ABSTRACT
Background
Overhydration (OH) is associated with mortality in chronic kidney disease (CKD). A relative overhydration adjusted for extracellular water (OH/ECW) measured by bioimpedance >15% has shown an increased mortality risk in haemodialysis but few studies have been developed in advanced CKD. Our objective was to evaluate the effect of OH on mortality in patients with Stage 4 or 5 non-dialysis CKD.
Methods
We performed a prospective study of 356 patients enrolled in 2011 and followed up until 2016. At baseline we collected general characteristics, serum inflammatory and nutrition markers, cardiovascular events (CVEs) and body composition using bioimpedance spectroscopy. During a median follow-up of 50 (24–66) months we collected mortality data.
Results
The mean creatinine was 3.5 ± 1.3 mg/dL, median proteinuria was 0.5 [interquartile range (IQR) 0.2–1.5] g/24 h, median OH was 0.6 (IQR −0.4–1.5) L and mean relative OH (OH/ECW) was 2.3 ± 0.8%. We found that 32% of patients died. The univariate Cox analysis showed an association between mortality and age, diabetes, previous CVEs, Charlson comorbidity index, low albumin and pre-albumin, high C-reactive protein (CRP), low lean tissue and high OH/ECW. Multivariate Cox analysis confirmed an association between mortality and age {exp(B) 1.1 [95% confidence interval (CI) 1.0–1.3]; P = 0.001}, Charlson comorbidity index [exp(B) 1.1 (95% CI 1.0–1.2); P = 0.01], CRP [exp(B) 1.1 (95% CI 1.0–1.2); P = 0.04], OH/ECW [exp(B) 3.18 (95% CI 2.09–4.97); P = 0.031] and low lean tissue [exp(B) 0.82 (95% CI 0.69–0.98); P = 0.002]. Kaplan–Meier analysis confirmed higher mortality in patients with OH/ECW >0% (log rank 11.1; P = 0.001).
Conclusion
Any grade of relative OH measured by OH/ECW >0% is associated with long-term mortality in patients with Stage 4 or 5 non-dialysis CKD.
Keywords: bioimpedance, cardiovascular events, chronic kidney disease, mortality, overhydration
INTRODUCTION
Patients with advanced chronic kidney disease (CKD) have an increased risk of developing cardiovascular events (CVEs) and mortality [1, 2]. Chronic fluid overload has been shown to be a mortality and morbidity risk factor in patients undergoing haemodialysis [3, 4]. An association between fluid overload and morbidity in CKD has also been reported [5–7]. However, hydration status may be difficult to assess due to the imprecision of clinical findings such as arterial hypertension, pulmonary and peripheral oedema and heart failure, which is not always present on examination. Therefore, objective tools to estimate hydration status and body composition are needed. Bioimpedance spectroscopy (BIS) is a simple and non-invasive technique based on tissue resistance to the flow of an alternating current ranging from 5 to 1000 kHz in frequency [8–11]. It has been validated using reference methods. BIS is therefore a useful and reliable tool for assessing body composition and hydration status [12–14].
BIS allows one to estimate overhydration adjusted for extracellular water (OH/ECW). Values >15% have been shown to be a risk factor for mortality and morbidity in patients on haemodialysis [3]. A study demonstrated an association between fluid overload and traditional and novel risk factors for CV disease such as inflammation, proteinuria, male sex, age or diabetes in the CKD population [15]. Another study showed an association between relative overhydration >7% and mortality in patients with advanced CKD [7].
The objective of this study was to evaluate the effect of OH on mortality in patients with Stage 4 or 5 non-dialysis CKD.
MATERIALS AND METHODS
Design of the study
This is a prospective study that started in January 2011 and patients were followed up until December 2016. Variables were collected at the beginning of the study and at the end of the study we collected mortality data. Patients who started renal replacement therapy during follow-up were excluded.
Study population
A total of 370 Stage 4 or 5 non-dialysis CKD patients were enrolled in this study at a single centre in Madrid, Spain. CKD was defined and staged according to Kidney Disease Outcomes Quality Initiative guidelines using the Modification of Diet in Renal Disease four-variable equation (MDRD4) [16]. Inclusion criteria were clinical stability with no recent hospitalization in the past 3 months, outpatient follow-up for >3 months, age >18 years and informed consent obtained. Exclusion criteria were an inability to understand the study, contraindication to BIS (patients with pacemakers or limb amputation), loss of laboratory parameters or loss to follow-up. Of these, 17 patients were finally excluded.
