Abstract
Introduction:
Approximately 17 million Americans and 300 000 veterans have an alcohol use disorder (AUD). Both oral naltrexone (NTX) and long-acting, injectable naltrexone (LAI NTX) are FDA-approved to treat AUD. LAI NTX is often reserved for patients with adherence concerns due to considerable differences in drug cost and administration requirements. To date, there are no randomized trials comparing efficacy of LAI NTX to oral NTX. This retrospective cohort study compared clinical outcomes in veterans treated with LAI NTX or oral NTX.
Methods:
Health care utilization in veterans at 1 Veterans Affairs facility treated for AUD with oral NTX and LAI NTX was compared. The primary outcome was 90-day alcohol-related hospital admissions per patient (ARA90). Secondary outcomes included 90-day outpatient clinic and emergency department visits and 30-day alcohol-related admissions (ARA30). Inclusion criteria included first-time prescription of NTX for AUD from January 1, 2015, through December 1, 2015. Veterans receiving concurrent acamprosate or disulfiram were excluded.
Results:
Seventy-nine patients were included with 65 in the oral NTX group and 14 in the LAI NTX group. The ARA90 was 0.17 for the oral NTX group and 0.64 for the LAI NTX group (P = .06). The oral NTX group had significantly fewer ARA30 than the LAI NTX group (P < .01). Oral NTX also had significantly lower health care utilization for all other parameters.
Discussion:
Oral NTX was associated with lower health care utilization compared to LAI NTX in this veteran population. This indicates that LAI NTX may not provide additional benefit justifying the cost. This study had several limitations. Randomized trials comparing efficacy between oral NTX and LAI NTX are needed.
Keywords: naltrexone, Vivitrol, alcohol use disorder, AUD, substance use disorder, alcohol, SUD, alcohol dependence, long-acting injection
Introduction
Alcohol use disorder (AUD) affects a large percentage of Americans with an estimated 17 million Americans with active AUD in 2014.1 Within the veteran population, it is estimated there are more than 300 000 veterans with an AUD.2 Approximately 88 000 people will die prematurely each year from acute and chronic complications of excessive alcohol consumption.3 In addition to mortality, AUD poses a significant financial burden to the health care system. Excessive drinking cost the United States more than $223 billion in 2006, largely in lost productivity as well as health care costs due to the complications from alcohol use.4 This disease has a major impact on public health, yet it is still undertreated. In a national survey conducted in 2012-13, only 19.8% of respondents with AUD had ever been offered treatment.5 Clearly, there is a need for effective treatment options to be available and offered to persons suffering from AUD.
Currently, 3 medications are approved by the Food and Drug Administration to treat alcohol dependence: oral and long-acting, injectable (LAI) naltrexone (NTX), disulfiram, and acamprosate. Additionally, several medications are used off-label for AUD, such as gabapentin and topiramate. The Department of Veterans Affairs and Department of Defense (VA/DoD) published a clinical treatment guideline for the management of substance use disorders, which was updated in 2015.3 This guideline recommends offering pharmacotherapy for alcohol dependence in addition to nonpharmacologic treatments for patients with moderate-to-severe AUD. The VA/DoD guideline recommends oral NTX be routinely considered as one of the first-line options for pharmacological treatment of AUD. Long-acting, injectable naltrexone is recommended when there are significant concerns about medication adherence. Although acamprosate, disulfiram, and topiramate are also first-line options for treatment in the VA/DoD guideline, NTX is more commonly prescribed at the facility in which this study took place due to superior efficacy and safety profile.6-8 At the study facility, acamprosate, disulfiram, and NTX are formulary options with no restrictions for ordering.
Naltrexone has been found to be effective and well-tolerated pharmacotherapy for AUD. Several studies have found that oral NTX can decrease alcohol cravings,9 number of drinking days,9,10 and heavy drinking11,12 and reduce relapses.8-10 Bryson et al6 demonstrated that patients are more likely to discontinue pharmacological treatment for AUD with acamprosate or disulfiram compared to NTX. Patients were also significantly more likely to discontinue oral NTX compared to LAI NTX. In another study,12 LAI NTX significantly decreased the number of heavy drinking days by 25% compared to placebo. Long-acting, injectable naltrexone has been shown to prolong the time to the first drinking day compared to placebo.13 Both oral and LAI NTX are efficacious and are recommended as part of a comprehensive treatment plan for veterans with AUD. However, to date, there have been no randomized clinical trials directly comparing efficacy of oral NTX to LAI NTX. It is also important to note the large difference in cost between dosage forms. The average wholesale price in late 2016 for one injection of LAI NTX was $1570.80, and for a 30-day supply of generic oral NTX 50-mg tablets, the average wholesale price was $128.25.14
Although both oral and LAI NTX are considered effective, first-line options for pharmacotherapy of AUD, there are significant differences in price and a lack of direct comparisons. The purpose of this study was to compare the differences in health care utilization between oral NTX and LAI NTX.
