Abstract
Parkinson disease (PD) is a common neurodegenerative disorder in older adults characterized by motor and nonmotor symptoms and complications. Impulse control disorders (ICDs), such as pathological gambling, compulsive shopping, compulsive sexual behavior (hypersexuality), and binge eating disorder, affect 13.6% of the PD population. Use of dopamine receptor agonists (DRAs) is considered a major risk factor for ICD development. Amantadine and a high dose of levodopa were linked to ICDs to a lesser extent than DRAs. Based on the severity of behavior(s), ICDs can negatively impact social, professional, and familial lives of patients and their families. Ideally, all PD patients taking DRAs, high doses of levodopa, and/or amantadine should be routinely asked about or monitored for ICDs during therapy initiation and continuation. Dose decrease or withdrawal of the offending agent, primarily DRAs, is usually the most effective first step in ICD management. Careful dose adjustment with close monitoring is warranted due to risk for worsening of motor symptoms or emergence of dopamine agonist withdrawal syndrome (DAWS). About 1/3 of PD patients with ICD who decrease or discontinue DRA experienced DAWS. The lowest dose of DRA will need to be continued to balance ICDs and DAWS as it is not alleviated by other dopaminergic or psychotropic medications. Other therapies with low empiric evidence, such as amantadine, naloxone, cognitive behavior therapy, deep brain stimulation, and psychopharmacotherapy showed mixed results for ICD management. It is crucial that clinicians are familiar with the psychiatric complications of PD, including ICDs, beyond mere recognition and management of motor symptoms.
Keywords: impulse control disorders, dopamine dysregulation syndrome, dopamine agonist withdrawal syndrome, dopamine receptor agonist, Parkinson disease, levodopa, amantadine
Introduction
Parkinson disease (PD) is the most common type of primary parkinsonism and the second most common progressive neurodegenerative disorder. It affects about 1% of the population over age 50 years and about 2.5% of the population over age 70.1 The lifetime risk for PD development is 2.0% in men and 1.3% in women.2 Idiopathic, also known as sporadic PD, is the most common form of PD, affecting primarily older adults. In general, PD is associated with motor symptoms, such as resting tremor, bradykinesia/akinesia, and rigidity as a result of dopamine deficiency in the basal ganglia due to neurodegeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc).3
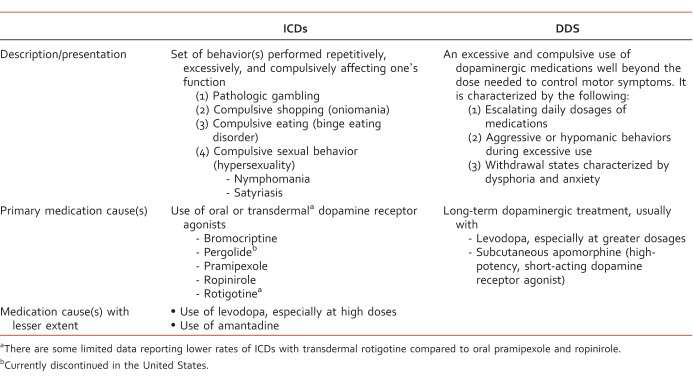
In addition to motor symptoms, nonmotor symptoms and complications, such as neuropsychiatric or neurobehavioral problems, autonomic dysfunction, and sensory problems, are also considered an important part of PD.1 Neuropsychiatric or neurobehavioral complications such as depression, anxiety, rapid eye movement sleep behavior disorder, and dementia are all very common in PD and are likely to occur due to neurodegenerative processes in different neurotransmitters systems in various brain regions.4 Other complications, such as psychosis, delirium, and compulsive/impulsive spectrum disorders, such as impulse control disorders (ICDs), dopamine dysregulation syndrome (DDS), and punding, are also relatively common.4-10 Impulse control disorders are a group of psychiatric disorders associated with impulsive behavior or drive or temptation to be involved in pleasurable activity of an excessive and harmful nature to the individual (and his or her family).5,6 The four major ICD behaviors in PD patients are pathologic gambling, compulsive shopping, compulsive sexual behavior (hypersexuality), and binge eating disorder.5-7 Important and contrasted information between ICDs and DDS in PD are listed in the Table. As the US population is aging and growth of the population of individuals 65 years and older will continue over the coming years, it is crucial that clinicians are familiar with the complications of PD beyond mere recognition and management of motor symptoms.
TABLE: .
