Abstract
Adolescence is a time of increased social-affective sensitivity, which is often related to heightened health-risk behaviors. However, moderate levels of social sensitivity, relative to either low (social vacuum) or high levels (exceptionally attuned), may confer benefits as it facilitates effective navigation of the social world. The present fMRI study tested a curvilinear relationship between social sensitivity and adaptive decision-making. Participants (ages 12–16; N = 35) played the Social Analogue Risk Task, which measures participants’ willingness to knock on doors in order to earn points. With each knock, the facial expression of the house’s resident shifted from happy to somewhat angrier. If the resident became too angry, the door slammed and participants lost points. Social sensitivity was defined as the extent to which adolescents adjusted their risky choices based on shifting facial expressions. Results confirmed a curvilinear relationship between social sensitivity and self-reported adaptive decision-making at the behavioral and neural level. Moderate adolescent social sensitivity was modulated via heightened tracking of social cues in the temporoparietal junction, insula and dorsolateral prefrontal cortex and related to adaptive decision-making. These findings suggest that social-affective sensitivity may positively impact outcomes in adolescence and have implications for interventions to help adolescents reach mature social goals into adulthood.
Keywords: adolescence, social sensitivity, risk taking, facial expression, adaptive decision-making
Introduction
Adolescence, the developmental period between childhood and adulthood, is characterized by heightened social-affective sensitivity (Crone and Dahl, 2012; Blakemore and Mills, 2014). An important developmental milestone during this time is to learn to effectively navigate the social world (Blakemore and Mills, 2014). There are tremendous maturational changes in how the brain codes and generates responses to social information throughout adolescence (Nelson et al., 2005; Nelson and Guyer, 2011; Nelson et al., 2016). These changes in the brain equip adolescents with the tools to navigate the increasingly complex social world. Indeed, adolescents show a uniquely heightened sensitivity to social experiences, and moderate levels of adolescent social sensitivity may be considered adaptive in order to meet this social developmental milestone. However, being in a social vacuum (low social sensitivity) or being exceptionally attuned to one’s social environment (high social sensitivity) likely limits an individual’s ability to successfully interact with others (Nelson and Guyer, 2011; Blakemore and Mills, 2014). Hence, this study tests whether moderate levels, as compared to low or high levels of social sensitivity, confer benefits in the context of risky decision-making.
Social sensitivity and the developing brain
Adolescence is a period of changing social dynamics, with an increased saliency of social acceptance and rejection, changing social relationships with parents and peers, and a tendency to explore societal boundaries in many different ways (Crone and Dahl, 2012; Nelson et al., 2016). Adolescents begin to make increasingly independent decisions about how to navigate the complex social world based on limited experiences (Ellis et al., 2012; McLaughlin et al., 2015). Indeed, strategic exploration (i.e. making choices that provide new experiences and information) emerges rapidly in adolescence and is related to the propensity for risk-taking behaviors in daily life (Somerville et al., 2017). Exploration during adolescence has recently been linked to adaptive behaviors in a risky context, such as learning and lower perceptions of real-world risk taking (Goldenberg et al., 2017; McCormick and Telzer, 2017).
In order to effectively interact with a wider range of social agents, adolescents need to develop more complex social cognitive abilities (Blakemore and Mills, 2014). For example, they interact with different teachers for each course in school, develop more dynamic peer relationships and begin exploring romantic interests without direct scaffolding from parents. In order to navigate these more complex social relationships, social cognitive abilities are required, which include being attuned to and adequately processing social cues, engaging in mentalizing processes to consider what others are thinking and feeling, as well as flexibly adjusting behaviors based on social feedback (Nelson and Guyer, 2011; Somerville, 2013). Within the broader domain of social cognition, social sensitivity can be defined as a shifting motivation that intensifies the attention, salience, and emotion implicated in processing social cues (Somerville, 2013). Significant developmental changes in the adolescent brain may partly underlie this shifting motivation in social sensitivity.
A collection of brain regions, including basic affective regions, social brain regions and cognitive control regions likely work in concert in the process of social sensitivity. In regards to affective regions, a key aspect of social sensitivity is processing social cues from facial expressions. Recruitment of the amygdala, anterior insula (AI), and superior temporal sulcus allow individuals to recognize emotions in others (Cohen Kadosh et al., 2013b; Fuhrmann et al., 2016). Indeed, neural processing of facial expressions develops throughout adolescence, with sensitivity to social feedback peaking in this network during adolescence (Guyer et al., 2009, 2012; Jones et al., 2014). A higher-order aspect of social sensitivity is understanding and acting on more complex social emotions. That is, emotions that require the representation of other people’s mental states, or mentalizing (Burnett et al., 2009). Activation in the social brain network required for mentalizing, including the medial prefrontal cortex (mPFC) and temporoparietal junction (TPJ), is greater in adolescence than children or adults when evaluating social emotions (Burnett et al., 2009; Somerville, 2013). Finally, this network of affective and social brain regions works together with the cognitive-regulatory network (e.g. lateral prefrontal cortex, lPFC) to support the execution of goal-directed and flexible social behaviors, for example after social feedback (Nelson and Guyer, 2011; Casey, 2015). In sum, social sensitivity is supported by a network of regions including affective processing, social cognition and cognitive control.
A curvilinear relationship between social sensitivity and adaptive outcomes
A recent neurobiological susceptibility model posits that adolescent development is shaped by brain-based individual differences in sensitivity to social context (Scriber and Guyer, 2017). In particular, the degree to which adolescents are tuned to their environment may be calibrated through individual differences in structural and functional neural characteristics. Moderate levels of social sensitivity and recruitment of social-affective neurocircuitry are likely related to adaptive outcomes during adolescence, because this is crucial for competently interacting with others and flexible social behavior, as well as normative exploration and risk taking (Nelson and Guyer, 2011; Scriber and Guyer, 2017). However, too much or too little social sensitivity may be related to maladaptive psychosocial outcomes, as each hinders effectively navigating the social world.
On the more extreme end of the continuum, high social sensitivity may be related to maladaptive outcomes, as research has shown that greater social-affective sensitivity is related to heightened health-risk behaviors (Chein et al., 2011; Heller and Casey, 2016). Drawing from clinical work, highly socially anxious individuals are hyper-attentive to social cues and tend to have lower thresholds to detect angry faces, which results in impaired social functioning in daily life (Gilboa-Schechtman and Shachar-Lavie, 2013). In line with these findings, chronically victimized adolescent girls as compared to non-victimized girls show greater risk-taking behavior after an episode of exclusion which is mediated by greater activation in regions involved in affective sensitivity, social cognition and cognitive control (Telzer et al., 2017). On the other end of the continuum, detriments in social sensitivity are apparent in individuals along the autism spectrum, who experience psychosocial difficulties because of impairments in social cognition (Lai, Lombardo and Baron-Cohen, 2014). Individuals along the autism spectrum tend to show reduced sensitivity to social cues, which is modulated by diminished activity in social cognition and affective brain regions (for a meta-analysis, see Philip et al., 2012). Taken together, empirical and theoretical work suggests a curvilinear relationship between social sensitivity and adaptive decision-making—either too little or too much social sensitivity may be maladaptive, whereas a moderate level of social sensitivity may be associated with adaptive outcomes. While prior work suggests impairments in social sensitivity at both the extremely high end (e.g. socially anxious) or extremely low end (e.g. autism spectrum), we do not currently know how variability in social sensitivity within a normative adolescent sample is linked to adaptive outcomes in a risky context.