Methods
This study was approved by the University of Utah/VA Salt Lake City Health Care System Institutional Review Board. It was a retrospective, observational cohort study conducted at a single VA health care system. Outcomes were set a priori. The primary outcome was 90-day alcohol-related hospital admissions per patient (ARA90). Secondary outcomes included 90-day outpatient clinic and emergency department (ED) visits, 30-day alcohol-related admissions (ARA30), and medication adherence. Medication adherence was determined using a medication possession ratio (MPR) based on medication refill dates as well as medication administration chart notes.
Veterans were identified by review of an archived database of the electronic medical record (EMR) system within the VA. First-time prescriptions and inpatient orders for both oral and LAI NTX were identified by querying the EMR. Inclusion criteria were first-time receipt of oral NTX or LAI NTX from January 1, 2015, through December 31, 2015, and use of NTX for AUD. First-time use could be either an outpatient prescription or inpatient medication order. Exclusion criteria were concurrent use of acamprosate or disulfiram and use of NTX for any diagnoses other than AUD. Veterans were divided into 2 groups: oral NTX and LAI NTX. Veterans in the LAI NTX group could receive up to 30 days of oral NTX prior to being initiated on LAI NTX. Manual retrospective chart review of the EMR was conducted to gather data using a prespecified data collection form.
After subject identification, baseline data was collected by manual chart review. Data included age, sex, ethnicity, and mental health diagnoses. In addition, baseline utilization of health care services for AUD was collected, encompassing the 12 months prior to NTX initiation. This included ARA, clinic visits, and ED visits. Clinic visits were defined as a visit with a mental health prescriber and identified by notes in the EMR from the prescriber. Admissions were identified through history and physical notes in the chart as well as scanned discharge summaries from non-VA facilities. Admissions were determined to be alcohol-related by admission diagnosis in the history and physical note. Additional data collected included ARA, clinic visits, ED visits, and MPR through 6 months following NTX initiation. For the oral NTX group, MPR was calculated using the following equation: number of days' supply dispensed/number of days' supply prescribed. For the LAI NTX group, MPR was calculated using the following equation: number of doses administered/number of doses prescribed. This information was obtained using the internal VA pharmacy database. Veterans fill the majority of their medications with the VA pharmacy, and this dispensing information is available in the EMR. Up to a 90-day supply may be dispensed. If a veteran used an outside pharmacy, this information was not available and not utilized.
Data were extracted and analyzed by the first author utilizing Microsoft Excel (Redmond, WA). Demographic data were analyzed using descriptive statistics. The primary and secondary outcomes were compared using the Student t test for independent samples.
Results
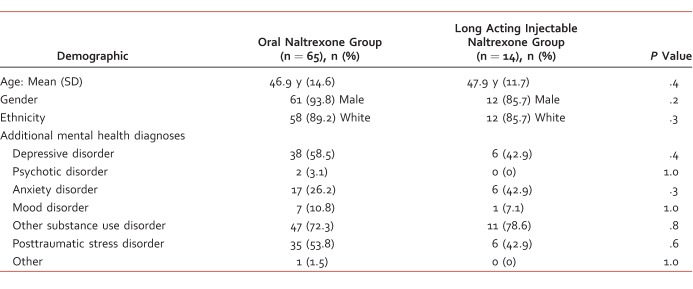
A total of 108 patients were identified as having received a first-time order for NTX in the study period. A total of 65 patients in the oral NTX group and 14 in the LAI NTX group met inclusion criteria. Of the 29 veterans excluded, 14 were prescribed NTX for opioid use disorder, 1 prescribed acamprosate, and 1 disulfiram. Baseline demographics and health care utilization in the 12 months prior to NTX initiation appeared similar between the groups as shown in Tables 1 and 2, respectively. Every veteran included had more than 1 mental health diagnosis, most commonly an additional substance use disorder, mainly tobacco use disorder. The average length of oral NTX overlap in the LAI NTX group was 19 days.
TABLE 1.
Baseline demographics
TABLE 2.