Impulse Control Disorder (ICD) in PD
The Dominion Study (Phase I), a large cross-sectional multicenter study of 3090 patients (mean age 63.8 ± SD 8.0 years) with idiopathic PD from 46 movement disorder centers across the United States (n = 33) and Canada (n = 13), assessed the prevalence of ICDs. Presence of individual ICDs were assessed using Massachusetts Gambling Screen (pathologic gambling), the Minnesota Impulsive Disorders Interview (compulsive sexual and buying behavior), and Diagnostic and Statistical Manual of Mental Disorders IV criteria for binge eating disorder.8 At least 1 ICD was identified in 13.6% of patients, and frequencies of individual ICDs were pathological gambling (5.0%), compulsive sexual behavior (3.5%), compulsive buying (5.7%), and binge eating disorder (4.3%).8 Twenty-eight percent of PD patients with an ICD experienced 2 or more ICDs.8
It appears that ICDs are related more to dopaminergic treatment than to PD itself. The Parkinson's Progression Markers Initiative studied the frequency of individual ICDs in newly diagnosed and untreated PD patients (n = 168; mean age 61.5 ± SD 9.5 years) and unmatched healthy controls (n = 143; mean age 59.1 ± SD 12 years) from 21 different academic movement disorders centers in the United States (n = 16) and Europe (n = 5).23 The presence of ICDs was assessed using the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Short Form using previously reported criteria and cut-off values.24 Prevalence of ICDs did not differ between de novo untreated PD patients and unmatched healthy controls for compulsive gambling (1.2% vs 0.7%), compulsive buying (3.0% vs 2.1%), compulsive sexual behavior (4.2% vs 3.5%), or binge eating disorder (7.1% vs 10.5%).23
The use of oral dopamine receptor agonists (DRAs), including ergot derivatives (eg, bromocriptine, pergolide) and nonergot derivatives (eg, pramipexole, ropinirole) is currently considered the most important risk factor for developing ICDs in PD patients.5-8,11-20 A recent 4-year prospective cohort study of nondemented outpatients with idiopathic PD and no previous history of PD neurosurgery or ICDs studied the prevalence of new-onset ICDs in this population. Forty-six out of 164 patients were treated with DRAs.12 Twenty-five subjects were newly treated, and 21 subjects received ongoing therapy with a DRA. Eighteen patients (39.1%) treated with a DRA developed new-onset ICDs over the course of the study. The timing of onset of an ICD was highly variable, ranging from 3 to 114 months after initiation of DRA with median onset time of 23 months. The most common ICD was binge eating disorder, which affected 16 of 18 subjects with new-onset ICD (44% women). The study concluded that timing for new-onset ICD after DRA treatment is highly variable among PD patients. In addition, high lifetime prevalence of cigarette smoking (44.2% vs 14.3%; P = .04) and greater baseline prevalence of caffeine consumption (100% vs 66.7%; P = .007) were associated with high prevalence of new-onset ICD while on a DRA.12
Garcia-Ruiz et al25 conducted a multicenter transversal study with 233 PD patients (mean age 66 ± SD 9.7 years) chronically treated with a single DRA (transdermal rotigotine, oral pramipexole, or oral ropinirole) for at least 6 months. The patients were recruited from 5 different Spanish PD centers, and the mean exposure time to a select single DRA was 5.9 years (SD 4.1 years). A total of 116 patients were treated with pramipexole, 81 patients with ropinirole, and 36 patients with rotigotine. Thirty-nine percent of patients on a single DRA fulfilled the clinical criteria for an ICD25 using the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease form.24 Oral DRA treatment either with pramipexole or ropinirole was associated with higher risk for ICD development in comparison to transdermal rotigotine (42% vs 19%; Fisher exact test P < .01). Univariate analysis of data revealed that younger age (P < .01), treatment with rasagiline (P < .05), and treatment with oral DRA (pramipexole or ropinirole; P < .01) were significantly associated with ICDs. No difference was observed between extended-release and immediate-release formulations of pramipexole or ropinirole (Fisher exact test P > .05). Multivariate analysis revealed that oral DRAs are associated with 3-fold increased risk of ICD development (odds ratio [OR] = 3.14; 95% confidence interval [CI] = 1.26-7.83; P = .014). In addition, concomitant use of rasagiline with a DRA was associated with a 2-fold increased risk for ICD development (OR = 2.12; 95% CI = 1.07-4.21; P = .032).25 This study reported that transdermal rotigotine might be associated with lower risk for development of ICD compared to oral pramipexole or ropinirole. The significance of these differences is not well understood. There is no clear explanation for whether this is due to difference in drug pharmacology, route of administration, or other factors. Due to study design, it was not possible to determine relative risk for ICD development. In addition, this study included fewer subjects treated with rotigotine and shorter exposure time to rotigotine therapy in comparison to oral DRAs.25 There is need for further investigation using prospective studies in order to confirm lower risk for ICD development during rotigotine use in comparison to oral pramipexole or ropinirole.
