halepensis) trees. These species are generally found in a Mediterranean climate, although in this particular area some, such as J. phoenicea and Q. coccifera, are close to their tolerance limits with respect to aridity and low winter temperatures. This vegetation develops on leptosols over marls and limestones that are rich in gypsum.
Recording field canopy dieback
One particularly severe drought period lasted from 2009 to summer 2012 (Fig. 1B, C). This drought triggered widespread leaf loss (canopy dieback) in most woody species in the community. A pulse of rainfall occurred in autumn 2012, although temperatures remained above average and the drought persisted (Sapes et al., 2017), as indicated by the standardized precipitation–evapotranspiration index (SPEI) (Vicente-Serrano et al., 2010). We selected two sampling times: April 2012, at the peak of the drought period, and February 2013, which followed the autumn rainy season.
Adults from nine woody species were selected according to the following criteria: (1) perennial species that comprise a wide range of life forms, from suffruticose and small shrubs to trees; (2) species amenable to reliable visual estimates of drought-induced leaf loss; and (3) species comprising a wide range of leaf morphologies and wood densities. The species used in this study were P. halepensis (Ph), J. phoenicea (Jp), Q. coccifera (Qc), R. lycioides (Rl), R. officinalis (Ro), L. fruticosum (Lf), G. scorpius (Gs), T. tinctoria (Ti) and T. vulgaris (Th) (Table 1).
Table 1.
Description of studied species. Values of height, specific leaf area and wood density were obtained as explained in Materials and methods. Values of mean green canopy and plant density were obtained from Sapes et al. (2017), except for P. halepensis, L. fruticosum and T. tinctoria. For these three species, mean green canopy was obtained from 50 plants randomly selected from the sampled population, while values of plant density were obtained from five 100-m2 plots located in the study area. The biogeographical range description follows Bolòs et al., 2005 (W, western; SW, south-western)
| Species | Family | Biogeographical range | Mean green canopy (%) | Plant density (individuals ha−1) | Height (cm) | Specific leaf area (cm2 g−1) | Wood density (mg mm−3) |
|---|---|---|---|---|---|---|---|
| Pinus halepensis | Pinaceae | Mediterranean | 67.2 | 20.0 | 690 | 57.6 | 0.647 |
| Juniperus phoenicea | Cupressaceae | Mediterranean | 40.7 | 343.6 | 172 | 23.3 | 0.723 |
| Quercus coccifera | Fagaceae | W Mediterranean | 71.0 | 30.4 | 165 | 59.7 | 0.726 |
| Rhamnus lycioides | Rhamnaceae | SW Mediterranean | 64.6 | 172.4 | 136 | 91.0 | 0.763 |
| Genista scorpius | Fabaceae | W Mediterranean | 50.2 | 1443.6 | 52 | 134.8 | 0.765 |
| Rosmarinus officinalis | Lamiaceae | Mediterranean | 49.6 | 4164.0 | 93 | 69.4 | 0.663 |
| Thymus vulgaris | Lamiaceae | W Latemediterranean | 68.2 | 4308.8 | 21 | 124.7 | 0.670 |
| Lithospermum fruticosum | Boraginaceae | W Mediterranean | 32.9 | 116.0 | 37 | 129.6 | 0.666 |
| Thymelaea tinctoria | Thymelaceae | Latemediterranean | 76.6 | 36.0 | 38 | 74.5 | 0.627 |
Visual estimates of green canopy level were considered a proxy for species’ response to drought. This method is standard practice for measures of drought-induced impact on ecosystems dominated by woody vegetation (e.g. Carnicer et al., 2011; Galiano et al., 2011; Nakajima et al., 2011; Boehmer et al., 2013). In order to facilitate comparisons between species that may exhibit different leaf phenologies, the green canopy was estimated by considering the percentage of young, thin tips of branches with no signs of decay and regularly holding up leaves (as well as checking recent leaf scars in species that drop leaves early after unfolding, such as G. scorpius). Thus, although we considered the current levels of leaf loss, these also reflected the drought experienced in the study area in the previous years (Fig. 1B, C). The visual estimates of green canopy in this community correlated well with the actual amounts of green leaves (leaves/branch weight ratio) (for a more detailed description of green canopy estimates see Sapes et al., 2017). Individuals with signs of decay prior to the drought period (stumps, decomposing stems, branches with no thin tips or buds) were excluded from the sampling. Then, for each species, we selected 20 undefoliated plants (>80 % green canopy, except in R. lycioides and L. fruticosum, in which these values were >70 and 40 %, respectively, because regardless of drought they commonly show tips of branches with no leaves) and 20 plants affected by canopy dieback (<50 % green canopy, except in L. fruticosum, in which this value was set to 25 %, as explained above) and we categorized them as ‘undefoliated’ or with ‘leaf loss’, respectively. All the sampled plants affected by leaf loss maintained some green leaves, vital buds and green bark under the periderm and were considered to be alive in most cases, as only 1.7 % of the plants in the leaf loss category appeared as dead (without the above-mentioned signs) at the end of the study.
Half the plants from each canopy dieback category were randomly sampled in April 2012, at the peak of the drought period (hereafter, drought), while the other half were sampled in February 2013, after the autumn rain pulse (hereafter, post-rain) (Fig. 1C), just before the start of the new growing season. Our study does not focus on phenological NSC dynamics, but rather on its variations in water availability during an extreme drought episode and after subsequent rainfall. At each sampling time, leaf loss levels were visually estimated as a percentage for each individual plant in relation to the undefoliated individuals found in the study area, as in Sapes et al. (2017).
Functional trait measures
Study species were sampled to collect information about several functional traits: (1) wood density (WD), which is related to conductive efficiency, as well as to growth potential and resistance to several stress and disturbance agents (Chave et al., 2009; Pérez-Harguindeguy et al., 2013); (2) plant height (Hmax), which is related to growth form, competitive vigour and potential lifespan; and (3) specific leaf area (SLA, leaf area per unit of dry leaf mass), which is related to resource acquisition and leaf turnover (Wright et al., 2004). Functional trait data were obtained from six representative individuals with no signs of canopy dieback per species of the studied community in May 2014. All trait measurements were conducted following standardized protocols (Pérez-Harguindeguy et al., 2013). Wood density (mg mm−3) was measured after removing the bark from 10-cm long stem segments from each plant. In the case of P. halepensis, we used cores obtained with tree-ring borers. We used the water displacement method to determine fresh volume and we dried all the samples in an oven at 70 °C for 72 h until a constant weight was obtained. Plant height (cm) was measured as the shortest distance between the upper limit of the main photosynthetic tissues (excluding inflorescences) and the ground level, and Hmax was determined as the maximum of these values for a given species. We estimated SLA (cm2 g−1) for each individual as the mean value of 20 fully expanded, undamaged sun leaves from the current year. Fresh leaves were digitally scanned and analysed with ImageJ software (US National Institutes of Health; http://www.nih.gov/, accessed 22 February 2013). Leaves were then oven-dried at 70 °C for at least 72 h and weighed to the nearest 0.0001 g. We calculated SLA as the ratio between the area of the leaf lamina and its dry mass.