Present study
The goal of the present fMRI study was to test the hypothesized curvilinear relationship between social sensitivity on a behavioral and neural level and adaptive decision-making in adolescence. We were specifically interested in individual differences in social sensitivity in the context of risky decision-making and employed the novel Social Analogue Risk Task (SART), a social adaptation of the well-validated Balloon Analogue Risk Task (Lejuez et al., 2002; Humphreys et al., 2016). The SART is a ‘trick-or-treat game’ that measures participants’ willingness to knock on doors in order to earn points. With each knock on a door, points increase while the facial expression of the house’s resident morphs from happy to somewhat angrier. Knocking is associated with an increasing risk, because if the resident gets too angry and slams the door, all points for that door are lost. Crucially, some residents are faster to get angry than others, and so adolescents need to flexibly adapt their risk-taking behavior (i.e. number of knocks on each door) in the context of each new resident in order to collect the most points.
Social sensitivity was defined as the extent to which adolescents adjusted their risk-taking behavior based on information about the anger level of the house’s resident. As such, social sensitivity in the SART likely taps into social cues from facial expressions, as well as mentalizing about the thoughts and feelings of the resident. That is, greater social sensitivity would entail knocking less on doors of residents who changed from happy to angry relatively fast, and knocking more on doors of residents who were slow to get angry. We hypothesized that relatively moderate social sensitivity (i.e. normative exploration and cashing-out right before the resident gets too angry and slams the door) would be related to greater self-reported adaptive decision-making. Low or high levels of social sensitivity, relative to moderate levels, are likely related to lower self-reported adaptive decision-making.
At the neural level, we expected that moderate social sensitivity would be related to enhanced tracking of the changes in emotional expression in affective, social cognition and cognitive control regions during risky decision-making, as prior work suggests that these brain regions are involved in attuning to social cues and flexible social behavior (Nelson and Guyer, 2011; Somerville, 2013; Rosen et al., 2017). We expected to find a curvilinear effect in these regions, such that compared to moderate levels of social sensitivity, low and high levels of social sensitivity would result in less neural tracking of the changes in emotional expression in these regions. Finally, we predicted that enhanced neural tracking in these regions would be linked to greater self-reported adaptive decision-making.
Materials and methods
Participants and procedure
This study included 35 healthy adolescents between 12 and 16 years (MAge = 15.28 years, s.d. = 1.34; 61% female). One additional participant was excluded due to excessive movement (> 2 mm movement between slices on >10% of slices). Within the sample, 74% identified as European-American, 14% as mixed ethnicity, 6% as African-American, 3% as Latin-American and 3% as Asian-American. Participants took part in a larger cross-sectional fMRI study about the development of decision-making. Given that previous work using SART has shown developmental changes (McCormick et al., 2018), and adolescence is uniquely characterized as a phase of social reorientation, we selected the adolescent sample a priori. All adolescent participants that had SART task data as well as Flinders questionnaire data (see below) were included in this study.
Participants were recruited through a participant database, word of mouth and flyers. We screened all participants to make sure that they were free of psychiatric disorders, neurological disorders and MRI contraindications. A training session was completed outside the scanner to train participants on how to perform the scan task correctly. The actual scan session lasted ∼1.5 h. Participants received a $50 endowment and selected prizes from a prize box based on the points they earned on the tasks during the scan. Participants and their parents provided written informed consent and assent prior to the start of the study. All procedures were approved by the Institutional Review Board of the University of Illinois.
Measures
Social analogue risk task
Participants played an adapted version of the Balloon Analogue Risk Task (BART), which in its original form is a well validated and widely-used task to assess risk-taking behaviors across development (Lejuez et al., 2002; McCormick and Telzer, 2017). Risk taking on the BART is correlated with real-life risk taking in adolescents, both concurrently (Telzer et al., 2015) and longitudinally (Qu et al., 2015). Similar to the BART, the SART involves sequential risk-taking in pursuit of points. The novel aspect of the SART is a social component, which makes it more comparable to real-world adolescent risk-taking behavior that tends to occur in an interactive social environment. This social environment was created by displaying dynamically changing facial expressions in response to participants’ risky decisions. The current version of the SART was based off of previous behavioral studies (e.g. Humphreys et al., 2016), but was modified in several ways as outlined in more detail below.
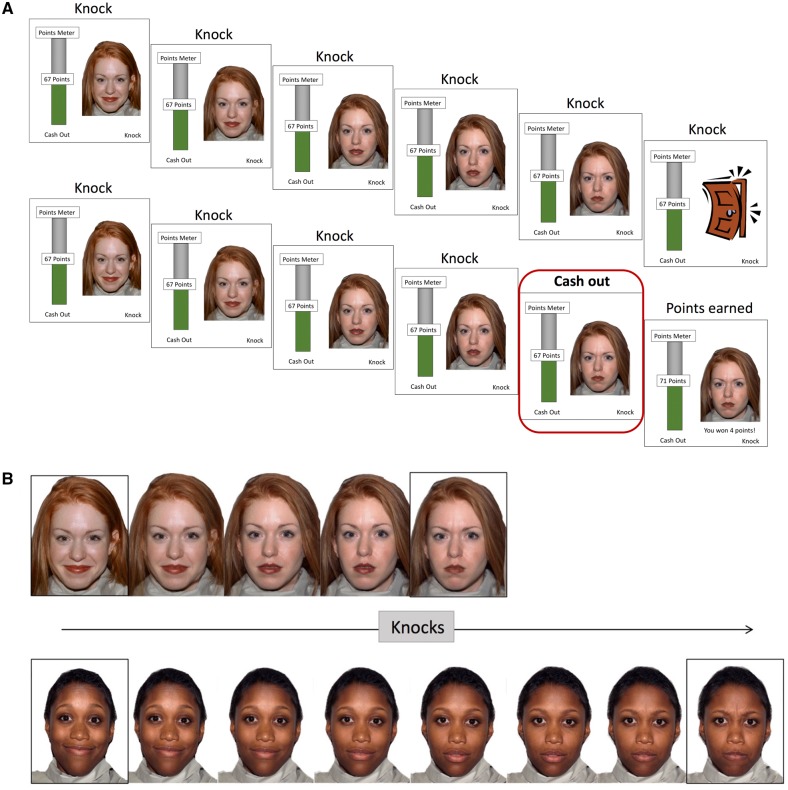
The task was explained as a ‘trick-or-treat’ game, in which participants were presented with a series of 24 people at their houses. Participants could knock on the door at each house to earn points for every knock (i.e. more knocks resulted in more points). They were instructed to earn as many points as possible, which could be cashed in for prizes after the study. All residents initially started out with a happy facial expression and grew increasingly angry with each successive knock, and would eventually slam the door, at which point the participant would lose all points earned for that respective door. Alternatively, participants had the option to cash out the points they earned for that respective door at any point. During training, participants were shown what these two options (i.e. knocking and cashing out) and their outcomes would look like. Each knock decision was accompanied by a knocking sound, slam events were accompanied by a loud slamming sound, and cash-out decisions were accompanied by a short note that indicated point receipt. A running total of points earned was presented on a points meter throughout the task (Figure 1A).