Additional medications, such as amantadine and high doses of levodopa, were also reported to be associated with an increased risk for ICDs in PD populations, however, to a lesser extent than oral DRAs.8,10,21-22 The Dominion study (Phase I) reported that use of DRAs (pergolide, pramipexole, and ropinirole) versus levodopa was associated with a 2.6-fold increased risk for ICD development (OR = 2.60; 95% CI = 1.97-3.43; P < .001).8 It also revealed that use of oral DRAs and levodopa are both independently associated with elevated risk for all of the 4 studied ICDs. The use of DRA versus no DRA was associated with a 2.7-fold risk increase for ICDs (OR = 2.72; 95% CI = 2.07-3.57; P < .001), and levodopa versus no levodopa use was associated with a 1.5-fold increase (OR = 1.51; 95% CI = 1.09-2.09; P < .01).8 No significant difference was observed for the propensity of pramipexole (n = 1286) or ropinirole (n = 651) to induce ICD behaviors (17.7% vs 15.5%; P = .14). The rate of ICDs did not significantly differ from pramipexole compared with ropinirole (17.7% vs 15.5%; P = .14; OR = 1.22; 95% CI = 0.94-1.57).8 Use of pergolide (n = 50) was associated with a 22% overall increase in ICD frequency.8 Importantly, the highest risk for development of ICD was observed during concomitant therapy of an oral DRA and levodopa. This combination therapy, compared to DRA treatment alone, increased the odds of having an ICD by 1.4-fold (OR = 1.42; 95% CI = 1.08-1.98; P < .001).8 Interestingly, higher dosages of levodopa were associated with an increased risk for ICD (P = .008), and no dose-dependent effect was observed for oral DRAs.8 In contrast to these findings, a recent retrospective chart analysis study by Rana et al26 reported strong association with the dose of DRA as well as levodopa and the onset of ICD in PD patients.26 Of the 140 community dwelling PD patients from a single community-based PD and movement disorder clinic, a total of 8 patients treated with antiparkinson medications developed symptoms of ICD. The symptoms included compulsive buying, compulsive gambling, binge eating, and increased creativity and hypersexuality. Out of 42 PD patients treated with DRA, a total of 7 patients developed symptoms consistent with ICDs. In contrast, only 1 patient out of 92 PD patients treated with levodopa developed such symptoms. Interestingly, all patients who developed ICD(s) were given high doses of either DRA (pramipexole or amantadine) or levodopa. In addition, those who developed ICDs in this study had other common characteristics: male sex, early stages of PD (Stage 1 to 2), and early-onset of PD (between age 30 to 60 years).26
Although ICDs are more prevalent in PD patients treated with oral DRAs, a high dose of levodopa and amantadine, other clinical and demographic factors (eg, male sex, younger age of onset of PD, preexisting impulsivity or impulse control disorder or substance use disorder, and family history of gambling problems) were shown to increase the likelihood of development of ICD in patients on DRA therapy.8,11,27 The Dominion study (Phase I)8, the largest cross-sectional study to date, reported that men were more likely to develop compulsive sexual behavior than women (5.2% vs 0.5%; OR = 11.98; 95% CI = 4.87-29.48; P < .001) and less likely to develop compulsive shopping (4.5% vs 7.8%; OR = 0.55; 95% CI = 0.40-0.74; P < .001) and binge eating disorder (3.4% vs 5.8%; OR = 0.57; 95% CI = 0.40-0.81; P = 0.002).8 In addition, the US patients had about a 2-fold increased risk for development of compulsive buying in comparison to Canadians (OR = 2.16; 95% CI = 1.42-3.28; P < .001).8 Comparable findings for compulsive shopping and gambling were reported in a small prospective study with PD outpatients with the exception of binge eating, which was identified in 56% (9/16 subjects) in males versus 44% females (7/16 subjects).12
As evident from the data presented and discussed above, PD patients are at increased risk for development of one or more ICD behaviors while on dopaminergic therapy. These behaviors can widely differ in their severity and can have various degrees of impact on the social, professional, and familial lives of patients and their families.28-31 The Dominion Study (Phase II)11 demonstrated a significant impact of presence of an ICD on a variety of aspects of life when comparing PD patients with an ICD (n = 282; mean age 61.34 ± SD 0.50 years) and those without (n = 282; mean age 60.89 ± SD 0.50 years).11 Parkinson disease patients with an ICD had more functional impairment (P < .001) and greater depressive (P < .0001) and anxiety (P < .0001) scores compared to those without.11
Management of ICDs in PD
Clinicians should be directed to educate patients and their families on risk of ICDs, especially upon initiation of a DRA or combination treatment of levodopa and DRA. Ideally, all PD patients taking a DRA, a high dose of levodopa, and/or amantadine should be routinely asked about ICDs during initiation and continuation of the therapy. A cross-sectional study with 150 PD patients compared patients' self-assessments using a novel ICD screening questionnaire and ratings by their caregivers. The comparison of self-rated frequencies of ICDs by PD patients and the estimation by their caregiver was significantly different in regard to reported frequency of pathological gambling (P = .01), hypersexuality (P < .001), and compulsive shopping (P < .001).32 Reports of frequency of compulsive eating did not differ between patients and caregivers.32 Thus, the need for reporting symptoms should be stressed not only to the patient, but also to his or her family or caregiver in order to treat ICD behaviors early.