Carbohydrate sampling and analyses
For each plant, we collected stem samples that were located within the first 10 cm above the ground surface. Similarly, root samples were collected within the first 10 cm below the ground surface. Root and stem samples were transported in a cooler over ice until sample processing. On the day of collection, samples were microwaved for 90 s to stop enzyme activity and subsequently oven-dried for 72 h at 65 °C. Heartwood and inner bark were removed from the sample before grinding into fine powder for the NSC analyses. We defined NSC as low-molecular-weight SS (glucose and fructose, sucrose, and other free sugars) plus starch, and they were analysed following the procedures described by Hoch et al. (2002) and Galiano et al. (2012). Sapwood powder (~12–14 mg) was extracted with 1.6 mL of distilled water at 100 °C for 60 min. After centrifugation, an aliquot of the extract was used to determine SS content after the enzymatic conversion of sucrose and fructose into glucose (using invertase from Saccharomyces cerevisiae and glucose hexokinase [GHK] assay reagent; I4504 and G3293, Sigma-Aldrich, Spain). The content of NSC was obtained from another aliquot that was incubated in amyloglucosidase from Aspergillus niger (10115, Sigma-Aldrich) at 50 °C overnight, to break down all NSC (starch included) to glucose. The concentration of free glucose was determined photometrically in a 96-well microplate reader (Sunrise™ Basic Tecan, Männedorf, Switzerland) after enzymatic (GHK assay reagent) conversion of glucose into gluconate-6-phosphate. Then, the dehydrogenation of glucose causes an increase in optical density at 340 nm. All the NSC and SS contents were expressed as percentages of dry matter.
We computed several indices at the species level to describe the proportion of NSC and SS in the different organs, canopy dieback states, and periods. We calculated the ratio of NSC and SS in roots relative to stems (considering the values for all plants, regardless of leaf loss) in both the drought and post-rain periods; this ratio indicates the differences in carbon allocation between organs in the various species. We also calculated the ratio of NSC and SS between undefoliated plants and those with leaf loss (by averaging stems and root values) for both periods; this ratio indicates differences associated with canopy dieback in the various species. Finally, we calculated the species’ difference and relative change (value in the post-rain period minus value in the drought period/value in the drought period) in NSC and SS by averaging the stem and root values of all the plants, regardless of leaf loss) and green canopy (again by averaging all the plants) between the two studied periods.
Statistical analyses
We tested the existence of differences in NSC and SS concentrations between roots and stems, between species, and between green and defoliated plants using general linear mixed models (GLMMs). Separate models were built for samples collected in April 2012 (drought) and in February 2013 (post-rain). Both models included either NSC (log-transformed) or SS concentration (log-transformed) as the dependent variable and organ (root, stem), canopy dieback status (undefoliated, leaf loss) and species as fixed factors. Two-way interactions between fixed factors were included in the model to test whether canopy dieback status influenced NSC and SS contents differentially between organs and species, and whether differences in NSC and SS concentrations at the organ level were species-specific. Plant identity was also included in the models as a random factor to account for plant effects on NSC concentration in the different organs of a single individual. Post-rain models also included percentage of green canopy, since we assumed that this percentage largely reflected autumn rain and could explain NSC concentrations after rain. Samples in which chemical analyses failed to obtain photometrical readings above zero (probably due to low NSC concentrations) were excluded from data analyses.
We tested for differences in NSC and SS concentration between drought and post-rain periods using GLMMs. The models included NSC and SS (log-transformed) as dependent variables; sampling time (drought, post-rain), organ, canopy dieback status during the drought period, and species as fixed factors; and plant identity as a random factor. Two-way interactions were included in the models, for the reasons explained above. We conducted within-species comparisons of the main factors of the different models for each sampling date and between sampling dates using least-squares means difference (LSMD) Student’s t-tests.
We also tested for relationships between the recovery of species’ green canopy between the two periods (difference in green foliage) and both NSC and SS concentration in the drought period and changes in NSC and SS between the two periods, using Spearman’s non-parametric correlations. Relationships between species’ functional traits (log-transformed) and NSC and SS concentrations (averaging the stem and root values of all plants) during drought and post-rain periods were explored using phylogenetic generalized least squares (PGLS). We separately analysed each variable describing species’ functional traits since no significant correlation was observed between them. We also used PGLS to relate functional traits to (1) the proportion of NSC and SS existing in roots relative to stems; (2) the ratio of NSC and SS between undefoliated plants and those with leaf loss; (3) the species’ difference and relative change in NSC and SS between the two studied periods, determined as explained above.
Statistical analyses were performed with JMP10.0 (SAS Institute), except for PGLS analyses, which were performed with R software (version 3.1, R Foundation for Statistical Computing, Vienna, Austria) using the phylotools (Zhang et al., 2012), ape (Paradis et al., 2004) and caper (Orme et al., 2013) packages. Phylogenetic relatedness between species was estimated with Phylomatic (Webb and Donoghe, 2004) and Phylocom (Webb et al., 2008).
RESULTS
Carbohydrates during the drought period
Plants with leaf loss exhibited a significantly lower NSC concentration (15.7 %) compared with undefoliated plants (averaging the stem and root values) (Fig. 2, Supplementary Data Table S1). This effect was consistent in both stems and roots. The species in which these differences were largest were Q. coccifera (LSMD t ratio = 2.59, P = 0.010), R. lycioides (LSMD t ratio = 2.90, P = 0.029), G. scorpius (LSMD t ratio = 3.02, P = 0.003) and R. officinalis (LSMD t ratio = 4.70, P < 0.001) (Table 2, Supplementary Data Table S1). In Q. coccifera low NSC values were mostly due to low root concentration in plants with leaf loss, while in G. scorpius they were due to stems. In R. lycioides and R. officinalis the differences between plants with leaf loss and undefoliated ones were not significant when the organs were considered separately.
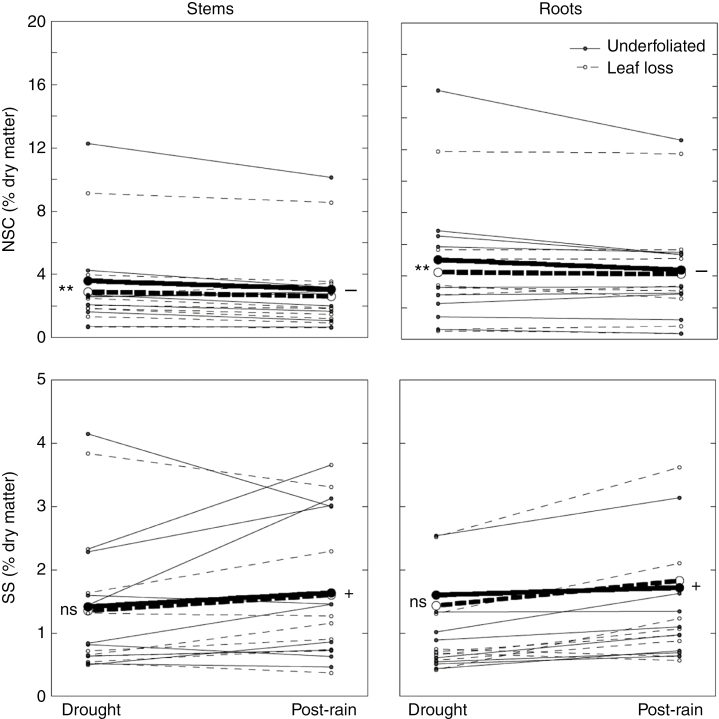
Fig. 2.
Changes between drought (April 2012) and post-rain (February 2013) periods of non-structural carbohydrate (NSC) and soluble sugar (SS) concentrations of the different species in stems and roots of plants with leaf loss and in undefoliated plants. Bold lines correspond to average values for all nine species (for detailed information for each species see Supplementary Data Fig. S1). The + and − symbols indicate a significant increase or decrease (P < 0.001), respectively, between periods, overall considering undefoliated plants and plants with leaf loss. **Significant difference (P < 0.001) between undefoliated plants and plants with leaf loss, overall considering drought and post-rain periods; ns, no significant difference (see Table 3 for the respective model results).
Table 2.