Baseline variables
At baseline, demographic and clinical data were collected, including age, sex, CKD aetiology, presence of diabetes mellitus, hypertension (defined using the Eighth Report of the Joint National Committee) [17], dyslipidaemia (defined using Adult Treatment Panel III guidelines) [18], Charlson comorbidity index [19] and a history of prior heart disease including congestive heart failure (CHF) and myocardial infarction, peripheral vascular disease (PVD) and stroke.
Fluid status was assessed at baseline using BIS (body composition monitor, Fresenius Medical Care, Bad Homburg, Germany). Measurements were taken after a 10-min resting period in the supine position. Hydration parameters were total body water [TBW (in litres)], ECW (in litres), intracellular water [ICW (in litres)] and fluid overload [OH (in litres)], defined as water not included in extracellular and intracellular spaces and considered as excess water. We estimated relative OH by dividing OH by ECW (OH/ECW) [6]. We also collected lean tissue index [LTI (in kg/m2)] and fat tissue index [FTI (in kg/m2)] data.
Laboratory variables recorded included creatinine and glomerular filtration rate (MDRD4 equation), proteinuria, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), nutritional and inflammatory parameters (pre-albumin and albumin), and C-reactive protein (CRP).
Follow-up
During follow-up {50 [interquartile range (IQR) 24–66] months}, new CVEs and mortality were recorded. CVEs were defined as ischaemic or haemorrhagic cerebrovascular accident (diagnosed by computed tomography), myocardial infarction (diagnosed by cardiac marker elevation and electrocardiography and confirmed by coronary angiography), CHF (diagnosed by the presence of acute pulmonary oedema and an echocardiogram with ventricular systolic dysfunction and left ventricular ejection fraction <45%) and peripheral vascular events (diagnosed by stenosis of major arteries or lower limbs confirmed by arteriography and/or the need for amputation).
Mortality aetiology was classified as CV, tumoural, infection, rejection to start renal replacement therapy and other.
Statistical analysis
All variables were analysed using a Kolmogorov–Smirnov test to classify them as normally or non-normally distributed. Values are given as mean ± SD or median (IQR). Univariate analysis was performed using logistic regression to assess factors associated with mortality and CVEs. A multivariate Cox regression analysis was performed in order to establish independent predictors of mortality and CVEs. The models included factors that showed a significant association or those considered confounding factors. Outcomes were analysed using Kaplan–Meier plots and survival curves were compared using a log-rank test. All statistical analyses were performed with SPSS 18.0 software (SPSS, Chicago, IL, USA). P-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics
We included 356 patients; 64% were male with a mean age of 67 ± 13 years. Baseline characteristics are shown in Table 1. The mean creatinine was 3.5 ± 1.3 mg/dL, mean glomerular filtration rate (MDRD equation) was 16 mL/min/1.73 m2 and median proteinuria was 0.5 (IQR 0.2–1.5) g/24 h. Forty-three percent of patients were receiving diuretics. The median OH was 0.6 (IQR −0.4–1.5) L and mean relative OH (OH/ECW) was 2.3 ± 0.8%. The percentage of patients with a lower mean relative OH (OH/ECW <2.3 ± 0.8%) was 44% and the percentage with a higher mean relative OH (OH/ECW >2.3 ± 0.8%) was 56%.
Table 1.