If possible, the first step in the management of new-onset ICDs is to reduce the dose or discontinue the offending dopaminergic agent.33,34 A study by Mamikonyan et al33 showed that PD patients with DRA-induced ICDs experienced remission or significant reduction of ICD behaviors after significant dose decrease or discontinuation.33 Therefore, if tolerated, the lowest effective dose of DRA or even levodopa should be considered as an initial treatment of ICDs. Not all PD patients with ICDs are able to tolerate DRA dose reduction or discontinuation due to motor symptom worsening. For those who cannot tolerate modification of DRA treatment due to reappearance or worsening of motor symptom(s), substitution with an adequate dose of levodopa or addition of other antiparkinson medications can be used to ameliorate this issue.33,34 It was demonstrated that discontinuation or significant decrease in DRA daily exposure, in the presence of substitution with levodopa, was associated with full or partial remission of their ICD symptoms without worsening motor symptoms in the majority of patients.33 When DRA reduction or discontinuation is pursued in PD patients with ICDs, one needs to keep in mind that this can result in severe nonmotor drug withdrawal syndrome called dopamine agonist withdrawal syndrome (DAWS).34-37 It seems that DAWS is more common in PD patients who decrease or discontinue DRA therapy due to ICD rather than PD patients who decrease/discontinue DRA for other reasons.35,37 Therefore, careful titration with close monitoring is warranted. In addition, cumulative DRA exposure (DRA levodopa equivalent daily doses [LEDD] were defined as the LEDD for DRAs only; cumulative DRA exposure was approximated by multiplying the maintenance dose of DRA [in LEDD] by the duration of therapy) was suggested to be a risk factor for DAWS.35
As such, early recognition or diagnosis of ICDs and titration or discontinuation of the offending DRA could reduce the risk of this complication. Based on reported data from two studies performed by Rabinak and Nirenberg35 and Pondal et al,37 approximately 1/3 of PD patients with ICDs developed DAWS upon modification of DRA therapy (5/15 patients and 13/42 patients, respectively).35,37 Dopamine agonist withdrawal syndrome clinical manifestations resemble psychostimulant withdrawal syndromes and include psychiatric (eg, anxiety, dysphoria, depression, panic attack, insomnia, agitation, and irritability) and autonomic symptoms (eg, orthostasis, diaphoresis, and nausea).35 Dopamine agonist withdrawal syndrome is typically ameliorated by reinitiation of withdrawn DRA, but substitution of DRA with levodopa or another antiparkinson therapy has not been shown to be effective.34-37 In addition, use of psychotropic agents (eg, antidepressants, anxiolytics) does not seem to improve symptoms associated with DAWS.34-37 A prospective cohort study of outpatients with idiopathic PD and new-onset of ICD after initiation of a DRA demonstrated that, of the 18 subjects, ICDs completely resolved in all 10 individuals who completely discontinued DRA and in 3 of 5 individuals who reduced daily DRA dose. No improvement in ICD symptoms was observed in 3 individuals who continued the same dosage of DRA.12 Dopamine agonist withdrawal syndrome was reported in 6 individuals: 4 patients who discontinued DRA, 1 patient who decreased DRA dose, and 1 patient who was unable to decrease DRA dose because of severe DAWS symptoms. Four individuals with DAWS then developed secondary DDS during self-adjustment of levodopa dose in unsuccessful attempts to alleviate DAWS.12
If a patient cannot tolerate DRA dose adjustment or discontinuation, there are potentially other options, however, with less empiric support.33,37 It is currently unknown whether switching from one DRA to another provides any clinical benefit. It was previously reported that both pramipexole and ropinirole carry similar risk for ICD development in PD patients.8 There are limited data supporting lower rates of ICDs with transdermal rotigotine compared to pramipexole and ropinirole.25 In addition, there are mixed results on efficacy of use of amantadine,21,22,38 naltrexone,39 and cognitive behavioral therapy40,41 for the alleviation of ICDs in the PD population. A recent