Results of GLMM analysis of non-structural carbohydrate (NSC) and soluble sugar (SS) concentrations in the drought (April 2012) and post-rain (February 2013) periods as a function of organ (stem, root), dieback state (undefoliated, with leaf loss), species and second-level interactions between these factors. NSC and SS values were log-transformed. Bold type indicates statistical significance (P < 0.05). Cohen’s f2b effect size was calculated following Selya et al. (2012)
| Drought | ||||||
|---|---|---|---|---|---|---|
| NSC (R2 = 0.906) | SS (R2 = 0.787) | |||||
| F | P | f 2 b | F | P | f 2 b | |
| Organ | 33.56 | <0.001 | 0.2527 | 1.65 | 0.201 | 0.0039 |
| Dieback state | 22.55 | <0.001 | 0.0135 | 3.71 | 0.056 | 0.0016 |
| Species | 101.18 | <0.001 | -0.0336 | 68.39 | <0.001 | −0.0094 |
| Organ × dieback | 0.28 | 0.596 | −0.0068 | 0.51 | 0.477 | −0.0023 |
| Organ × species | 12.01 | <0.001 | 0.6070 | 3 456 | 0.001 | 0.1340 |
| Dieback × species | 2.46 | 0.016 | 0.0199 | 3.14 | 0.003 | 0.0226 |
| Post-rain | ||||||
| NSC (R2 = 0.842) | SS (R2 = 0.803) | |||||
| F | P | f 2 b | F | P | f 2 b | |
| Organ | 32.67 | <0.001 | 0.2126 | 0.20 | 0.659 | −0.0100 |
| Dieback state | 2.76 | 0.099 | 0.0014 | 5.20 | 0.024 | 0.0051 |
| Species | 66.80 | <0.001 | 0.0191 | 64.19 | <0.001 | −0.1043 |
| Green canopy | 25.82 | <0.001 | −0.0157 | 15.40 | <0.001 | 0.0071 |
| Organ × dieback | 3.62 | 0.059 | 0.0191 | 1.75 | 0.188 | 0.0012 |
| Organ × species | 9.63 | <0.001 | 0.4074 | 4.43 | <0.001 | 0.2062 |
| Dieback × species | 0.49 | 0.65 | −0.0017 | 0.28 | 0.971 | 0.0113 |
In contrast to NSC, the overall effect of leaf loss on SS was only marginally significant. In plants with leaf loss SS decreased by only 5.9 % compared with undefoliated plants (averaging the stem and root values) (Fig. 2, Supplementary Data Table S1). The differences were only significant in R. lycioides (LSMD t ratio = 2.85, P = 0.005) and R. officinalis (LSMD t ratio = 4.18, P < 0.001), mostly due to lower SS values in the roots of plants with leaf loss (Table 2, Supplementary Data Fig. S1).
Overall, NSC concentration was 1.4 times higher in roots than in stems (Table 2, Fig. 2, Supplementary Data Table S1). The exceptions were L. fruticosum and T. vulgaris, where NSC concentration did not differ in the roots and stems, and in R. officinalis, where the values were higher in stems (LSMD t ratio = 4.23, P < 0.001) (Supplementary Data Fig. S1).
The overall SS concentration did not vary significantly between organs. The exceptions were R. lycioides and R. officinalis, which had a lower SS content in roots than in stems (LSMD t ratio = 2.28, P = 0.024 and LSMD t ratio = 3.50, P < 0.001, respectively), and T. tinctoria, which exhibited the opposite pattern (LSMD t ratio = 2.25, P = 0.020) (Table 2, Supplementary Data Fig. S1).
Carbohydrates and green foliage in the post-rain period
After the rain, plants with leaf loss in the drought period did not differ significantly in NSC concentration from undefoliated ones (Table 2, Fig. 2). In contrast, the SS concentration was higher overall in plants that exhibited leaf loss, compared with undefoliated ones (Table 2, Fig. 2), particularly in stems of J. phoenicea (LSMD t ratio = 2.91, P = 0.002) and roots of Q. coccifera (LSMD t ratio = 2.09, P = 0.019) (Supplementary Data Fig. S1).
As during the drought period, the overall NSC concentration was 1.4 times higher in roots relative to stems (averaging all the plants) but this pattern varied strongly between species: in T. vulgaris, the roots had lower NSC than the stems (LSMD t ratio = 4.33, P < 0.001) but the differences were not significant in Q. coccifera, L. fruticosum and R. officinalis (Table 2, Supplementary Data Fig. S1).
Overall, SS concentration did not vary significantly between organs, but some species did present different patterns. The SS concentration was lower in roots, compared with stems, in J. phoenicea (LSMD t ratio = 2.14, P = 0.034) and Q. coccifera (LSMD t ratio = 2.21, P = 0.029), but the reverse was true in P. halepensis (LSMD t ratio = 3.41, P < 0.001) and T. tinctoria (LSMD t ratio = 3.36, P = 0.001) (Table 2, Supplementary Data Fig. S1.
In the post-rain sampling, the percentage of green canopy had a stronger positive effect on both NSC and SS concentrations than canopy dieback status (Table 2).
Comparison between periods
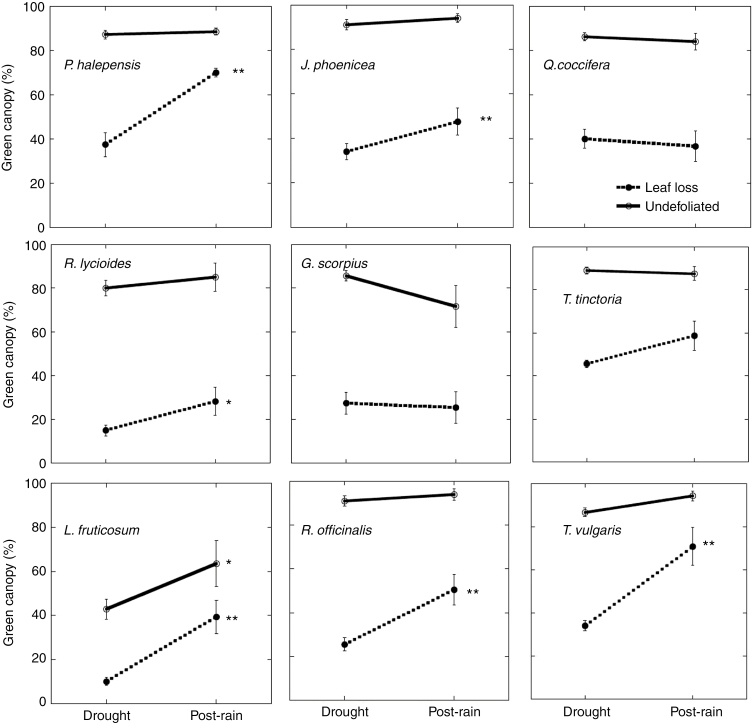
Green canopy increased more in plants with leaf loss. This recovery was particularly strong in P. halepensis, L. fruticosum, R. officinalis and T. vulgaris. In contrast, G. scorpius showed a clear decrease in the green foliage of undefoliated plants (Fig. 3).
Fig. 3.
Mean percentage of green canopy during the drought (April 2012) and post-rain (February 2013) periods of plants of the different species. Values were obtained for the plants that were sampled in the post-rain period (N = 10 for each dieback state category). Error bars indicate s.e. *P < 0.05, **P < 0.01 (drought versus post-rain periods). Differences between undefoliated plants and those with leaf loss (dieback) were always significant (P < 0.01).