Fig. 1.
(A) Illustration of the SART. The cash-out decisions, highlighted in the red square, were the focus of the current analyses. Each decision was self-paced and there was a jitter (500–4000 ms) between each event. Participants played a ‘Trick-or-Treat’ game during which they knocked on doors in order to earn points. With each knock, the facial expression of the house’s resident morphed from happy to somewhat angrier. At 50% angry, the resident slammed the door and all points for that door were lost. Participants could also cash out at any moment. (B) Example of variable anger increments between residents. The upper resident slams the door after four knocks, whereas the lower resident is slower to anger and takes seven knocks to slam the door.
The door always slammed at a 50% angry facial expression, which was not explicitly explained to participants. Yet, some residents were slower to get angry than others. Thus, although all faces morphed along the same continuum from 100% happy to 100% neutral to 50% angry, the threshold for slamming the door varied between 3 and 10 knocks (Figure 1B for an illustration). Therefore, some residents morphed into 50% anger after 3 knocks, whereas others morphed more slowly and became 50% angry after 10 knocks. Hence, participants could use the socioaffective information in the faces to guide their decisions to determine whether they should keep knocking or cash out points. Table 1 shows descriptives concerning number of cash-out decisions, slams as well as average and range of knocks on the task.
Table 1.
Descriptives for SART task behavior
| Task parameters | Mean | s.d. | Range |
|---|---|---|---|
| Number of cash-out decisions | 21.89 | 1.66 | 17–24 |
| Number of door slams | 2.11 | 1.66 | 0–7 |
| Average # of knocks on cash-out trials | 4.81 | 1.55 | 3.19–6.5 |
| Average reaction time (s) all trial types | 1.18 | 0.21 | 0.75–1.73 |
Note: Slams and cash-out decisions are opposite to each other and add up to 24 trials in total.
The task consisted of one run with 24 self-paced trials (Table 1 lists display of reaction times). The run lengths ranged between 6.49 and 12.65 min, with a median of 9.28 min. Note that the run length mostly affects the data for the knocks condition, because participants with shorter runs knock fewer times than those with longer runs. Trials and each consecutive facial expression (i.e. angrier expression following a decision to knock; new face after decision to cash-out; new face following a slam trial) were separated with a random jitter (500 –4000 ms). The faces were drawn from the NimStim face database and modeled off of a study by Humphreys and colleagues (Tottenham et al., 2009; Humphreys et al., 2016). Participants saw 12 individual faces (4 European-American, 4 African-American and 4 Asian-American; all faces were female) twice during the task. For each participant, faces were presented in the same order and with fixed but previously randomly determined slam thresholds. As such, the current fMRI task was adapted from previous work by including more faces, each of which was shown twice, as well as having variable probabilities of door slamming for each individual trial. Finally, the timing was also adjusted such that the task could be administered in the MRI scanner.
Social sensitivity
Social sensitivity during the SART was operationalized as the extent to which individuals adjusted their number of knocks on the current trial based on information about the anger level of the house’s resident. More specifically, social sensitivity was operationalized as knocking more on houses in which the resident morphed to 50% angry more slowly and knocking less on houses in which the resident morphed to 50% angry more quickly. We employed hierarchical linear modeling (HLM; Raudenbush and Bryk, 2002) to obtain this social sensitivity index. We modeled 24 nested trials for each participant, with number of knocks as our outcome variable, and the anger level of the face as the predictor. The level 1 equation was as following:
Total knocks on a particular trial (i) for a particular adolescent (j) was modeled as a function of the average number of knocks across the task (b0j) and the anger level of the face on the current trial b1j(Anger(N)) (i.e. # of knocks required to get to 50% anger). We also included three controls. The main control variable was whether the previous trial (b2j) was a cash-out or slam [coded Cash-Out(N−1) = 0; Slam(N−1) = 1], which represents non-social feedback learning. This variable allowed us to test how likely participants were to use information from the previous trial to guide their knocks on the next trial, adjusting their behavior when their previous decision resulted in maladaptive outcomes. We included this variable as a control to make sure that our measure actually reflected social sensitivity, and not general feedback learning (see McCormick and Telzer, 2017 for a feedback learning approach). Two additional controls included were whether the current trial resulted in a cash-out or a slam [b3j; coded Cash-Out(N−1) = 0; Slam(N−1) = 1] and the trial number (b4j), which are controls often used in modeling of BART behavior (e.g., McCormick and Telzer, 2017). Trial number controls for cumulative learning over time, and this parameter did not predict the number of knocks/risk-taking (P = 0.680; see Supplementary for an overview of the entire HLM model). Note that social sensitivity effects were similar when we controlled for gender in our HLM level 2 equation. Therefore, we did not control for gender in further behavioral and neural analyses.
To use the index of social sensitivity (variable of interest) and non-social feedback learning (control) in our analyses, Empirical Bayes estimates were extracted for each participant. These estimates represent optimally weighted averages that are computed through a combination of estimates on an individual and group level, and shrinks the individual’s estimates towards the overall mean (Diez-Roux, 2002; McCormick and Telzer, 2017). The extracted estimate provides an individual difference measure indicating whether participants changed their behavior as a function of anger level. Values larger than 0 indicate that participants adjusted their risk-taking behavior based on social sensitivity (i.e. knocked more on houses where the face morphed more slowly and knocked less on houses where the face morphed more quickly), whereas values around 0 indicate little or no adjustment based on social sensitivity.