Baseline health care utilization in the 12 months prior to naltrexone initiation
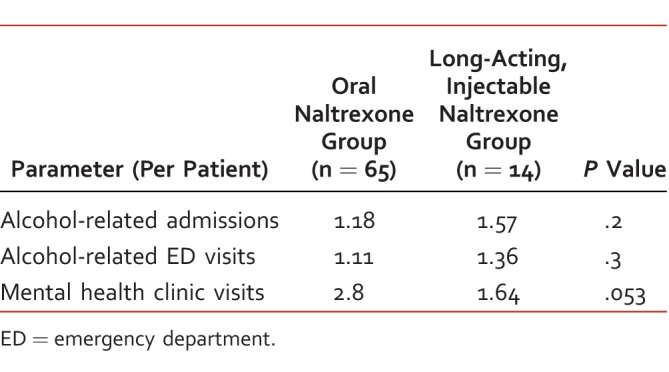
ARA90 were similar between the oral NTX and LAI NTX groups. There were 0.17 ARA90 per patient in the oral NTX group and 0.64 per patient in the LAI NTX group (P = .06). Veterans in the oral NTX group had significantly fewer ARA30 than those in the LAI NTX group with 0.05 admissions per patient compared to 0.36 (P < .01). Veterans in the oral NTX group also had significantly fewer 90-day clinic visits and 90-day ED visits than those in the LAI NTX group (P < .01). See Table 3 for the full data set.
Table 3.
Health care utilization after naltrexone initiation
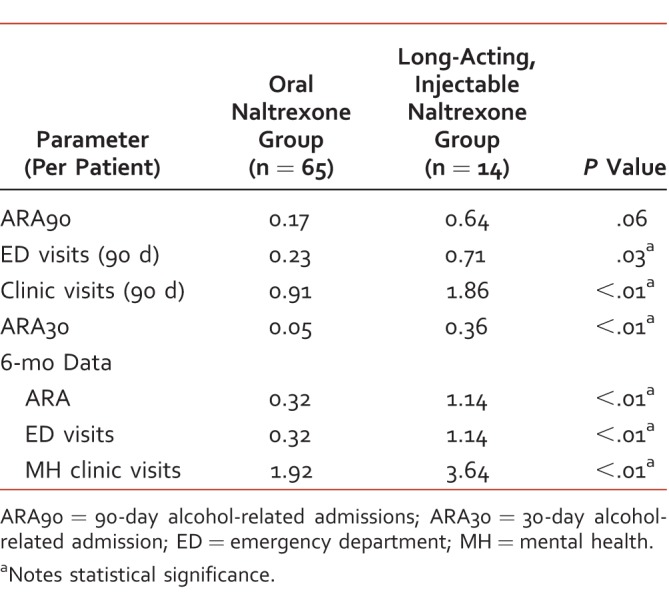
Six-month data was also obtained for admissions, ED visits, and mental health clinic visits as well as 6-month adherence data. Oral NTX had significantly lower health care utilization per patient at 6 months compared to LAI NTX. Six-month ARA totaled 0.32 per patient in the oral NTX group and 1.14 per patient in the LAI NTX group (P < .01). The oral NTX group also had significantly fewer ED visits at 6 months with 0.32 visits per patient compared to 1.14 per patient in the LAI NTX group (P < .01). The oral NTX group had 1.92 clinic visits per patient, compared to 3.64 per patient in the LAI NTX group (P < .01). Adherence data showed similar adherence over 6 months between the two groups. The average MPR for the oral NTX group was 65.14%. The average proportion of doses administered (MPR) in the LAI NTX group was 67%.
Discussion
This study aimed to compare health care utilization between oral NTX and LAI NTX. The 2 groups were similar at baseline. Overall, oral NTX had lower health care utilization at 90 days than LAI NTX. There was, however, a statistically significant difference in 90-day mental health clinic visits between oral NTX and LAI NTX, and this persisted at 6 months. This is expected, as veterans typically have an appointment with a mental health prescriber the day of their NTX injection, leading to a visit approximately every 28 days. This increase in clinic visits adds to the overall increased health care utilization with LAI NTX. Of note, if a veteran did not meet with a prescriber on the date of NTX injection, this was not considered a clinic visit. Oral NTX also had significantly fewer ARA30 per patient compared to LAI NTX. At 6 months, adherence was similar for the 2 groups. Overall, the data suggest that patients receiving oral NTX are less likely to be readmitted, have an ED visit, or need frequent mental health follow-up compared to those receiving LAI NTX. Based on these results, the benefits of LAI NTX may not outweigh the costs. Oral NTX should be considered as the standard of care until further studies are completed.