Baseline characteristics of the study population (n = 356)
| Characteristics | Value |
|---|---|
| General characteristics (%) | |
| Sex (male) | 64 |
| Age (years), mean ± SD | 67 ± 13 |
| Charlson comorbidity index, mean ± SD | 7.2 ± 2.7 |
| Diabetes | 36 |
| Hypertension | 87 |
| Dyslipidaemia | 72 |
| CKD aetiology (%) | |
| Glomerular | 23 |
| Diabetes | 19 |
| Vascular | 28 |
| Interstitial | 13 |
| Polycystic | 10 |
| Other | 7 |
| Previous CVE (%) | |
| Myocardial Infarction | 28 |
| CHF | 27 |
| Stroke | 15 |
| Peripheral vascular disease (%) | 12 |
| Laboratory parameters | |
| Creatinine (mg/dL), mean ± SD | 3.5 ± 1.5 |
| MDRD (mL/min/1.73 m2), mean ± SD | 16.0 ± 5.5 |
| Proteinuria (g/24 h), median (IQR) | 0.5 (0.2–1.5) |
| Albumin (g/dL), mean ± SD | 4.1 ± 0.4 |
| NT-proBNP (ng/dL), median (IQR) | 84 (37–181) |
| CRP (mg/dL), median (IQR) | 0.3 (0.1–0.7) |
| Pre-albumin (mg/dL), median (IQR) | 32 (27–38) |
| Hydration statement and corporal composition | |
| BMI (kg/m2), mean ± SD | 28.0 ± 5.2 |
| FTI (kg/m2), mean ± SD | 12.3 ± 5.6 |
| LTI (kg/m2), mean ± SD | 15.7 ± 3.4 |
| OH (L), median (IQR) | 0.6 (−0.4–1.5) |
| ECW (L), mean ± SD | 17.0 ± 3.5 |
| ICW (L), mean ± SD | 19.7 ± 4.7 |
| ECW/ICW, mean ± SD | 0.8 ± 0.1 |
| OH/ECW (%), mean ± SD | 2.3 ± 0.8 |
BMI, body mass index; CHF, congestive heart failure; CKD, chronic kidney disease; CRP, C-reactive protein; ECW, extracellular water; FTI, fat tissue index; ICW, intracellular water; LTI, lean tissue index; MDRD, MDRD equation to estimate glomerular filtration rate; Nt-ProBNP, N-terminal prohormone of brain natriuretic peptide; OH, overhydration; PVD, Peripheral vascular disease.
A total of 236 patients (66%) had relative OH (OH/ECW) >0% and 120 patients (34%) had a relative OH (OH/ECW) <0%.
CVEs at the end of follow-up
A total of 150 patients (42%) experienced a CVE during follow-up. The most common CVE was CHF (47%), as well as myocardial infarction (28%), peripheral vascular disease (13%) and cerebrovascular accident (12%).
Univariate analysis showed that older age, diabetes, hypertension, dyslipidaemia, previous CVE, higher Charlson comorbidity index, proteinuria, CRP, NT-proBNP levels, lower albumin levels and impaired kidney function were associated with CVEs. Multivariate analysis confirmed an independent association between proteinuria [exp(B) 1.1; P = 0.001], CRP [exp(B) B 1.2; P = 0.02], NT-proBNP [exp(B) 1.2; P = 0.01], impaired kidney function [exp(B) 0.7; P = 0.03] and previous CVEs [exp(B) 2.7; P = 0.001] and development of CVEs during follow-up.
Mortality
We found that 113 patients (32%) died during follow-up. Mortality causes were CV, 19%; tumoural, 7%; infections, 3%; conservative treatment, 1% and other, 2%.
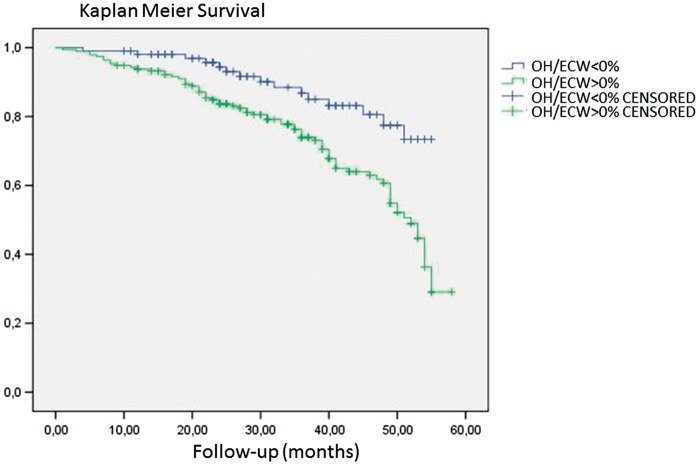
The univariate Cox analysis showed an association between mortality and age, diabetes, Charlson comorbidity index, previous CVE, low albumin level, low pre-albumin, high levels of CRP, high OH, low LTI and high OH/ECW (Table 2). Multivariate Cox analysis showed an independent association with mortality and age, Charlson comorbidity index, higher CRP levels, low LTI and relative OH (OH/ECW) as seen in Table 2. We divided patients into two groups (OH/ECW <0% and OH/ECW >0%). Kaplan–Meier analysis confirmed higher mortality in patients with OH/ECW >0% (log rank 11.1; P= 0.001) as seen in Figure 1.