Overall, the NSC concentration decreased from the drought to the post-rain period (Table 3, Supplementary Data Table S1). Generally speaking, this decrease was consistent between organs, canopy dieback status during the drought, and species (Table 3), in spite of some discrepancies in specific species (Supplementary Data Fig. S1). Exceptions to this overall pattern were found, for example, in R. officinalis, where root NSC increased in plants with leaf loss (LSMD t ratio = 5.47, P < 0.001), and in P. halepensis, where NSC decreased in stems (LSMD t ratio = 2.19, P = 0.014) but not in roots (LSMD t ratio = 0.32, P = 0.745).
Table 3.
Results of GLMM analysis of non-structural carbohydrate (NSC) and soluble sugar (SS) concentrations as a function of period (drought, post-rain), organ (stem, root), dieback state (undefoliated, with leaf loss), species and second-level interactions between these factors. NSC and SS values were log-transformed. Bold type indicates statistical significance (P < 0.05). Cohen’s f2b effect size was calculated following Selya (2012)
| NSC (R2 = 0.863) | SS (R2 = 0.780) | |||||
|---|---|---|---|---|---|---|
| F | P | f 2 b | F | P | f 2 b | |
| Period | 10.27 | <0.001 | −0.0026 | 27.82 | <0.001 | 0.0002 |
| Organ | 59.60 | <0.001 | 0.1929 | 0.08 | 0.781 | −0.0026 |
| Dieback state | 24.52 | <0.001 | 0.0012 | 2.82 | 0.094 | 0.0006 |
| Species | 145.40 | <0.001 | −0.0008 | 129.74 | <0.001 | −0.0503 |
| Period × organ | 1.85 | 0.174 | 0.0030 | 2.41 | 0.122 | 0.0011 |
| Period × dieback | 0.43 | 0.512 | 0.1974 | 0.85 | 0.358 | 0.0011 |
| Period × species | 1.44 | 0.180 | 0.0091 | 3.60 | <0.001 | −0.0006 |
| Organ × dieback | 0.76 | 0.385 | 0.0019 | 2.41 | 0.122 | −0.0031 |
| Organ × species | 17.08 | <0.001 | 0.3971 | 6.30 | <0.001 | 0.1354 |
| Dieback × species | 1.91 | 0.057 | 0.0019 | 2.58 | 0.010 | 0.0030 |
In contrast to NSC, SS concentration generally increased in the post-rain period (Supplementary Data Table S1). This trend occurred in both organs and plants with different canopy dieback status in the drought period. However, the SS increase varied between species (Table 3): it was significant in Q. coccifera (LSMD t ratio = 5.82, P < 0.001), R. lycioides (LSMD t ratio = 4.23, P < 0.001), G. scorpius (LSMD t ratio = 2.89, P = 0.004) and L. fruticosum (LSMD t ratio = 2.95, P = 0.003) (Supplementary Data Fig. S1). In R. officinalis, SS increased in plants with leaf loss (LSMD t ratio = 3.13, P = 0.002) but not in undefoliated ones (LSMD t ratio = 0.39, P = 0.700).
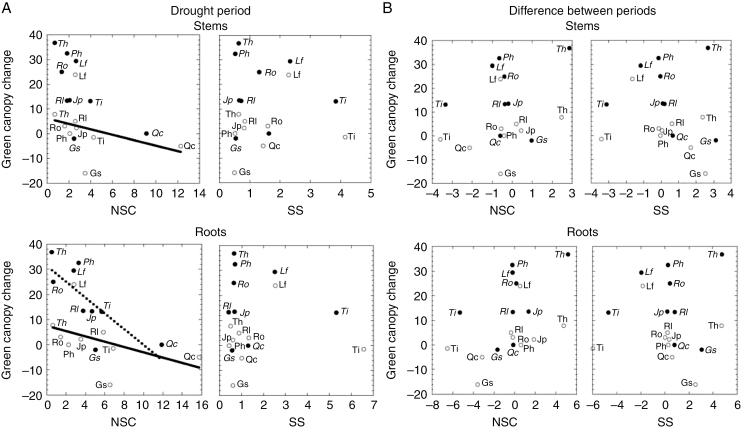
The recovery of green canopy in plants with leaf loss was negatively correlated with root NSC concentration in the drought period across the species (Spearman ρ = −0.882, P = 0.002) (Fig. 4A). This pattern was also observed in the stems (Spearman ρ = −0.683, P = 0.042) and roots (Spearman ρ = −0.683, P = 0.042) of undefoliated plants. The recovery of species’ green canopy was not significantly related to SS concentration in the drought period or to the change in either NSC or SS between periods.
Fig. 4.
Relationship between change in green canopy (difference in percentage between the post-rain and drought periods) and (A) mean non-structural carbohydrates (NSC) (left panels) and soluble sugars (SS) (right panels) during the drought period, and (B) change in NSC (left panels) and SS (right panels) between periods. Upper panels correspond to stems and lower panels to roots. NSC and SS values correspond to percentage dry matter content. Open and black circles correspond to undefoliated and plants with leaf loss, respectively. Only statistically significant relationships are shown (Spearman’s non-parametric correlation, P < 0.05). Ph, P. halepensis; Jp, J. phoenicea; Qc, Q. coccifera; Rl, R. lycioides; Gs, G. scorpius; Ti, T. tinctoria; Lf, L. fruticosum; Ro, R. officinalis; Th, T. vulgaris. Species abbreviations in italics indicate plants with leaf loss.
Relationships between traits and carbohydrate content
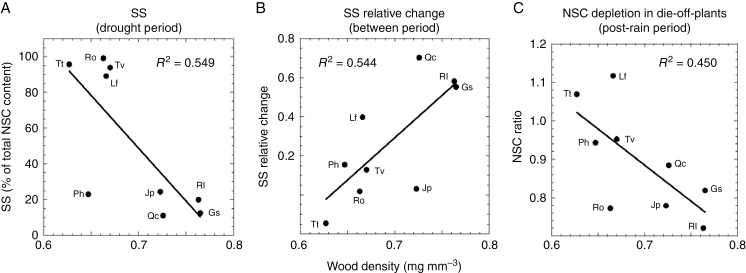
Wood density was related to some aspects of carbon dynamics. During the drought period, low-WD species (particularly T. tinctoria, L. fruticosum, R. officinalis and T. vulgaris) had a significantly higher fraction of NSC composed of SS compared with high-WD species (P. halepensis, J. phoenicea, Q. coccifera, R. lycioides and G. scorpius; PGLS, λ = 0.993, t = 2.45, P = 0.044) (Fig. 5A). This relationship exhibited a phylogenetic signal, probably associated with the different behaviour of the gymnosperm P. halepensis. Similarly, higher WD was associated with greater relative increases in SS from the drought to the post-rain periods, irrespective of lineage effect (PGLS, λ = 0, t = 3.04, P = 0.023) (Fig. 5B). Finally, during the post-rain period lower-WD species exhibited greater depletion of NSC in plants with leaf loss compared with undefoliated ones, irrespective of lineage effect (PGLS, λ = 0, t = 2.40, P = 0.047) (Fig. 5C). No other significant relationship was found between WD and NSC or green canopy change.
Fig. 5.
Relationship between wood density (WD) and (A) soluble sugars (SS) during the drought period (as a percentage of total non-structural carbohydrates, NSC); (B) relative change in SS between post-rain and drought periods (value in the post-rain period minus value in the drought period/value in the drought period); and (C) NSC depletion in plants with leaf loss, estimated as the ratio of NSC between undefoliated plants and plants with leaf loss in the post-rain period for each species. NSC and SS values were obtained by averaging stem and root values of all undefoliated and plants with leaf loss. R2 values correspond to linear correlation coefficients. Species abbreviations are as in Fig. 4.