There were several limitations to this study. It was retrospective, nonrandomized, and limited to the veteran population. The LAI NTX group had a small sample size of only 14 patients. Factors that could affect prognosis and adherence, such as medical comorbidities and homelessness, were not accounted for. Specifics on co-occurring substance use disorders were also not taken into account. For 6 months of the study period, LAI NTX was a nonformulary item at the study facility, which may have influenced prescribing patterns. Another influence may be that patients considered appropriate for LAI NTX may have more severe AUD and be more prone to relapse, which is why they were trialed on LAI NTX to start. The 2 groups were similar at baseline, but additional factors may have influenced the choice of medication for each group. There may be differences among patients who initiated NTX in an inpatient setting versus an outpatient setting, which was not taken into account. Although there is some documentation in the VA EMR of veterans receiving care outside of the VA, it is possible that not all ED visits or hospitalizations were accounted for. The use of off-label pharmacotherapy for AUD, such as gabapentin or topiramate, was not considered. Contact with additional care, including group therapy and psychology appointments, was not taken in to account. Future studies would benefit from considering all of these limitations and factoring them into the study design.
In conclusion, based on the results of this study, oral NTX appears to be associated with lower health care utilization and fewer readmissions compared to LAI NTX at this VA facility. Because LAI NTX has a significantly higher cost and did not show advantage in terms of reducing overall health care utilization, LAI NTX should be reserved for cases in which a patient cannot take oral medications or there is significant concern for adherence. It is important for patients receiving LAI NTX to be motivated for treatment as they must present every 28 days for an injection. More data is needed, however, to further elucidate the differences in efficacy between oral NTX and LAI NTX. It would be beneficial to have randomized, controlled trials conducted in the general population comparing efficacy of the 2 dosage forms head to head. Future studies should include time to first drink and number of heavy drinking days as outcomes.
Footnotes
Disclosures: All authors of this manuscript have nothing relevant to disclose.
References
- 1. Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. HHS Publication No. SMA 14-4927. Available from: http://www.samhsa.gov/data
- 2. Harris AHS, Bowe T, Del Re AC, Finlay AK, Oliva E, Myrick HL, et al. Extended release naltrexone for alcohol use disorders: Quasi-experimental effects on mortality and subsequent detoxification episodes. Alcohol Clin Exp Res. 2015; 39 1: 79- 83. DOI: 10.1111/acer.12597. PubMed PMID: 25623408. [DOI] [PubMed] [Google Scholar]
- 3. Department of Veteran Affairs, Department of Defense. VA/DoD clinical practice guideline for management of substance use disorders (SUD). 2015. Available from: http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGrevised22216.pdf
- 4. Centers for Disease Control and Prevention. Excessive drinking costs US $223.5 billion. c2014 [cited 2017. Jan 4]. Available from: http://www.cdc.gov/features/alcoholconsumption/.
- 5. Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015; 72 8: 757- 66. DOI: 10.1001/jamapsychiatry.2015.0584. PubMed PMID: 26039070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bryson WC, McConnell KJ, Korthius PT, McCarty D. . Extended-release naltrexone for alcohol dependence: persistence and healthcare costs and utilization. Am J Manag Care. 2011; 17 Suppl 8: S222- 34. [PMC free article] [PubMed] [Google Scholar]
- 7. Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006; 295 17: 2003- 17. DOI: 10.1001/jama.295.17.2003. PubMed PMID: 16670409. [DOI] [PubMed] [Google Scholar]
- 8. Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, et al. Naltrexone versus acamprosate in the treatment of alcohol dependence: a multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006; 101 10: 1451- 62. DOI: 10.1111/j.1360-0443.2006.01555.x. PubMed PMID: 16968347. [DOI] [PubMed] [Google Scholar]
- 9. Volpicelli JR. . Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992; 49 11: 876- 80. DOI: 10.1001/archpsyc.1992.01820110040006. PubMed PMID: 1345133. [DOI] [PubMed] [Google Scholar]
- 10. O'Malley SS, Jaffe AJ, Chang G, Schottenfield RS, Meyer RE, Rounsaville B. . Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch Gen Psychiatry. 1992; 49 11: 881- 7. PubMed PMID: 1444726. [DOI] [PubMed] [Google Scholar]
- 11. O'Malley SS, Corbin WR, Leeman RF, DeMartini KS, Fucito LM, Ikomi J, et al. Reduction of alcohol drinking in young adults by naltrexone: a double-blind, placebo-controlled, randomized clinical trial of efficacy and safety. J Clin Psychiatry. 2015; 76 2: e207- 13. DOI: 10.4088/JCP.13m08934. PubMed PMID: 25742208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005; 293 13: 1617- 25. DOI: 10.1001/jama/293.13.1617. PubMed PMID: 15811981. [DOI] [PubMed] [Google Scholar]
- 13. Kranzler HR, Wesson DR, Billot L. . Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004; 28 7: 1051- 9. DOI: 10.1097/01.ALC.0000130804.08397.29. PubMed PMID: 15252291. [DOI] [PubMed] [Google Scholar]
- 14. Naltrexone. Lexi-Comp, Inc (Lexi-Drugs™). Hudson (OH): Lexi-Comp, Inc; October 2016. Available at http://online.lexi.com