study found evidence of benefit from switching patients from oral levodopa and other dopaminergic medications to jejunal levodopa infusion for improvement of ICDs in patients with advanced PD with severe impulsivity and motor symptoms.42 In some cases, environmental modifications, such as limiting patient access to money or the Internet may help in reducing some ICD behaviors, such as pathologic gambling or compulsive buying.34 Subthalamic nucleus deep brain stimulation (STN-DBS) might also be considered an option for those individuals for whom reduction of DRA is not feasible while experiencing severe ICDs.43,44 One needs to keep in mind that results have been contradictory as new-onset ICDs or no improvement were also reported after STN-DBS.45,46 Psychotropic medications (eg, antidepressants, antipsychotics, or mood stabilizers) are often used to treat ICD behaviors but with mixed results and in the absence of controlled clinical trials.34 Before any options with low evidentiary support can be recommended as a standard therapy for ICDs, more data are needed. However, in cases in which dose decrease or discontinuation of offending agents is not possible due to motor problems, DAWS, or patient refusal, the clinician may carefully consider their use in individual patients based on occasional case reports of successful resolutions or improvement of ICDs.34,36
Conclusions
Impulse control disorders, pathological gambling, compulsive shopping, compulsive sexual behavior, and binge eating disorder are fairly common, affecting 13.6% of the PD population. Based on behavior severity, ICDs can have a negative impact on the social, professional, and familial lives of patients and their families. Current thought is that ICDs are not significant complications of PD, but are rather behavioral manifestations of PD dopaminergic therapy, particularly oral DRAs and high doses of levodopa and their combinations. It has been suggested that the rate of ICDs in untreated de novo PD patients is not greater than that observed in a healthy population. It seems that use of DRA presently is the most important risk factor for development of ICDs in PD. It needs to be noted that not every individual treated with DRA will develop an ICD as a complication of dopaminergic medication(s). Other clinical and demographic factors play a role in ICD development. A variety of factors were identified to be associated with increased frequency of ICD development in the PD population including male sex, younger age of onset of PD, cigarette smoking, high caffeine consumption at baseline, family history of gambling, and preexisting impulsivity or substance use disorder and history of deep brain stimulation. At this time, however, there is no clear understanding of which PD patients on DRA therapy are at the highest risk for ICD development nor why. In addition, the best way to prevent these unwanted and potentially harmful consequences of use of DRAs is unknown.
Patients and their families should be educated about this potential complication to facilitate early identification and treatment. When ICDs emerge, decreasing dose or withdrawal of an offending dopaminergic agent, particularly DRA, should be the first step. During modification of an antiparkinson regimen, it is important to ensure close monitoring for motor symptom worsening and improvement of ICDs. As about 1/3 of patients cannot tolerate decreased dose or complete discontinuation of DRA due to DAWS, it is important to educate patients about this and carefully monitor for emergence of symptoms associated with DAWS. Dopamine agonist withdrawal syndrome symptoms do not respond to other PD dopaminergic agents or psychototropic medications. The lowest dose of DRA will need to be continued while maintaining a careful balance between improvement of DAWS and worsening of ICD behavior(s). Select ICD behaviors can be also somewhat regulated by modification of a patient's environment, such as decreased access to finances; credit cards; and shopping, gambling, or eating opportunities. Several other potential strategies for which results have lower evidentiary support are also available. However, until stronger evidence becomes available, clinicians may use such therapies if other options with empiric evidence fail.