Species’ Hmax and SLA were not statistically related to carbon concentration and dynamics or to canopy recovery. However, small species tended to have higher concentrations of SS in undefoliated plants than in plants with leaf loss after the rain period (negative relationship between Hmax and ratio of SS in undefoliated plants to plants with leaf loss; PGLS, λ = 1, t = 2.28, P = 0.057) and higher NSC concentration in stems than in roots (positive relationship between Hmax and ratio of root to stem; PGLS, λ = 1, t = 2.32, P = 0.054).
DISCUSSION
Our community-level study revealed that NSC as a whole and the SS fraction responded differently to a sequence of intense drought followed by rain. There was a decrease in the overall NSC stocks in shrubs with symptoms of canopy dieback due to prolonged drought, compared with those of undefoliated shrubs. On average, NSC stocks continued to decrease after the rain, likely because canopy recovery incurs initial C costs. In contrast to NSC, SS concentrations were similar between plants with and without leaf loss during the drought, and they increased after the rain event. Such SS dynamics are consistent with the critical role of SS in metabolism and water relationships during drought (O’Brien et al., 2014; Sevanto et al., 2014; García-Forner et al., 2016). Despite these general trends, coexisting species presented different carbon dynamics, which were in part related to wood density, a trait related to growth rates and long-distance water transport.
Carbohydrate dynamics
As hypothesized, plants exhibiting foliage loss (canopy dieback) during the drought (with foliage loss) had lower NSC concentrations in both stems and roots than undefoliated plants, as previously observed in the roots of other Mediterranean trees under water stress (Klein et al., 2014). This decrease occurred in both stems and roots. Roots were the main NSC storage organ, as expected in Mediterranean systems where above-ground disturbances are common (Martínez-Vilalta et al., 2016). NSC depletion was similar in both organs, suggesting that carbon demand or mobilization was also similar in above- and below-ground organs. These lower NSC concentrations in plants with leaf loss during drought occurred despite the potential NSC accumulation that can occur when drought decreases growth more than photosynthesis (Körner, 2003).
NSC concentration in most species did not increase after the autumn rain period. On the contrary, on average, NSC decreased regardless of the degree of canopy dieback at the peak of the drought. This suggests that newly assimilated C after seasonal pulses of rain was insufficient to meet C demand for new tissue growth after a prolonged drought. The low NSC concentrations found in the post-rain period (February) before the start of the new growing season could also reflect temperature limitations on wintertime C assimilation (Terradas, 1986; Camarero et al., 2010). Temperature-limited C assimilation is relatively common in some (De Lillis and Fontanella, 1992; Llorens et al., 2003; Asensio et al., 2007; Vaz et al., 2010), though not all (Körner, 2003), Mediterranean-type ecosystems.
The significant increase in SS after the autumn rain does suggest a general increase in physiological activity, as sugars were mobilized for growth and metabolic demands. An autumnal increase in physiological activity occurs in Mediterranean-type ecosystems with strong seasonal trends in precipitation, resulting in peaks of assimilation in both spring and autumn, due to the high rainfall and mild temperatures (Larcher, 2000; Llorens et al., 2003). The seasonality of Mediterranean-type ecosystems is essential to any interpretation of our results, which were probably influenced by both the specific conditions of the year of the study (within a multi-year drought period) and the timing of the second sampling, after a peak of autumn activity and before a second peak of potential productivity in spring.
The observed values of SS in plants with leaf loss agree with experimental evidence suggesting that NSC storage mitigates the effects of drought (Woodruff and Meinzer, 2011; O’Brien et al., 2014). One consequence of drought is turgor loss (Bartlett et al., 2012), along with potential loss of water transport, desiccation and death (Tyree et al., 2003). A main mechanism by which cells maintain turgor under drought is via the accumulation of osmotically active compounds such as SS (O’Brien et al., 2014; Sala and Mencuccini, 2014; Sevanto et al., 2014), which are converted from starch (McDowell et al., 2011; García-Forner et al., 2016). Furthermore, starch-derived SS might also play a critical role in xylem repair and vascular transport (De Baerdemaeker et al., 2017). Our results are consistent with this interpretation because, compared with NSC, SS remained relatively stable across species when plants with leaf loss were compared with their undefoliated counterparts, even though SS is a source of carbon for respiration (cf. Hartmann and Trumbore, 2016; Martínez-Vilalta et al., 2016). Recent pulse-labelling experiments further suggest that SS may be actively accumulated during water shortage and subsequent recovery at the expense of short-term growth, presumably to optimize growth and survival in the long term (Hartmann et al., 2015; Galiano et al., 2017). In our case, such accumulation appeared to be species- and organ-specific. For instance, in R. lycioides and R. officinalis root SS values were lower during the drought period than after the rain.
The SS concentration increased after the rain pulse, which was not the case for total NSC. This increase is expected when physiological activity resumes and sugars are used for growth and metabolic demands, and may be due to (1) temporary increases in assimilation rates and/or reductions in C demand or (2) the conversion of starch into SS. In the first case, these adjustments would yield a net C gain, which would be observed first as SS, given that SS are both the direct product of assimilation and the substrate of most anabolic reactions. In the second case, a conversion of starch from stems and roots into SS would yield higher SS concentrations without any increases in total NSC. Our results are consistent with this second interpretation, as NSC either declined or remained constant in nearly all the species in our study.
Recovery of green foliage
We hypothesized that the recovery of green foliage after the autumn rainy period would consume stored NSC and would thus result in lowered NSC concentrations in both stems and roots. Consistent with this, the NSC average across species did show lower concentrations after the drought period, compared with those found during drought (Fig. 2), coinciding with an increase in green foliage in most species (Fig. 3). Therefore, the decrease in NSC from the drought to the post-rain period appeared to correspond to growth-related C demands. Previous studies have documented reductions in NSC concentrations associated with the production of new foliage after drought (Bréda et al., 2006; Galiano et al., 2011, 2012) or other disturbances (e.g. Canadell and López Soria, 1998; Palacio et al., 2012). The NSC concentrations were particularly low in plants with leaf loss, probably because they grew more leaves in the autumn. There was no relationship, however, between the drop in NSC from drought to post-rain and the canopy recovery across species (i.e. species with a higher canopy recovery did not show stronger decreases in NSC), probably because the balance between assimilation and growth or other demands varied across species.
Interestingly, foliage recovery tended to correlate negatively with NSC concentration during the drought. This could occur if species that approached their lowest NSC thresholds during drought subsequently maximized the production of new leaves. This response would minimize the risk of complete depletion of reserves in the mid-term (Martínez-Vilalta et al., 2016). In fact, plants with leaf loss did not show greater reductions in NSC between the two study periods, despite the fact that these plants had less NSC at the peak of the drought and recovered more green foliage on average (Fig. 4). That is, plants that experienced leaf loss appeared to minimize NSC depletion during canopy recovery perhaps due to greater (or faster) returns from new leaves. Variability between species, however, was substantial. In general, NSC continued to decrease after the drought, concurrent with a lack of canopy recovery (see P. halepensis, Q. coccifera G. scorpius and T. vulgaris in Supplementary Data Fig. S1), perhaps reflecting a legacy of drought effects. In R. officinalis, which had severe leaf loss during the drought, root NSC increased during canopy recovery, consistent with reports of a fast replenishment of C stores after disturbance (e.g. Palacio et al., 2012). Our study shows that NSC use for rebuilding canopies generally exceeded any new formation of reserves derived from relatively high rates of assimilation in autumn. Therefore, despite occasional rain events, if drought persists in the long term, plants may fall into a feedback loop in which storage is eventually insufficient to grow new leaves, in turn reducing canopy assimilation and NSC formation. Such dynamics could ultimately compromise the capacity to resist frequent droughts (Galiano et al., 2011).