Adaptive decision-making
The concept of social sensitivity applies to general decision-making strategies in real-life as well as more specifically to social situations, as most decisions are made in a social context or with input from social others, especially during adolescence when individuals are especially attuned to the social context (Albert et al., 2013; Blakemore and Mills, 2014; Scriber and Guyer, 2017). The Flinders Adolescent Decision Making scale assesses adolescent decision-making patterns and distinguishes between adaptive and maladaptive decision-making (Mann et al., 1989; Tuinstra et al., 2000). Adaptive decision-making encompasses the subscales Vigilant (precision and deliberation in making decisions; e.g. ‘I like to think about my decision before I make it’) and Self-confident decision-making (confidence and efficacy when making decisions; e.g. ‘The decisions I make turn out well’), representing careful and deliberated decision-making. Within the revised 22-item scale (Tuinstra et al. 2000, p. 280), the Vigilant scale is measured with three items and the Self-confident decision-making scale with five items. Since we were interested in overall adaptive decision-making, we averaged these subscales to have a more potent measure of adaptive decision-making with eight items total (α = 0.79). A maladaptive pattern of decision-making is indexed by 14 items with the subscales Panic, Evasiveness and Complacency. Reflecting the original measure, the responses are coded on a 4-point scale ranging from ‘Never’ to ‘Always’ and the total score is calculated as the average of the respective items. Higher scores are indicative of more adaptive decision-making, whereas lower scores are indicative of less adaptive decision-making. This scale has been used to link neural processing to adaptive decision making in adolescents (e.g. Telzer et al., 2013).
fMRI data acquisition
Imaging data were obtained with a 3-T Siemens Trio MRI scanner, using a 12-channel head coil. The task consisted of one self-paced run. Functional data were collected using T2*-weighted echoplanar images (EPI) (slice thickness = 3 mm; 38 slices; TR = 2 s; TE = 25 ms; matrix = 92×92; FOV = 230 mm; voxel size 2.5×2.5×3 mm3). To provide an anatomical reference, structural scans were obtained, including a T2*weighted, matched-bandwidth (MBW; TR = 4 s; TE = 64 ms; FOV = 230; matrix = 192×192; slice thickness = 3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1.9 s; TE = 2.3 ms; FOV = 230; matrix = 256×256; sagittal plane; slice thickness = 1 mm; 192 slices). The MBW and EPI scans were acquired with an oblique axial orientation.
fMRI data preprocessing and analysis
We used the SPM8 software package (Wellcome Department of Cognitive Neurology, UK) for preprocessing and data analysis. Preprocessing involved correction for head motion with spatial realignment, co-registration to a high-resolution T1* MPRAGE structural scan, and segmentation into grey matter, white matter and cerebrospinal fluid. For one participant, the co-registration was conducted with the MBW scan because the T1* was missing. We applied the resulting transformation matrices to the MBW and EPI images in order to warp them into the standard stereotactic space as defined by Montreal Neurological Institute (MNI). EPI images were spatially smoothed with an 8-mm FWHM isotropic Gaussian kernel. The fMRI time series for each trial were convolved with the hemodynamic response function. To remove low-frequency scanner drift across time we applied a 128 s high-pass filter, and estimated serial autocorrelations using a restricted maximum likelihood algorithm with an autoregressive model order of 1.
We conducted statistical analyses on individual subjects’ data using the general linear model in SPM8. In the fixed-effects model, knock decisions, cash-out decisions and slam events were modeled as separate events of interest. The jitter between events was not modeled and utilized as an implicit baseline. The trials were modeled from the onset of the trial to when participants made their decision using the reaction time, and as such represent the decision-making phase. A parametric modulator (PM) was included to model increasing risk across knock decisions. The cash-out decision, which we focus on in the current manuscript, represents neural activity at the moment participants decided to cash out when the face was ‘too’ angry, and thus, when social sensitivity likely affects decisions. PM values signified the number of knocks for the entire trial (i.e. the total risk that is taken for each house), and were centered within a person around the average number of knocks for each door. We focus on the cash-out PM condition rather than the knocks condition, such that we were able to examine neural tracking of increasingly angry faces at the moment when participants decided to cash out. The resulting contrast images, computed at the individual level, were submitted to random-effects group-level analyses. At the group level, analyses were conducted using GLMFlex, which removes outliers and sudden activation changes in the brain, partitions error terms, analyzes all voxels containing data, and corrects for variance-covariance inequality (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex).
We first looked at the main effect and then performed whole-brain regression analyses on the cash-out PM condition, in which social sensitivity was entered as a quadratic term, given our hypothesis that a moderate amount of social sensitivity would be most beneficial. In this analysis, we controlled for social sensitivity as a linear predictor as well as the index for nonsocial feedback-learning. Given the use of GLM-Flex, we corrected for multiple comparisons using a Monte Carlo simulation through 3dclustsim (updated version November 2016) in the software package AFNI (Ward, 2000), and computed the smoothness of the data with the acf function within the 3dFWHMx command. For the main effect, the simulation resulted in a voxel-wise threshold of P < 0.005 and minimum cluster size of 79 voxels for the whole-brain, which corresponds to P < 0.05, FWE cluster-corrected. For the regression, the simulation resulted in a voxel-wise threshold of P < 0.005 and minimum cluster size of 107 voxels for the whole-brain.
Results
Behavioral results
First, we employed HLM to estimate how adolescents adjusted their decision-making based on social sensitivity to shifting facial expressions, as well as non-social feedback learning on the task. As expected, adolescents showed significant social sensitivity on average, as indexed by a strong association between number of knocks and anger level (b = 0.545, SE = 0.041, P < 0.001). Thus, adolescents knocked more when residents’ facial expressions were slower to change from happy to angry, and knocked less when residents’ facial expressions were faster to change from happy to angry. Moreover, adolescents also showed significant non-social feedback learning, as indexed by knocking less if the previous trial was a slam (b = −0.587, SE = 0.118, P < .001). As such, they learned to adjust their behavior when this previously resulted in a maladaptive slam outcome. The complete set of results of the first level model can be found in Supplementary Table S1. Next, we extracted the Empirical Bayes estimates for social sensitivity and non-social feedback learning for each participant. There was considerable variability in the Empirical Bayes estimate of social sensitivity, M = 0.545, s.d. = 0.224, range 0.129–0.909. Whereas some participants showed relatively low social sensitivity (i.e. estimates closer to 0), other adolescents showed relatively high social sensitivity (i.e. estimates closer to 1). Non-social feedback learning also showed individual variability (M = −0.587, s.d. = 149), range −0.842 to −0.183. Some participants showed a larger decrease in the number of knocks after the previous outcome was a slam (i.e. estimates closer to −1), while others showed a small decrease in knocks after a slam (i.e. estimates closer to 0).