Table 2.
Cox proportional hazards regression analysis for mortality
| Baseline characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Sex (male, %) | 1.35 (0.76–1.79) | 0.34 | ||
| Age (years) | 1.08 (1.01–1.20) | 0.001 | 1.1 (1.0–1.3) | 0.001 |
| Diabetes (%) | 1.50 (1.20–1.98) | 0.024 | 1.30 (0.62–1.87) | 0.32 |
| Hypertension (%) | 1.65 (0.33–1.98) | 0.76 | ||
| Dyslipidaemia (%) | 1.43 (0.54–1.87) | 0.45 | ||
| Charlson comorbidity index | 1.27 (1.03–1.74) | 0.02 | 1.1 (1.0–1.2) | 0.01 |
| Previous global CVEs | 1.50 (1.19–1.99) | 0.001 | 1.59 (0.89–3.30) | 0.26 |
| Creatinine (mg/dL) | 1.02 (0.89–1.18) | 0.34 | ||
| Proteinuria (g/24 h) | 1.001 (0.99–1.01) | 0.69 | ||
| Albumin (g/dL) | 0.38 (0.12–0.79) | 0.001 | 0.84 (0.68–1.12) | 0.54 |
| Prealbumin (mg/dL) | 0.94 (0.56–0.99) | 0.001 | 0.99 (0.89–1.23) | 0.31 |
| CRP (mg/dL) | 1.20 (1.03–1.78) | 0.01 | 1.3 (1.1–1.9) | 0.04 |
| NT-proBNP (ng/dL) | 1.00 (0.99–1.01) | 0.677 | ||
| Cholesterol | 0.99 (0.98–1.01) | 0.07 | ||
| OH (L) | 1.1 (1.02–1.19) | 0.01 | 1.10 (0.99–1.20) | 0.08 |
| ECW (L) | 0.96 (0.88–1.05) | 0.640 | ||
| ICW (L) | 0.88 (0.81–1.04) | 0.12 | ||
| OH/ECW (%) | 3.10 (1.88–5.01) | 0.001 | 3.18 (2.09–4.97) | 0.031 |
| BMI (kg/m2) | 0.98 (0.93–1.04) | 0.650 | ||
| FTI (kg/m2) | 1.04 (0.89–1.08) | 0.124 | ||
| LTI (kg/m2) | 0.85 (0.33–0.97) | 0.001 | 0.82 (0.69–0.98) | 0.002 |
Bold significance is for P-values <0.05.
BMI, body mass index; CRP, C-reactive protein; CVev, cardiovascular events; ECW, extracellular water; FTI, fat tissue index; ICW, intracellular water; LTI, lean tissue index; Nt-ProBNP, N-terminal prohormone of brain natriuretic peptide; OH, overhydration; OH/ECW, Relation between overhydration and extracellular water.
FIGURE 1:
Kaplan–Meier analysis for mortality and relative OH. Log rank 11.1; P = 0.001.
Start of renal replacement therapy
A total of 125 patients (35%) needed dialysis during follow-up.
DISCUSSION
This study shows that any grade of relative OH in patients with advanced CKD is a long-term independent mortality risk factor. Thus minimal OH, even difficult to detect on examination, is related to mortality. The majority of studies related to OH and mortality have been performed in patients receiving renal replacement therapy. Wizemann et al. [9] studied the relation between relative OH and mortality in patients receiving chronic haemodialysis and established a cut-off of relative overhydration >15%. We found in a transverse study in haemodialysis patients an association between CVEs and relative OH. This association was maintained even when we decreased the cut-off to 10% [20]. In patients receiving peritoneal dialysis, the same association between mortality and relative OH >15% has been found, so this association could not be dependent on the type of renal replacement therapy chosen by patients [21]. Tsai et al. [7] studied relative OH in advanced CKD non-dialysis patients and found a significant cut-off for mortality of 7%. Based on our results, we propose to decrease the cut-off to as low as possible, because any degree of relative OH in CKD is harmful and, if possible, should be avoided.