Relationships between traits and carbohydrate content
We did not find any clear association between acquisitive leaf traits (high SLA) or plant longevity (high Hmax) and higher NSC variability, as we had hypothesized. Only WD could explain, to some extent, differences in carbon dynamics across species. Low WD is generally correlated with high growth rates (Poorter et al., 2008; Greenwood et al., 2017), low resistance to xylem embolism, but more efficient water transport relative to denser wood, which is more mechanically stable (Baas et al., 2004; Chave et al., 2009). The greater proportion of SS relative to total NSC in low-WD species relative to high-WD species may therefore reflect their overall greater metabolic activity. Consistently, most of the small trees and large shrubs in the community (i.e. largest but presumably slower-growing plants) had high WD and a low proportion of SS relative to the total NSC (Fig. 5A). An exception was P. halepensis, a fast-growing gymnosperm tree which, despite its low WD, exhibited low SS concentration compared with the total NSC. However, relative to angiosperms, gymnosperms have much less parenchyma tissue, and consequently lower carbon storage capacity and subsequent SS depolymerization (Johnson et al., 2012; Morris et al., 2016). Within low-WD species, we also found that during the drought NSC was similar between plants with and without leaf loss, but after the rain plants with leaf loss exhibited lower NSC than undefoliated plants. The generally faster growth of lower WD species may predispose those that become defoliated under drought to consume more C for foliage recovery after the autumn rain.
Patterns of growth and related carbon dynamics were expected to differ in the contrasting life forms considered in our study (from suffruticose small shrubs to trees). Plant size, measured in our study by Hmax, constitutes one of the two major axes explaining trait variability at a global scale (Díaz et al., 2016), and it is often associated with faster growth rates (Díaz et al., 2016), higher longevity and higher competitive ability due to the pre-emption of light resources. In our community, shorter species (T. tinctoria, L. fruticosum, T. vulgaris) had a shorter lifespan and lower long-term competitive ability. We failed to find any clear relationship between Hmax and carbon dynamics, probably due to the low number of studied species and the multiple functions associated with this single trait. Nevertheless, we observed a negative but weak relationship between Hmax and concentration of SS in undefoliated plants after the rain period, which is consistent with a faster response to pulses of resources in smaller, short-lived species.
Specific leaf area indicates the C cost to replace the canopy after leaf loss. It is also positively related to growth rates and photosynthetic potential (Poorter et al., 2009). However, high SLA has recently been related to susceptibility to drought-induced mortality (Greenwood et al., 2017). However, we found no relationship between SLA and NSC or SS dynamics. This is likely because some species in arid ecosystems often use an avoidance-type strategy whereby rapid leaf turnover allows the regulation of leaf area and water loss (Mooney and Dunn, 1970). This rapid leaf turnover in drought-avoiders often places these species at the low-cost, rapid-investment-return end of the leaf-economy spectrum. Thus, plant communities in arid ecosystems tend to be composed of a mixture of drought-avoidant (i.e. high-SLA) and drought-tolerant (i.e. low-SLA) species, which makes the correlations between leaf-economy traits and climate rather weak. In fact, Wright et al. (2004) found a strong relationship between leaf mass per area (the inverse of SLA) and climate in evergreen species but not in deciduous species, including summer deciduous species. Summer deciduousness is common in Mediterranean communities like the one that we studied (Zunzunegui et al., 2005). In our case, fast foliage recovery in several potentially drought-avoidant small species (T. vulgaris, L. fruticosum) (see also Lloret et al., 2016) may have blurred any relationship between SLA and NSC dynamics. Furthermore, the relationships between traits (including SLA) and demographic rates often depend on the ontogenetic state and can also be related to plant size (Gibert et al., 2016). This limits inferences, particularly when considering small sets of species with a wide range of growth forms and phylogenies, as in our study.
Concluding remarks
Our study showed a decrease of NSC stocks in woody plants with canopy dieback in response to a prolonged drought. Subsequent growth stimulation after stochastic pulses of water availability coinciding with mild temperatures incurred further C costs. These results indicate that recovery of NSC reserves would require subsequent periods of average or above-average precipitation in the mid-term. Persistent long-term drought, even when interrupted by occasional rain pulses, could eventually deplete NSC stocks to the point that canopy recovery is no longer possible. Such an effect is likely to occur in many ecosystems predicted to experience increasing aridity and climatic variability in the future where short-term canopy recovery and subsequent C assimilation after rain events may not ensure long-term resilience. Because changes in plants’ carbon dynamics may be difficult to detect via integrative measures obtained at the stand or landscape levels, such as remote sensing imagery, the assessment of vegetation conditions over extensive temporal or spatial scales remains challenging.
Our study also shows substantial species-specific variability in carbon dynamics and highlights the importance of taking into account phenological effects. Additional research is therefore required to fully understand species-specific C dynamics under drought in Mediterranean species and their consequences for resilience to drought. Nevertheless, the variable responses we found in coexisting species suggests a range of adaptive abilities that could promote coexistence in a changing climate. Recent studies have indeed highlighted the role of functional diversity in enhancing resilience to extreme climatic episodes (De la Riva et al., 2017). Plant functional traits are increasingly used as a tool to compare species’ functional responses and to upscale to community and ecosystem levels, where environmental gradients are more conspicuous. In our study, however, only one specific trait (WD) was related to ecophysiological responses (carbon economy) to particular climatic events, highlighting the importance of selecting appropriate traits and the difficulty in making generalizations based on the relationships between sets of traits (e.g. acquisitive versus conservative classifications) and plants’ responses to climatic disturbance.
SUPPLEMENTARY DATA
Supplementary data are available online at htpps://academic.oup.com/aob and consist of the following. Supplementary Data Table S1: mean non-structural carbohydrate and soluble sugar concentrations in stems and roots of undefoliated plants and plants with leaf loss from the set of species during the drought and post-rain periods. Figure S1: non-structural carbohydrate and soluble sugar concentrations of the different species in stems and roots of undefoliated plants and those with leaf loss (dieback).
ACKNOWLEDGEMENTS
We thank Iris Cobacho, Daniel Ponce and Isabel Ourêlo for their field and laboratory support. This study was supported by Spanish Government grants CGL2012-32965, CGL2015-67419-R, CGL2014-53840-REDT and CGL2013-46808-R, and Catalonian Government grant AGAUR 2014-SGR-00453.