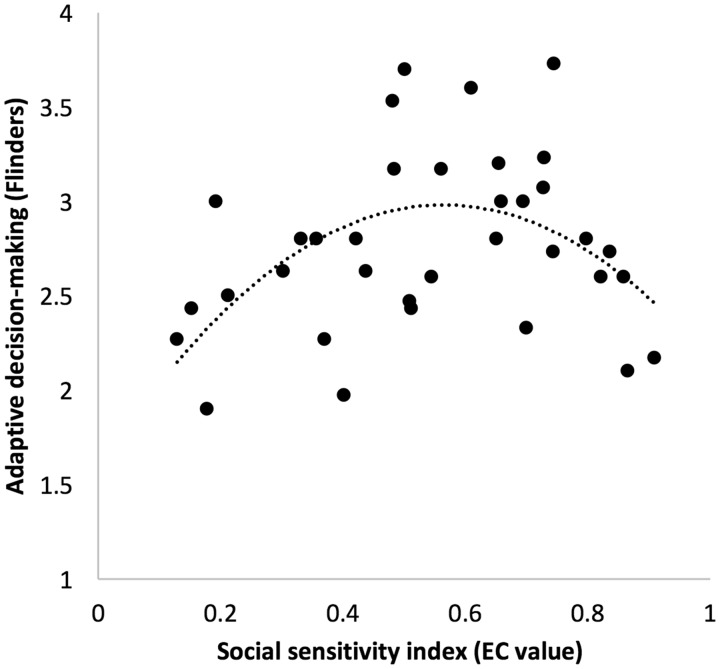
Next, we ran a regression model to examine whether social sensitivity showed curvilinear relations with adaptive decision making. We entered linear social sensitivity in model 1 as a baseline model, added social sensitivity2 in model 2, and non-social feedback learning in model 3. Kolmogorov–Smirnov tests of normality showed that all of these variables were normally distributed (P’s > 0.05). Model 2 (social sensitivity2 controlling for social sensitivity); and model 3 (social sensitivity2 controlling for social sensitivity and non-social feedback learning) were significant (Ps < 0.05), but only model 2 predicted significantly more variance than the baseline model (r2change = 0.209; P = 0.006). As such, model 2 was the best fit to the data, explaining 20% of the variance [(F(2, 34) = 5.249, P = 0.011]. As expected, there was a curvilinear relationship between social sensitivity and adaptive decision making, such that moderate levels of social sensitivity were associated with more adaptive decision making, whereas low and high levels of social sensitivity were associated with lower adaptive decision making [b = −4.392, SE = 1.475, β = −2.232, P = .006; Figure 2). Non-social feedback learning in model 3 did not contribute to the prediction of adaptive decision-making (b = 0.897, SE = 0.742, β = 0.286, P = 0.236). Together, these findings underscore the important adaptive role of moderate social sensitivity for adolescent decision-making processes.
Fig. 2.
Quadratic relationship between social sensitivity and self-reported adaptive decision-making, as indicated by the Flinders adolescent decision-making scale.
fMRI results
Neural tracking of cash-out decisions
We first examined the main effect of the cash-out decisions, during which participants decided to cash out points before the door was slammed. Results showed that the left precuneus and occipital gyrus tracked increasingly angry faces with corresponding increased risk when participants decided to cash out (Table 2). In addition, the right TPJ was recruited, but this region did not survive correction. As such, these findings show that social brain regions and visual regions are recruited more as participants knock more and see increasingly angry faces on trials where they decide to cash out.
Table 2.
Brain regions that displayed a main effect for the cash-out PM contrast
| Region label | Volume (mm3) | t-Value | MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| L Superior occipital gyrus | 88 | 4.058 | −21 | −79 | 40 |
| L Precuneus | 88 | 3.659 | −9 | −73 | 55 |
| R Superior temporal gyrus (TPJ) | 66a | 3.974 | 60 | −37 | 13 |
Note: Analysis for negative relationships showed no significant clusters of activation. P <0.05, FWE cluster-corrected.
Does not survive FWE-cluster correction.
Links between social sensitivity and neural tracking during cash-out decisions
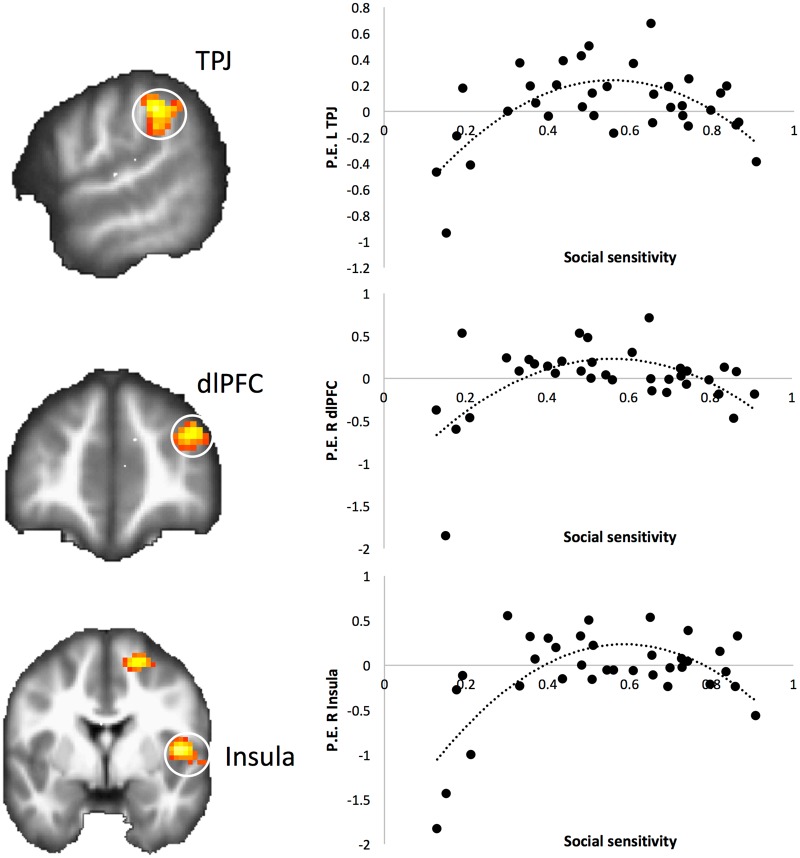
In our primary analyses, we conducted a whole-brain regression analysis, in which social sensitivity was entered as a quadratic predictor, and linear social sensitivity and non-social feedback learning were included as covariates. Results indicated a quadratic relationship for several regions implicated in social cognition and cognitive control, including the left TPJ, right insula and right dorsolateral prefrontal cortex (dlPFC), demonstrating that these brain regions track increasing anger the most at moderate levels of social sensitivity, but track less so at either low or high levels of social sensitivity (Figure 3; Table 3). Next, we ran a whole-brain regression analysis in which feedback learning was entered as a predictor, controlling for social sensitivity2 and social sensitivity, to examine whether this effect was unique to social sensitivity. This analysis yielded no significant results, demonstrating that these neural effects are indeed unique to social sensitivity.
Fig. 3.
Quadratic relationship between social sensitivity and tracking of increasing anger in left TPJ (MNI −60 −40 40), right dlPFC (MNI 36 47 25) and right insula (MNI 45 −1 7) during decisions to cash-out (P <0.05, FWE-cluster corrected; for visualization purposes only).
Table 3.