The most common mortality cause in advanced CKD, as in our group of patients, is CV. Some known CV factors (traditional and novel ones), such as male sex, age and diabetes, are associated and increase in prevalence with overhydration. All of these, including inflammatory and cardiac biomarkers and a history of CV disease have been demonstrated as independent risk factors for CVEs and mortality in the CKD population [22–24]. The main finding of this study is that OH by itself is also an independent long-term mortality risk factor. Therefore BIS can help detect high-risk patients, as it is an easy and useful tool [17]. Probably, and although our study lacks serial measurements, performing BIS more frequently, for example, at each clinical visit, could improve patient survival [25]. Those patients with OH could then receive closer follow-up, adjusting diuretics if necessary and receiving more frequent visits to the nephrologist.
Body composition may influence mortality in advanced CKD as we previously described [26]. Low LTI is associated with mortality. Lean tissue is related to physical activity, so we encourage our patients to practice exercise regularly. Other significant factors such as age, Charlson comorbidity index and high levels of CRP are frequent variables related to mortality in the general population and in CKD [27, 28]. Another interesting finding is that the association between mortality and other risk factor such as previous CVEs, diabetes and low pre-albumin level were not significant in our study and relative OH and low lean tissue had an independent association.
CVEs were recorded in 150 patients. Nearly half of these patients had CHF, and this frequent association contributes to the development of cardiorenal syndrome, which explains the morbidity of this association [29]. A significant number of patients suffered from myocardial infarction. Chronic disease has been shown to increase mortality in patients with ischaemic heart disease [30]. Monitoring of serum cardiac biomarkers may therefore be helpful to detect these conditions, as NT-proBNP was associated to the development of CVEs in our study. As regards mortality, CVEs were also the most important cause of death. For these reasons, we suggest that new strategies to detect early CVEs should be developed to improve the survival of our CKD patients. Meanwhile, the use of BIS to monitor fluid overload may be helpful.
Our study has some limitations. Only one BIS measurement was available for each patient and no additional BIS measurements could be done to evaluate their changes and consequences. We did not measure urine sodium and sodium intake, which could be involved in oedema formation.
In conclusion, any grade of relative OH measured by OH/ECW >0% is associated with long-term mortality in patients with Stages 4 and 5 CKD.
ACKNOWLEDGEMENTS
The authors would like to thank Thomas O’Boyle for proofreading the manuscript.
AUTHORS’ CONTRIBUTIONS
A.V., S.A., I.A., N.M. and J.L. designed the study. A.G-P., T.L., E.T. and A.H. performed BIS. N.M. gave informed consent. A.V., S.A. and I.A. collected data. A.V. wrote the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- 1. Holzmann M, Jernberg T, Szummer K. et al. Long-term cardiovascular outcomes in patients with chronic kidney disease undergoing coronary artery bypass graft surgery for acute coronary syndromes. J Am Heart Assoc 2014; 3: e000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Betriu A, Martinez-Alonso M, Arcidiacono MV. et al. Prevalence of subclinical atheromatosis and associated risk factors in chronic kidney disease: the NEFRONA study. Nephrol Dial Transplant 2013; 29: 1415–1422 [DOI] [PubMed] [Google Scholar]
- 3. Wizemann V, Wabel P, Chamney P. et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009; 24: 1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hecking M, Karaboyas A, Antlanger M. et al. Significance of interdialytic weight gain versus chronic volume overload: consensus opinion. Am J Nephrol 2013; 38: 78–90 [DOI] [PubMed] [Google Scholar]
- 5. Caravaca F, Martínez del Viejo C, Villa J. et al. Hydration status assessment by multi-frequency bioimpedance in patients with advanced chronic kidney disease. Nefrologia 2011; 31: 537–44 [DOI] [PubMed] [Google Scholar]