Footnotes
Disclosures: Nothing to disclose
References
- 1. de Lau LML, Breteler MMB. . Epidemiology of Parkinson's disease. Lancet Neurol. 2006; 5 6: 525- 35. DOI: 10.1016/S1474-4422(06)70471-9. PubMed PMID: 16713924. [DOI] [PubMed] [Google Scholar]
- 2. Oguh O, Videnovic A. . Inpatient management of Parkinson disease: Current challenges and future directions. Neurohospitalist. 2012; 2 1: 28- 35. DOI: 10.1177/1941874411427734. PubMed PMID: 23983860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Lanciego JL, Artieda J, Gonzalo N, et al. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 2000; 23 Suppl 10: S8- 19. [DOI] [PubMed] [Google Scholar]
- 4. Marsh L. . Neuropsychiatric aspects of Parkinson's disease. Psychosomatics. 2000; 41 1: 15- 23. DOI: 10.1016/S0033-3182(00)71169-8. PubMed PMID: 10665264. [DOI] [PubMed] [Google Scholar]
- 5. Wolters EC, van der Werf YD, van den Heuvel OA. . Parkinson's disease-related disorders in the impulsive-compulsive spectrum. J Neurol. 2008; 255 Suppl 5: 48- 56. DOI: 10.1007/s00415-008-5010-5. PubMed PMID: 18787882. [DOI] [PubMed] [Google Scholar]
- 6. Weintraub D, David AS, Evans AH, Grant JE, Stacy M. . Clinical spectrum of impulse control disorders in Parkinson's disease. Mov Disord. 2015; 30 2: 121- 7. DOI: 10.1002/mds.26016. PubMed PMID: 25370355. [DOI] [PubMed] [Google Scholar]
- 7. Weintraub D. . Dopamine and impulse control disorders in Parkinson's disease. Ann Neurol. 2009; 64 Suppl 2: S93- 100. DOI: 10.1002/ana.21454. PubMed PMID: 19127573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010; 67 5: 589- 95. DOI: 10.1001/archneurol.2010.65. PubMed PMID: 20457959. [DOI] [PubMed] [Google Scholar]
- 9. Giovannoni G, O'Sullivan JD, Turner K, Manson AJ, Lees AJ. . Hedonistic homeostatic dysregulation in patients with Parkinson's disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry. 2000; 68 4: 423- 8. PubMed PMID: 10727476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beaulieu-Boire I, Lang AE. . Behavioral effects of levodopa. Mov Disord. 2015. January; 30 1: 90- 102. [DOI] [PubMed] [Google Scholar]
- 11. Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, et al. Impulse control disorders in Parkinson disease: a multicenter case-control study. Ann Neurol. 2011; 69 6: 986- 96. DOI: 10.1002/ana.22356. PubMed PMID: 21416496. [DOI] [PubMed] [Google Scholar]
- 12. Bastiaens J, Dorfman BJ, Christos PJ, Nirenberg MJ. . Prospective cohort study of impulse control disorders in Parkinson's disease. Mov Disord. 2013; 28 3: 327- 33. DOI: 10.1002/mds.25291. PubMed PMID: 23283708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006; 63 7: 969- 73. DOI: 10.1001/archneur.63.7.969. PubMed PMID: 16831966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voon V, Thomsen T, Miyasaki JM, de Souza M, Shafro A, Fox SH, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007; 64 2: 212- 6. DOI: 10.1001/archneur.64.2.212. PubMed PMID: 17296836. [DOI] [PubMed] [Google Scholar]
- 15. Driver-Dunckley E, Samanta J, Stacy M. . Pathological gambling associated with dopamine agonist therapy in Parkinson's disease. Neurology. 2003; 61 3: 422- 3. PubMed PMID: 12913220. [DOI] [PubMed] [Google Scholar]
- 16. Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. . Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol. 2005; 62 9: 1377- 81. DOI: 10.1001/archneur.62.9.noc50009. PubMed PMID: 16009751. [DOI] [PubMed] [Google Scholar]
- 17. Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. . Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson's disease and multiple system atrophy. Parkinsonism Relat Disord. 2005; 11 6: 381- 6. DOI: 10.1016/j.parkreldis.2005.06.005. PubMed PMID: 16109498. [DOI] [PubMed] [Google Scholar]