Brain regions that displayed a quadratic relationship with social sensitivity when adolescents chose to cash out, controlling for nonsocial feedback learning and the linear predictor for social sensitivity
| Region label | Volume (mm3) | t-Value | MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| R Insula | 401 | −5.264 | 45 | −1 | 7 |
| R Superior temporal gyrus | 401 | −3.952 | 69 | −19 | 1 |
| L Cerebellum | 354 | −5.328 | −12 | −52 | −26 |
| L Supramarginal gyrus (TPJ) | 157 | −5.280 | −60 | −40 | 40 |
| R Superior frontal gyrus (premotor) | 122 | −4.767 | 21 | −1 | 64 |
| R Middle frontal gyrus (dlPFC) | 108 | −4.758 | 36 | 47 | 25 |
| R Cerebellum | 129 | −4.594 | 15 | −49 | −32 |
Note: Analysis for positive relationships showed no significant clusters of activation.
P <0.05, FWE cluster-corrected.
Neural activity and adaptive decision-making
Finally, we extracted the parameter estimates (averaged over the whole cluster) from the left TPJ, right insula and right dlPFC which showed significant quadratic relations with social sensitivity and conducted a series of regressions to predict self-reported adaptive decision-making. For the neural activation and self-reported adaptive decision making, high scores on each are predicted to be adaptive (i.e. moderate social sensitivity related to high adaptive decision making and high neural activation). Therefore, taking out social sensitivity in the equation here, we expected high neural activity to be related to high adaptive decision-making, which should result in a linear relationship rather than a quadratic relationship. Separate regressions were run with a Bonferroni correction for multiple comparisons (0.05/3 = 0.017), for each brain region. The regression model for the TPJ was significant [F(1, 33) = 10.79; R2adj = 0.224, P = 0.002], such that increased tracking in the TPJ predicted greater adaptive decision-making (b = 2.729, SE = 0.235, β = 0.496; P = 0.002). The regression for dlPFC [F(1, 33) = 5.90; R2adj = .126, P = 0.021; b = 0.428, SE = 0.176, β = 0.389, P = 0.021] and for insula [F(1, 33) = 4.271; R2adj = 0.088, P = 0.047; b = 0.319, SE = 0.154, β = 0.339; P = 0.047] revealed similar patterns at the trend level, when we accounted for multiple comparisons (Figure 3 shows a display of these analyses; visualization purposes only). Taken together, these findings demonstrate that more neural tracking in these regions associated with social cognition and cognitive control is associated with higher levels of adaptive decision-making.
Discussion
This study tested whether moderate levels of social sensitivity to changing facial expressions was associated with adaptive decision-making in a risky context, on a behavioral and neural level. In order to do so, the novel SART, a ‘trick-or-treat’ game, was employed in a sample of 12–16-year-old adolescents. Behavioral findings revealed a curvilinear relationship between social sensitivity and adaptive real-world decision-making. During decisions to cash-out, activity in the TPJ, insula and dlPFC displayed a curvilinear relationship with social sensitivity, indicating that these brain areas tracked shifting facial expressions the most at a moderate level of social sensitivity, but less so at low or high levels of social sensitivity. Finally, brain activity in each of these regions was associated with greater levels of adaptive real-world decision-making, with the TPJ showing the strongest effect, and insula and dlPFC at the trend level after correcting for multiple comparisons. Findings suggest that moderate social sensitivity is related to adaptive decision-making in a risky context, and this is modulated via heightened tracking of social cues in the TPJ, insula and dlPFC.
Moderate social sensitivity and adaptive decision-making
Adolescence is a developmental period marked by a major social re-orientation, with changes in social as well as health-risk behaviors (Ellis et al., 2012; Nelson et al., 2016). Elevated adolescent social-affective sensitivity has traditionally been linked to increases in excessive health-risk behaviors. More recently however, research has started to highlight the more adaptive properties of normative exploration and risk-taking, as well as social sensitivity (Crone and Dahl, 2012). There is a large and meaningful within-group variability in adolescent risk taking compared to childhood or adulthood (Van Duijvenvoorde et al., 2016), which further illustrates the need to characterize normative risk taking vs excessive risk taking. For example, exploration facilitates learning and understanding of the (social) environment, with greater experience relating to more available information, which is helpful in consequent decision-making (Goldenberg et al., 2017; McCormick and Telzer, 2017; Somerville et al., 2017). Our behavioral findings resonate with and extend previous work by revealing that those with moderate social sensitivity to shifting facial expressions, relative to low or high social sensitivity, showed greater adaptive decision-making. Taken together, the present findings provide evidence that moderate adolescent social-affective sensitivity may confer advantages in a risky context.
Theoretical and empirical work shows evidence for intensified processing of social cues from the environment during adolescence (Somerville, 2013). Sensitivity to social cues, and in particular to changing emotional expressions, is key in navigating the social world (Neta and Whalen, 2011). Greater social sensitivity to facial expressions has been linked to less social anxiety and fewer social problems (Rosen et al., 2017). In this study, we assessed social sensitivity based on task behavior, as the extent to which adolescents adjusted their risky decision-making based on how quickly the facial expression changed from happy to angry. This enabled us to look at individual differences in social sensitivity to facial expressions, and for the first time, how this affects decision-making in a risky context. Our findings supported the hypothesis that being moderately sensitive to changing facial expressions facilitates goal-directed and adaptive risky decisions. Given that the adolescent period encompasses the time when individuals reach mature social goals (Cohen et al., 2016), these findings intuitively make sense. That is, being in a social vacuum (low social sensitivity) or being exceptionally attuned to one’s social environment (high social sensitivity) limits successful interactions with others (Nelson and Guyer, 2011; Blakemore and Mills, 2014). Moderate social sensitivity on the other hand likely enables adolescents to successfully interact with a wide range of social agents, including parents, teachers, and peers. Ultimately, successful social relationships may pave the way to reaching mature social goals in adulthood.
Neural correlates of moderate social sensitivity and adaptive decision-making
Changes in social and health-risk behaviors during adolescence are paralleled by dynamic restructuring of the neural architecture of affective, social and cognitive control networks (Crone and Dahl, 2012; Blakemore and Mills, 2014). A recent neurobiological susceptibility model poses that adolescent development is shaped by brain-based individual differences in sensitivity to social context (Scriber and Guyer, 2017). In line with this model, the current study examined individual differences in adolescent neural tracking related to social sensitivity. During decisions to cash-out, adolescents with moderate levels of social sensitivity showed increased neural tracking of shifting facial expressions from happy to angry with increasing risk in the TPJ, insula and dlPFC. These brain regions showed less tracking at either low or high levels of social sensitivity. Together, regions associated with affective, social cognition, and cognitive control processing showed differential tracking based on individual differences in social sensitivity.