- 6. Tsai Y-C, Tsai J-C, Chiu Y-W. et al. Is fluid overload more important than diabetes in renal progression in late chronic kidney disease? PLoS One 2013; 8: e82566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai YC, Chiu JC, Tsai JC. et al. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol 2015; 10: 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chazot C, Wabel P, Chamney P. et al. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol Dial Transplant 2012; 27: 2404–2410 [DOI] [PubMed] [Google Scholar]
- 9. Wizemann V, Rode C, Wabel P.. Whole-body spectroscopy (BCM) in the assessment of normovolemia in hemodialysis patients. Contrib Nephrol 2008; 161: 115–118 [DOI] [PubMed] [Google Scholar]
- 10. Machek P, Jirka T, Moissl U. et al. Guided optimization of fluid status in haemodialysis patients. Nephrol Dial Transplant 2010; 25: 538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Earthman C, Traughber D, Dobratz J. et al. Bioimpedance spectroscopy for clinical assessment of fluid distribution and body cell mass. Nutr Clin Pract 2007; 22: 389–405 [DOI] [PubMed] [Google Scholar]
- 12. Wabel P, Chamney P, Moissl U. et al. Importance of whole body bioimpedance spectroscopy for the management of the fluid balance. Blood Purif 2009; 27: 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotanko P, Levin W, Zhu F.. Current state of bioimpedance technologies in dialysis. Nephrol Dial Transplant 2008; 23: 808–812 [DOI] [PubMed] [Google Scholar]
- 14. Basile C, Vernaglione L, Di Iorio B. et al. Development and validation of bioimpedance analysis prediction equations for dry weight in hemodialysis patients. Clin J Am Soc Nephrol 2007; 2: 675–680 [DOI] [PubMed] [Google Scholar]
- 15. Hung S-C, Kuo K-L, Peng C-H. et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 2014; 85: 703–709 [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Coresh J, Greene T. et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254 [DOI] [PubMed] [Google Scholar]
- 17. James PA, Oparil S, Carter BL. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–520 [DOI] [PubMed] [Google Scholar]
- 18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421 [PubMed] [Google Scholar]
- 19. Charlson M, Szatrowski TP, Peterson J. et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251 [DOI] [PubMed] [Google Scholar]
- 20. Vega A, Quiroga B, Abad S. et al. Study on overhydration in dialysis patients and its association with inflammation. Nefrologia 2014; 34: 579–583 [DOI] [PubMed] [Google Scholar]
- 21. Jotterand Drepper V, Kihm LP, Kälble F. et al. Overhydration is a strong predictor of mortality in peritoneal dialysis patients - independently of cardiac failure. PLoS One 2016; 11: e0158741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bae EH, Lim SY, Cho KH. et al. GFR and cardiovascular outcomes after acute myocardial infarction: results from the Korea Acute Myocardial Infarction Registry. Am J Kidney Dis 2012; 59: 795–802 [DOI] [PubMed] [Google Scholar]
- 23. Lou Q-L, Ouyang X-J, Gu L-B. et al. Chronic kidney disease and associated cardiovascular risk factors in Chinese with type 2 diabetes. Diabetes Metab J 2012; 36: 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agrawal V, Marinescu V, Agarwal M. et al. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol 2009; 6: 301–311 [DOI] [PubMed] [Google Scholar]
- 25. Verdalles U, de Vinuesa SG, Goicoechea M. et al. Utility of bioimpedance spectroscopy (BIS) in the management of refractory hypertension in patients with chronic kidney disease (CKD). Nephrol Dial Transplant 2012; 27: iv31–iv35 [DOI] [PubMed] [Google Scholar]
- 26. Vega A, Abad S, Macías N. et al. Low lean tissue mass is an independent risk factor for mortality in patients with stage 4 and 5 non-dialysis chronic kidney disease. Clin Kidney J 2017; 10: 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gregg LP, Adams-Huet B, Li X. et al. Effect modification of chronic kidney disease on the association of circulating and imaging cardiac biomarkers with outcomes. J Am Heart Assoc 2017; 6: e005235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doi T, Yamamoto S, Morinaga T. et al. Risk score to predict 1-year mortality after haemodialysis initiation in patients with stage 5 chronic kidney disease under predialysis nephrology care. PLoS One 2015; 10: e0129180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ronco C, Di Lullo L.. Cardiorenal syndrome. Heart Fail Clin 2014; 10: 251–280 [DOI] [PubMed] [Google Scholar]
- 30. Sabroe JE, Thayssen P, Antonsen L. et al. Impact of renal insufficiency on mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. BMC Cardiovasc Disord 2014; 14: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]