- 18. Grosset KA, Macphee G, Pal G, Stewart D, Watt A, Davie J, et al. Problematic gambling on dopamine agonists: Not such a rarity. Mov Disord. 2006; 21 12: 2206- 8. DOI: 10.1002/mds.21110. PubMed PMID: 17013907. [DOI] [PubMed] [Google Scholar]
- 19. Ahlskog JE. . Pathological behaviors provoked by dopamine agonist therapy of Parkinson's disease. Physiol Behav. 2011; 104 1: 168- 72. DOI: 10.1016/j.physbeh.2011.04.055. PubMed PMID: 21557955. [DOI] [PubMed] [Google Scholar]
- 20. Voon V, Hassan K, Zurowski M, Duff-Canning S, de Souza M, Fox S, et al. Prospective prevalence of pathological gambling and medication association in Parkinson disease. Neurology. 2006; 66 11: 1750- 2. PubMed PMID: 16769956. [DOI] [PubMed] [Google Scholar]
- 21. Weintraub D, Sohr M, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol. 2010; 68 6: 963- 8. DOI: 10.1002/ana.22164. PubMed PMID: 21154480. [DOI] [PubMed] [Google Scholar]
- 22. Walsh RA, Lang AE. . Multiple impulse control disorders developing in Parkinson's disease after initiation of amantadine. Mov Disord. 2012; 27 2: 326 DOI: 10.1002/mds.23964. PubMed PMID: 21954056. [DOI] [PubMed] [Google Scholar]
- 23. Weintraub D, Papay K, Siderowf A. . Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology. 2013; 80 2: 176- 80. DOI: 10.1212/WNL.0b013e31827b915c. PubMed PMID: 23296128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weintraub D, Hoops S, Shea JA, Lyons KE, Pahwa R, Driver-Dunckley ED, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson's disease. Mov Disord. 2009; 24 10: 1461- 7. DOI: 10.1002/mds.22571. PubMed PMID: 19452562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, Herranz Barcenas A, Vela L, Sanchez Alonso P, et al. Impulse control disorder in patients with Parkinson's disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry. 2014; 85 8: 840- 4. DOI: 10.1136/jnnp-2013-306787. PubMed PMID: 24434037. [DOI] [PubMed] [Google Scholar]
- 26. Rana AQ, Mansoor W, Hussaini S, Al Mosabbir A, Rahman M, Rahman L. . Factors associated with the development of impulse compulsive disorders in Parkinson patients. Int J Neurosci. 2013; 123 7: 503- 6. DOI: 10.3109/00207454.2013.768243. PubMed PMID: 23336737. [DOI] [PubMed] [Google Scholar]
- 27. Isaias IU, Siri C, Cilia R, De Gaspari D, Pezzoli G, Antonini A. . The relationship between impulsivity and impulse control disorders in Parkinson's disease. Mov Disord. 2008; 23 3: 411- 5. DOI: 10.1002/mds.21872. PubMed PMID: 18067187. [DOI] [PubMed] [Google Scholar]
- 28. Ahlskog JE. . Pathological behaviors provoked by dopamine agonist therapy of Parkinson's disease. Physiol Behav. 2011; 104 1: 168- 72. DOI: 10.1016/j.physbeh.2011.04.055. PubMed PMID: 21557955. [DOI] [PubMed] [Google Scholar]
- 29. Leroi I, Harbishettar V, Andrews M, McDonald K, Byrne EJ, Burns A. . Carer burden in apathy and impulse control disorders in Parkinson's disease. Int J Geriatr Psychiatry. 2012; 27 2: 160- 6. DOI: 10.1002/gps.2704. PubMed PMID: 21462269. [DOI] [PubMed] [Google Scholar]
- 30. Phu AL, Xu Z, Brakoulias V, Mahant N, Fung VSC, Moore GD, et al. Effect of impulse control disorders on disability and quality of life in Parkinson's disease patients. J Clin Neurosci. 2014; 21 1: 63- 6. DOI: 10.1016/j.jocn.2013.02.032. PubMed PMID: 24035421. [DOI] [PubMed] [Google Scholar]
- 31. Kadastik-Eerme L, Rosenthal M, Paju T, Muldmaa M, Taba P. . Health-related quality of life in Parkinson's disease: a cross-sectional study focusing on non-motor symptoms. Health Qual Life Outcomes. 2015; 13: 83 DOI: 10.1186/s12955-015-0281-x. PubMed PMID: 26088201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baumann-Vogel H, Valko PO, Eisele G, Baumann CR. . Impulse control disorders in Parkinson's disease: don't set your mind at rest by self-assessments. Eur J Neurol. 2015; 22 4: 603- 9. DOI: 10.1111/ene.12646. PubMed PMID: 25598147. [DOI] [PubMed] [Google Scholar]