The TPJ is an integral part of the so-called social brain network which is implicated in social cognition and mentalizing (Blakemore and Mills, 2014). In particular, TPJ activation has been linked to perspective-taking and understanding more complex emotions, as well as more general attention processes in the social domain (Burnett et al., 2009; Van den Bos et al., 2011). Although TPJ activation did not survive stringent correction in the main effect when we solely examined neural tracking during decisions to cash-out independent of social sensitivity, it is an interesting finding given that the non-social BART does not elicit any TPJ activity for a similar contrast (McCormick and Telzer, 2017). However, the effect with the curvilinear relation to social sensitivity during decisions to cash-out did survive stringent correction. One interpretation for TPJ activity may be perspective-taking, i.e. trying to understand the resident’s thoughts and feelings. Alternatively, TPJ activation may represent a more general attention process of being attuned to the changing emotions displayed by the resident of the house. As such, relatively moderate social sensitivity may be facilitated by social attention or perspective-taking processes that guides adaptive decision-making. Because our analyses utilized a PM, the neural effects represent linear increases in the neural tracking of emotional expressions in the faces rather than mean level activation. Thus, those with low and high social sensitivity each showed low TPJ tracking of increasing anger, suggesting that these adolescents are less sensitive to the changing social cues in the facial expressions, which is associated with less adaptive decision-making.
The insula is considered a ‘hub’ between cognitive control and affective brain networks and has been linked to a wide range of functions across different contexts. Indeed, the insula is implicated in salience detection and attentional resource for goal-directed behavior (Menon and Uddin, 2010; Smith et al., 2014). In the affective literature, insula activity has been reported for basic face processing, perceiving emotional states of the self and others and integrating this internal information with external cues from the social environment (i.e. social feedback) (Guyer et al., 2009; Lamm and Singer, 2010). As such, the insula plays a critical role in guiding decision-making and has particular importance for risk-taking (Smith et al., 2014). In a similar task design, adolescents showed greater insula activation when taking risks in a social feedback relative to non-social feedback condition (Op de Macks et al., 2017). The present findings fit with previous work and suggest that for individuals with moderate levels of social sensitivity, shifting emotional expressions were most salient, eliciting increased allocation of attentional resources that may facilitate social learning from the facial expression. Perhaps for adolescents on both the low and high end of the social sensitivity continuum, facial expressions are not as salient, or potentially hypersalient, which hinders the social learning process from facial expression and is detrimental to adaptive decision-making in a social context.
Imbalance models of neurocognitive development denote the implication of lateral regions of the PFC in cognitive control and inhibition (Somerville et al., 2010; Casey, 2015; Shulman et al., 2016). In a social-affective context, the lPFC is linked to flexible social behaviors and recruited together with face processing regions when more cognitive processes are layered onto passively viewing facial expressions, like labeling expressions (Neta and Whalen, 2011; Flannery et al., 2017). In such cognitively-taxing affective tasks, the dlPFC facilitates working memory to guide goal-directed behaviors and ultimately meet task demands (Neta and Whalen, 2011). Thus, increased tracking of the dlPFC with moderate social sensitivity likely reflects increased cognitive control, guiding the decision to stop knocking on the door and instead cash-out when the facial expression becomes too angry. That is, the dlPFC plays a role in the ability to flexibly switch risk-taking behaviors in light of the social cues from the social environment. On the other hand, adolescents on the low and high end of social sensitivity show less tracking in this area implicated in cognitive control, which is associated with less adaptive decision-making.
Future directions and conclusions
A few limitations should be noted. First, this study only incorporated dynamically changing female adult faces, because the task was based on previous work (Humphreys et al., 2016). Future research should replicate and extend the current findings with male faces, as well as different emotional expressions (e.g. happy shifting to sad). Another interesting direction for future research would be to examine the relation between social sensitivity and neural responses to peer faces, for example using the recently validated duckEES dynamic facial expressions dataset (Giuliani et al., 2017). Given the value placed on peer relationships and the increase in risk-taking when adolescents are with their friends (Chein et al., 2011), the peer context represents an important and salient context to further investigate social sensitivity. Previous studies that directly compared adolescent neural responses to peer and adult faces have shown mostly overlapping brain regions, except for enhanced amygdala response to positive peer faces and angry adult faces (Marusak et al., 2013; Flannery et al., 2017).
The SART does not allow us to disentangle the neural response to increasing risk and shifting emotional expressions as they increase at the same time. Although we used a PM to specifically examine neural tracking with increasing risk, which in itself controls for the social and non-social aspects of the task, future research is needed to disentangle and study these two processes independently. It will also be important to extend the current findings with an additional non-social control condition, for example using shapes that change color signaling the rate of transition instead of faces, or balloons in which the size indicates the explosion threshold, unlike the traditional BART in which the balloon can explode at any time with no cue indicating the threshold. In addition, while self-report of decision-making strategies gets at adolescents’ own perception of their decisions, this method is therefore also limited. Future studies can employ different methods to get at other aspects of this decision-making concept, such as using observational methods to code actual decision-making and linking this to neural activity in an fMRI task. And lastly, we acknowledge that the sample size used to assess individual differences in the current paper is relatively small. Future studies should replicate the current findings to confirm that they hold in larger samples, and examine how they may differ in children or adults.
In conclusion, we show that moderate adolescent social sensitivity, relative to low or high social sensitivity, is related to greater adaptive decision-making, and is modulated via TPJ, insula and dlPFC activity in the brain. These findings shed light on how adolescent social-affective sensitivities may positively impact outcomes in adolescence, and emphasize the importance of studying social sensitivity in the context of decision-making. Moreover, the curvilinear relationship suggests that there is a moderate optimum of social sensitivity, which has implications for training and interventions during adolescence tailored to help adolescents reach mature social goals in adulthood.
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgments
We thank Nicholas Ichien and Inge Karosevica for collecting the data. We greatly appreciate the assistance of the Biomedical Imaging Center at the University of Illinois.
Funding
This research was supported by a grant from the National Institutes of Health (R01DA039923) and generous funds from the Department of Psychology at the University of Illinois.
Conflict of interest. None declared.