LITERATURE CITED
- Adams HA, Zeppel MJB, Anderegg WRL et al. . 2017. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nature Ecology and Evolution 1: 1285–1291. [DOI] [PubMed] [Google Scholar]
- Aguadé D, Poyatos R, Gómez M, Oliva J, Martínez-Vilalta J. 2015. The role of defoliation and root rot pathogen infection in driving the mode of drought-related physiological decline in Scots pine (Pinus sylvestris L.). Tree Physiology 35: 229–242. [DOI] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H et al. . 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology Management 259: 660–684. [Google Scholar]
- Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB. 2012. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proceedings of the National Academy Sciences of the USA 109: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio D, Peñuelas J, Ogaya R, Llusia J. 2007. Seasonal soil and leaf CO2 exchange rates in a Mediterranean holm oak forest and their responses to drought conditions. Atmospheric Environment 41: 2447–2455. [Google Scholar]
- Baas P, Ewers FW, Davis SD, Wheeler EA. 2004. Evolution of xylem physiology. In: Hemsley A, Poole I, eds. The evolution of plant physiology. Amsterdam: Academic Press, 273–295. [Google Scholar]
- De Baerdemaeker NJF, Salomon RL, De Roo L, Steppe K. 2017. Sugars from woody tissue photosynthesis reduce xylem vulnerability to cavitation. New Phytologist 216: 720–727. [DOI] [PubMed] [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L. 2012. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecology Letters 15: 393–405. [DOI] [PubMed] [Google Scholar]
- Boehmer HJ, Wagner HH, Jacobi JD, Gerrish GC, Mueller-Dombois D. 2013. Rebuilding after collapse: evidence for long-term cohort dynamics in the native Hawaiian rain forest. Journal of Vegetation Science 24: 639–650. [Google Scholar]
- Bolòs O, Vigo J, Masalles RM, Ninot JM. 2005. Flora manual dels Països Catalans, 3rd edn Barcelona: Pòrtic. [Google Scholar]
- Braun-Blanquet J, Bolòs O. 1957. The plant communities of the Central Ebro Basin and their dynamics. Anales Estación Experimental Aula Dei 5: 1–266. [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. 2006. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science 63: 625–644. [Google Scholar]
- Breshears DD, Cobb NS, Rich PM et al. . 2005. Regional vegetation die-off in response to global-change-type drought. Proceedings of the National Academy Sciences of the USA 102: 15144–15148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa KR, Van Der Schrier G, Jones PD. 2009. Wet and dry summers in Europe since 1750. International Journal of Meteorology 29: 1894–1905. [Google Scholar]
- Camarero JJ, Olano JM, Paras A. 2010. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytologist 185: 471–480. [DOI] [PubMed] [Google Scholar]
- Canadell J, López-Soria L. 1998. Lignotuber reserves support regrowth following clipping of two Mediterranean shrubs. Functional Ecology 12: 31–38. [Google Scholar]
- Carnicer J, Coll M, Ninyerola M, Pons X, Sánchez G, Peñuelas J. 2011. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proceedings of the National Academy of Sciences of the USA 108: 1474–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS, Schulze E-D, Mooney HA. 1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447. [Google Scholar]
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecology Letters 12: 351–366. [DOI] [PubMed] [Google Scholar]
- Dai A. 2012. Increasing drought under global warming in observations and models. Nature Climate Change 3: 52–58. [Google Scholar]
- Díaz S, Kattge J, Cornelissen JHC et al. . 2016. The global spectrum of plant form and function. Nature 529: 167–171. [DOI] [PubMed] [Google Scholar]
- Dobbertin M, Brang P. 2001. Crown defoliation improves tree mortality models. Forest Ecology and Management 141: 271–284. [Google Scholar]
- Galiano L, Martínez-Vilalta J, Lloret F. 2011. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytologist 190: 750–759. [DOI] [PubMed] [Google Scholar]
- Galiano L, Martinez-Vilalta J, Sabate S, Lloret F. 2012. Determinants of drought effects on crown condition and their relationship with depletion of carbon reserves in a Mediterranean holm oak forest. Tree Physiology 32: 478–489. [DOI] [PubMed] [Google Scholar]
- Galiano L, Timofeeva G, Saurer M et al. . 2017. The fate of recently fixed carbon after drought release: towards unravelling C storage regulation in Tilia platyphyllos and Pinus sylvestris. Plant, Cell & Environment 40: 1711–1724. [DOI] [PubMed] [Google Scholar]
- Gao XJ, Pal JS, Giorgi F. 2006. Projected changes in mean and extreme precipitation over the Mediterranean region from a high resolution double nested RCM simulation. Geophysical Research Letters 33: L03706. [Google Scholar]
- García-Forner N, Sala A, Biel C et al. . 2016. Individual traits as determinants of time to death under extreme drought in Pinus sylvestris L. Tree Physiology 36: 1196–1209. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos C, Le Sage P, Bindi M, Moriondo M, Kostopoulou E, Goodess CM. 2009. Climatic changes and associated impacts in the Mediterranean resulting from a 2 °C global warming. Global Planetary Change 68: 209–224. [Google Scholar]
- Gibert A, Gray EF, Westoby M, Wright IJ, Falster DS. 2016. On the link between functional traits and growth rate: meta-analysis shows effects change with plant size, as predicted. Journal of Ecology 104: 1488–1503. [Google Scholar]
- Greenwood S, Ruiz-Benito P, Martínez-Vilalta J et al. . 2017. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecology Letters 20: 539–553. doi:10.111/ele.12748. [DOI] [PubMed] [Google Scholar]
- Gruber A, Pirkebner D, Florian C, Oberhuber W. 2012. No evidence for depletion of carbohydrate pools in Scots pine (Pinus sylvestris L.) under drought stress. Plant Biology 14: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. [DOI] [PubMed] [Google Scholar]
- Hagedorn F, Joseph J, Peter M et al. . 2016. Recovery of trees from drought depends on belowground sink control. Nature Plants 2: 16111. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Trumbore S. 2016. Understanding the roles of nonstructural carbohydrates in forest trees – from what we can measure to what we want to know. New Phytologist 211: 386–403. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Ziegler W, Kolle O, Trumbore S. 2013. Thirst beats hunger – declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytologist 200: 340–349. [DOI] [PubMed] [Google Scholar]
- Hartmann H, McDowell NG, Trumbore S. 2015. Allocation to carbon storage pools in Norway spruce saplings under drought and low CO2. Tree Physiology 35: 243–252. [DOI] [PubMed] [Google Scholar]
- Hoch G, Popp M, Körner C. 2002. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 98: 361–374. [Google Scholar]
- Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC. 2012. Hydraulic safety margins and embolism reversal in stems and leaves: why are conifers and angiosperms so different? Plant Science 195: 48–53. [DOI] [PubMed]
- Jump AS, Ruiz-Benito P, Greenwood S et al. . 2017. Structural overshoot of tree growth with climate variability and the global spectrum of drought-induced forest dieback. Global Change Biology 23: 3742–3757. [DOI] [PubMed] [Google Scholar]
- Klein T, Hoch G, Yakir D, Körner C. 2014. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiology 34: 981–992. [DOI] [PubMed] [Google Scholar]
- Körner C. 2003. Carbon limitation in trees. Journal of Ecology 91: 4–17. [Google Scholar]
- Larcher W. 2000. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosystems 134: 279–295. [Google Scholar]
- De Lillis M, Fontanella A. 1992. Comparative phenology and growth in different species of the Mediterranean maquis of central Italy. Vegetatio 100: 83–96. [Google Scholar]
- Llorens L, Peñuelas J, Filella I. 2003. Diurnal and seasonal variations in the photosynthetic performance and water relations in two co-occurring in two Mediterranean shrubs, Erica multiflora and Globularia alypum. Physiologia Plantarum 118: 84–95. [DOI] [PubMed] [Google Scholar]
- Lloret F, Escudero A, Iriondo JM, Martinez-Vilalta J, Valladares F. 2012. Extreme climatic events and vegetation: the role of stabilizing processes. Global Change Biology 18: 797–805. [Google Scholar]
- Lloret F, de la Riva E, Pérez-Ramos I et al. . 2016. Climatic events inducing die-off in Mediterranean shrublands: are species responses related to their functional traits?Oecologia 180: 961–973. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Sala A, Asensio MD et al. . 2016. Dynamics of non- structural carbohydrates in terrestrial plants: a global synthesis. Ecological Monographs 86: 495–516. [Google Scholar]