- 33. Mamikonyan E, Siderowf AD, Duda JE, Potenza MN, Horn S, Stern MB, et al. Long-term follow-up of impulse control disorders in Parkinson's disease. Mov Disord. 2008; 23 1: 75- 80. DOI: 10.1002/mds.21770. PubMed PMID: 17960796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samuel M, Rodriguez-Oroz M, Antonini A, Brotchie JM, Chaudhuri KR, Brown RG, et al. Management of impulse control disorders in Parkinson's disease: controversies and future approaches. Mov Disord. 2015; 30 2: 150- 9. DOI: 10.1002/mds.26099. PubMed PMID: 25607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rabinak CA, Nirenberg MJ. . Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol. 2010; 67 1: 58- 63. DOI: 10.1001/archneurol.2009.294. PubMed PMID: 20065130. [DOI] [PubMed] [Google Scholar]
- 36. Lindahl AJ, MacMahon DG. . Managing dopamine agonist withdrawal syndrome in Parkinson's disease. Prog Neurol Psychiat. 2011; 15 4: 4. [Google Scholar]
- 37. Pondal M, Marras C, Miyasaki J, Moro E, Armstrong MJ, Strafella AP, et al. Clinical features of dopamine agonist withdrawal syndrome in a movement disorders clinic. J Neurol Neurosurg Psychiatry. 2013; 84 2: 130- 5. DOI: 10.1136/jnnp-2012-302684. PubMed PMID: 22933817. [DOI] [PubMed] [Google Scholar]
- 38. Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M. . Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol. 2010; 68 3: 400- 4. DOI: 10.1002/ana.22029. PubMed PMID: 20687121. [DOI] [PubMed] [Google Scholar]
- 39. Papay K, Xie SX, Stern M, Hurtig H, Siderowf A, Duda JE, et al. Naltrexone for impulse control disorders in Parkinson disease: a placebo-controlled study. Neurology. 2014; 83 9: 826- 33. DOI: 10.1212/WNL.0000000000000729. PubMed PMID: 25037206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiménez-Murcia S, Bove FI, Israel M, Steiger H, Fernández-Aranda F, Alvarez-Moya E, et al. Cognitive-behavioral therapy for pathological gambling in Parkinson's disease: a pilot controlled study. Eur Addict Res. 2012; 18 6: 265- 74. DOI: 10.1159/000337442. PubMed PMID: 22760081. [DOI] [PubMed] [Google Scholar]
- 41. Okai D, Askey-Jones S, Samuel M, O'Sullivan SS, Chaudhuri KR, Martin A, et al. Trial of CBT for impulse control behaviors affecting Parkinson patients and their caregivers. Neurology. 2013; 80 9: 792- 9. DOI: 10.1212/WNL.0b013e3182840678. PubMed PMID: 23325911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Catalán MJ, de Pablo-Fernández E, Villanueva C, Fernández-Diez S, Lapeña-Montero T, García-Ramos R, et al. Levodopa infusion improves impulsivity and dopamine dysregulation syndrome in Parkinson's disease. Mov Disord. 2013; 28 14: 2007- 10. DOI: 10.1002/mds.25636. PubMed PMID: 24123193. [DOI] [PubMed] [Google Scholar]
- 43. Ardouin C, Voon V, Worbe Y, Abouazar N, Czernecki V, Hosseini H, et al. Pathological gambling in Parkinson's disease improves on chronic subthalamic nucleus stimulation. Mov Disord. 2006; 21 11: 1941- 6. DOI: 10.1002/mds.21098. PubMed PMID: 16972268. [DOI] [PubMed] [Google Scholar]
- 44. Lhommée E, Klinger H, Thobois S, Schmitt E, Ardouin C, Bichon A, et al. Subthalamic stimulation in Parkinson's disease: restoring the balance of motivated behaviours. Brain. 2012; 135 Pt 5: 1463- 77. [DOI] [PubMed] [Google Scholar]
- 45. Broen M, Duits A, Visser-Vandewalle V, Temel Y, Winogrodzka A. . Impulse control and related disorders in Parkinson's disease patients treated with bilateral subthalamic nucleus stimulation: a review. Parkinsonism Relat Disord. 2011; 17 6: 413- 7. [DOI] [PubMed] [Google Scholar]
- 46. Moum SJ, Price CC, Limotai N, Oyama G, Ward H, Jacobson C, et al. Effects of STN and GPi deep brain stimulation on impulse control disorders and dopamine dysregulation syndrome. Plos One. 2012; 7 1: e29768 DOI: 10.1371/journal.pone.0029768. PubMed PMID: 22295068. [DOI] [PMC free article] [PubMed] [Google Scholar]