References
- Albert D., Chein J., Steinberg L. (2013). The teenage brain: peer influences. on adolescent decision making. Current Directions in Psychological Science, 22(2), 114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. (2014). Is ad olescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. [DOI] [PubMed] [Google Scholar]
- Burnett S., Bird G., Moll J., Frith C., Blakemore S.J. (2009). Development during adolescence of the neural processing of social emotion. Journal of Cognitive Neuroscience, 21(9), 1736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J. (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66(1), 295–319. [DOI] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.O., Breiner K., Steinberg L., et al. (2016). When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychological Science, 27(4), 549–62. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K., Johnson M.H., Dick F., Cohen Kadosh R., Blakemore S.-J. (2013a). Effects of age, task performance, and structural brain development on face processing. Cerebral Cortex, 23, 1630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Johnson M.H., Henson R.N.A., Dick F., Blakemore S.-J. (2013b). Differential face-network adaptation in children, adolescents and adults. NeuroImage, 69, 11–20. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–50. [DOI] [PubMed] [Google Scholar]
- Diez-Roux R. (2002). A glossary for multilevel analysis. Journal of Epidemiology and Community Health, 56(8), 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.J., Del Giudice M., Dishion T.J., et al. (2012). The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Developmental Psychology, 48(3), 598. [DOI] [PubMed] [Google Scholar]
- Flannery J.E., Giuliani N.R., Flournoy J.C., Pfeifer J.H. (2017). Neurodevelopmental changes across adolescence in viewing and labeling dynamic peer emotions. Developmental Cognitive Neuroscience, 25, 113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D., Knoll L.J., Sakhardande A.L., Speekenbrink M., Kadosh K.C., Blakemore S.-J. (2016). Perception and recognition of faces in adolescence. Scientific Reports, 6(33497), 33497–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa-Schechtman E., Shachar-Lavie I. (2013). More than a face: a unified theoretical perspective on nonverbal social cue processing in social anxiety. Frontiers in Human Neuroscience, 7, 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Flournoy J.C., Ivie E.J., Von Hippel A., Pfeifer J.H. (2017). Presentation and validation of the DuckEES child and adolescent dynamic facial expressions stimulus set. International Journal of Methods in Psychiatric Research, 26(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D., Telzer E.H., Fuligni A.J., Lieberman M.D., Gálvan A. (2017). Greater response variability in adolescents is associated with increased white matter development. Social Cognitive Affective Neuroscience, 12, 436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., McClure‐Tone E.B., Shiffrin N.D., Pine D.S., Nelson E.E. (2009). Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development, 80(4), 1000–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Pine D.S., Nelson E.E. (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7(1), 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Casey B.J. (2016). The neurodynamics of emotion: delineating typical and atypical emotional processes during adolescence. Developmental Science, 19(1), 3–18. [DOI] [PubMed] [Google Scholar]
- Humphreys K.L., Galan C.A., Tottenham N., Lee S.S. (2016). Impaired social decision-making mediates the association between ADHD and social problems. Journal of Abnormal Child Psychology, 44, 1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Somerville L.H., Li J., et al. (2014). Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 683–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.-C., Lombardo M.V., Baron-Cohen S. (2014). Autism. The Lancet, 383(9920), 896–910. [DOI] [PubMed] [Google Scholar]
- Lamm C., Singer T. (2010). The role of the anterior insular cortex in social emotions. Brain Structure and Function, 214(5–6), 579–91. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Read J.P., Kahler C.W., Richards J.B., Ramsey S.E., Stuart G.L. (2002). Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). Journal of Experimental Psychology: Applied, 8, 75.. [DOI] [PubMed] [Google Scholar]
- Mann L., Harmoni R., Power C. (1989). Adolescent decision making, the development of competence. Journal of Adolescence, 12(3), 265–78. [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Carré J.M., Thomason M.E. (2013). The stimuli drive the response: an fMRI study of youth processing adult or child emotional face stimuli. NeuroImage, 83, 679–89. [DOI] [PubMed] [Google Scholar]
- McCormick E.M., Telzer E.H. (2017). Adaptive adolescent flexibility: neurodevelopment of decision-making and learning in a risky context. Journal of Cognitive Neuroscience, 29, 413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick E.M., Perino M.T., Telzer E.H. (2018). Not just social sensitivity: adolescent neural suppression of social feedback during risk taking. Developmental Cognitive Neuroscience, 30, 134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Garrad M.C., Somerville L.H. (2015). What develops during emotional development? A component process approach to identifying sources of psychopathology risk in adolescence. Dialogues in Clinical Neuroscience, 17, 403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5–6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35(2), 163–74. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Guyer A.E. (2011). The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience, 1(3), 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Jarcho J.M., Guyer A.E. (2016). Social re-orientation and brain development: an expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M., Whalen P. (2011). Individual differences in neural activity during a facial expression vs. identity working memory task. NeuroImage, 56(3), 1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks Z.A., Bunge S.A., Bell O.N., Kriegsfeld L.J., Kayser A.S., Dahl R.E. (2017). The effect of social rank feedback on risk taking and associated reward processes in adolescent girls. Social Cognitive and Affective Neuroscience, 12(2), 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R.C., Dauvermann M.R., Whalley H.C., Baynham K., Lawrie S.M., Stanfield A.C. (2012). A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neuroscience and Biobehavioral Reviews, 36(2), 901–42. [DOI] [PubMed] [Google Scholar]
- Qu Y., Galván A., Fuligni A.J., Lieberman M.D., Telzer E.H. (2015). Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience, 35, 11308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S.W., Bryk A.S. (2002). Hierarchical Linear Models: Applications and Data Analysis Methods, Vol. 1 Newbury Park, CA: Sage. [Google Scholar]
- Rosen M.L., Sheridan M., Sambrook K.A., et al. (2017). Salience network response to changes in emotional expressions of others is heightened during early adolescence: relevance for social functioning. Developmental Science, e12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriber R.A., Guyer A.E. (2017). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19(1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman E.P., Smith A.R., Silva K., et al. (2016). The dual systems model: review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience, 17, 103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.R., Steinberg L., Chein J. (2014). The role of the anterior insula in adolescent decision-making. Developmental Neuroscience, 36(3–4), 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. (2010). A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H. (2013). The teenage brain: sensitivity to social evaluation. Current Directions in Psychological Science, 22(2), 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Sasse S.F., Garrad M.C., et al. (2017). Charting the expansion of strategic exploratory behavior during adolescence. Journal of Experimental Psychology: General, 146(2), 155–64. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. (2013). Meaningful family relationships: neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience, 25(3), 374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Miernicki M.E., Gálvan A. (2015). The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Social Cognitive Affective Neuroscience, 10, 389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Miernicki M.E., Rudolph K. (2017). Chronic childhood peer victimization heightens neural sensitivity to risk taking. Development and Psychopathology, 30, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra J., Van Sonderen F.L.P., Groothoff J.W., Van Den Heuvel W.J.A., Post D. (2000). Reliability, validity and structure of the Adolescent Decision Making Questionnaire among adolescents in The Netherlands. Personality and Individual Differences, 28(2), 273–85. [Google Scholar]
- Van den Bos W., van Dijk E., Westenberg M., Rombouts S.A., Crone E.A. (2011). Changing brains, changing perspectives the neurocognitive development of reciprocity. Psychological Science, 22(1), 60–70. [DOI] [PubMed] [Google Scholar]
- Van Duijvenvoorde A.C.K., Peters S., Braams B.R., Crone E.A. (2016). What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neuroscience and Biobehavioral Reviews, 70, 135–47. [DOI] [PubMed] [Google Scholar]
- Ward B.D. (2000). Simultaneous inference for fMRI data [WWW]. Available: https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf (17 November 2017, last accessed date).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.