- McDowell NG, Sevanto S. 2010. The mechanisms of carbon starvation: how, when, or does it even occur at all?New Phytologist 186: 264–266 [DOI] [PubMed] [Google Scholar]
- McDowell NG, Pockman WT, Allen CD et al. . 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought?New Phytologist 178: 719–739. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M. 2011. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends in Ecology and Evolution 26: 523–532. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Fisher R, Xu C et al. . 2013. Uncertainties and opportunities in modeling drought-associated vegetation mortality. New Phytologist 200: 304–321. [DOI] [PubMed] [Google Scholar]
- McKell CM. 1975. Shrubs – a neglected resource for arid lands. Science 187: 803–809. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, O’Grady AP, Tissue DT, White DA, Ottenschlaeger ML, Pinkard EA. 2013. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytologist 197: 862–872. [DOI] [PubMed] [Google Scholar]
- Mooney HA, Dunn L. 1970. Photosynthetic systems of Mediterranean-climate shrubs and trees of California and Chile. American Naturalist 104: 447–453. [Google Scholar]
- Morris H, Plavcová L, Cvecko P et al. . 2016. A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytologist 209: 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Kume A, Ishida M, Ohmiya T, Mizoue N. 2011. Evaluation of estimates of crown condition in forest monitoring: comparison between visual estimation and automated crown image analysis. Annals of Forest Science 68: 1333–1340. [Google Scholar]
- O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A. 2014. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nature Climate Change 4: 1–5. [Google Scholar]
- Orme D, Freckleton R, Thomas G et al. . 2013. CAPER: comparative analyses of phylogenetics and evolution in R. R package version 0.5. Methods in Ecology and Evolution 3: 145–151. [Google Scholar]
- Palacio S, Maestro M, Montserrat-Martí G. 2007. Relationship between shoot-rooting and root-sprouting abilities and the carbohydrate and nitrogen reserves of Mediterranean dwarf shrubs. Annals of Botany 100: 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio S, Hernández R, Camarero JJ, Maestro-Martí M. 2012. Fast replenishment of initial carbon stores after defoliation by the pine processionary moth and its relationship to the re-growth ability of trees. Trees 26: 1627–1640. [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E et al. . 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Petri MD, Collins SL, Swann AM, Ford PL, Litvak ME. 2015. Grassland to shrubland state transitions enhance carbon sequestration in the northern Chihuahuan Desert. Global Change Biology 21: 1226–1231. [DOI] [PubMed] [Google Scholar]
- Pivovaroff AL, Pasquini SC, De Guzman ME, Alstad KP, Stemke JS, Santiago LS. 2016. Multiple strategies for drought survival among woody plant species. Functional Ecology 30: 517–526. [Google Scholar]
- Poorter L, Wright SJ, Paz H et al. . 2008. Are functional traits good predictors of demographic dates? Evidence from five Neotropical forests. Ecology 89: 1908–1920. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar F. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182: 565–588. [DOI] [PubMed] [Google Scholar]
- Poyatos R, Aguadé D, Galiano L, Mencuccini M, Martínez-Vilalta J. 2013. Drought-induced defoliation and long periods of near-zero gas exchange play a key role in accentuating metabolic decline of Scots pine. New Phytologist 200: 388–401. [DOI] [PubMed] [Google Scholar]
- De la Riva E, Lloret F, Pérez-Ramos I et al. . 2017. The importance of functional diversity in the stability of Mediterranean shrubland communities after the impact of extreme climatic events. Journal of Plant Ecology 10: 281–293. [Google Scholar]
- Rosas T, Galiano L, Ogaya R, Peñuelas J, Martínez-Vilalta J. 2013. Dynamics of non-structural carbohydrates in three Mediterranean woody species following long-term experimental drought. Frontiers in Plant Science 4: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala A, Hoch G. 2009. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant, Cell & Environment 32: 22–30. [DOI] [PubMed] [Google Scholar]
- Sala A, Mencuccini M. 2014. Plump trees win under drought. Nature Climate Change 4: 666–667. [Google Scholar]
- Sala A, Piper F, Hoch G. 2010. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytologist 186: 274–281. [DOI] [PubMed] [Google Scholar]
- Sala A, Woodruff DR, Meinzer FC. 2012. Carbon dynamics in trees: feast or famine?Tree Physiology 32: 764–775. [DOI] [PubMed] [Google Scholar]
- Sapes G, Serra-Diaz JM, Lloret F. 2017. Species climatic niche explains drought-induced die-off in a Mediterranean woody community. Ecosphere 8: e01833. [Google Scholar]
- Sarris D, Christodoulakis D, Körner C. 2007. Recent decline in precipitation and tree growth in the eastern Mediterranean. Global Change Biology 13: 1287–1300. [Google Scholar]
- Saura-Mas S, Lloret F. 2007. Leaf and shoot water content and leaf dry matter content of Mediterranean woody species with different post-fire regenerative strategies. Annals of Botany 99: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. 2012. A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Frontiers in Psychology 3: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanto S, McDowell NG, Dickman LT, Pangle R, Pockman WT. 2014. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant, Cell & Environment 37: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley B. 2010. From plant traits to vegetation structure. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Shipley B, Lechowics MJ, Wright I, Reich PB. 2006. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87: 535–541. [DOI] [PubMed] [Google Scholar]
- Skelton RP, Westa AG, Todd E., Dawson TE. 2015. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proceedings of the National Academy of Sciences of the USA 115: 5744–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradas J. 1986. El paisatge vegetal dels Monegros: assaig d’interpretació. Orsis 2: 71–95. [Google Scholar]
- Trumbore S, Czimczik CI, Sierra CA, Muhr J, Xu X. 2015. Non-structural carbon dynamics and allocation relate to growth rate and leaf habit in California oaks. Tree Physiology 35: 1206–1222. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Engelbrecht BMJ, Vargas G, Kursar TA. 2003. Desiccation tolerance of five tropical seedlings in Panama. Relationship to a field assessment of drought performance. Plant Physiology 13: 1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz M, Pereira JS, Gazarini LC et al. . 2010. Drought-induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (Quercus ilex and Quercus suber). Tree Physiology 30: 946–956. [DOI] [PubMed] [Google Scholar]
- Venturas MD, Mackinnon ED, Dario HL, Jacobsen AL. 2016. Chaparral shrub hydraulic traits, size, and life history types relate to species mortality during California’s historic drought of 2014. PLoS ONE 11: e0159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Serrano SM, Beguería S, López-Moreno JI. 2010. A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. Journal of Climate 23: 1696–1718. [Google Scholar]
- Vicente-Serrano S, Zouber A, Lasanta T, Pueyo Y. 2012. Dryness is accelerating degradation of vulnerable shrublands in semiarid Mediterranean environments. Ecological Monographs 82: 407–428. [Google Scholar]
- Webb CO, Donoghe MJ. 2004. Phylomatic: tree assembly for applied phylogenetics. Molecular Ecology Resources 5: 181–183. [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. 2008. Phylocom: software for the analysis of phylogenetic community structure and character evolution. Bioinformatics 24: 2098–2100. [DOI] [PubMed] [Google Scholar]
- van Wilgen BW, Cowling RM, Burgers CJ. 1996. Valuation of ecosystem services. Bioscience 46: 184–189. [Google Scholar]
- Westoby M, Reich PB, Wright I. 2013. Understanding ecological variation across species: area- based vs mass-based expression of leaf traits. New Phytologist 199: 322–323. [DOI] [PubMed] [Google Scholar]
- Woodruff DR, Meinzer FC. 2011. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant, Cell & Environment 11: 1920–1930. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M et al. . 2004. The worldwide leaf economics spectrum. Nature 12: 821–827. [DOI] [PubMed] [Google Scholar]
- Zhang J, Pei N, Mi X. 2012. phylotools: Phylogenetic tools for eco-phylogenetics https://rdrr.io/cran/phylotools/man/phylotools-package.html.
- Zunzunegui M, Barradas MD, Ain-Lhout F, Clavijo A, Novo FG. 2005. To live or to survive in Doñana dunes: adaptive responses of woody species under a Mediterranean climate. Plant and Soil 273: 77–89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.