Abstract
Adrenal hemorrhage represents a relatively rare condition, usually associated with meningococcal septicemia. It is an underestimated cause of acute decompensation, multiorgan failure and death, usually diagnosed post-mortem. Depending on its etiology adrenal hemorrhage is categorized as traumatic and non-traumatic. The technical advancement of imaging modalities, has made diagnosis and management more efficient. Assessment of hemodynamic stability, exclusion of a hormonal active adrenal tumor and assessment for adrenal insuffi¬ciency, are of cardinal importance. Angiographic embolization has contributed significantly in better outcomes as emergency laparotomy is associated with high morbidity and mortality rates. Hereby we present two cases of spontaneous adrenal hemorrhage associated with extensive retroperitoneal bleeding and hemodynamic instability. Both underwent angiography with one requiring embolization with favorable outcome. Investigation for exclusion of underlying adrenal tumor, adrenal insufficiency and follow-up imaging are presented in detail for both cases.
INTRODUCTION
Adrenal hemorrhage (AH) represents a rare and underestimated cause of acute decompensation, multiorgan failure and death [1]. It is usually diagnosed post-mortem, due to the non-specific presentation in prominent concurrent illness [1]. In autopsy series, incidence ranges from 0.14 to 1.1% [2, 3].
Depending on etiology AH is categorized as traumatic and non-traumatic (Table 1) [1]. Spontaneous AH, commonly presents with acute abdominal pain, in the absence of trauma or use of anticoagulants [1]. Ruptured aortic aneurysm, renal tumors and renal artery aneurysm are included in the differential diagnosis [4]. As spontaneous AH is an obscure medical condition, it might remain unrecognized with lethal consequences [1, 5]. The technical advancement and widespread use of cross-sectional imaging, has made diagnosis and management more efficient [1, 5].
Table 1.
Classification of adrenal hemorrhage
| Traumatic | Non-traumatic |
|---|---|
| Blunt trauma | Spontaneous |
| Severe stress or sepsis associated (ICU) | |
| Anti-phospholipid antibody syndrome and heparin-associated thrombocytopenia | |
| Penetrating trauma | Postoperative |
| Anticoagulant-associated | |
| Incidental finding on imaging study |
In this report, we present two cases of unilateral spontaneous AH and discuss its potential mechanism, diagnosis and management, by reviewing current literature. Informed consent was obtained from both patients.
CASE REPORT 1
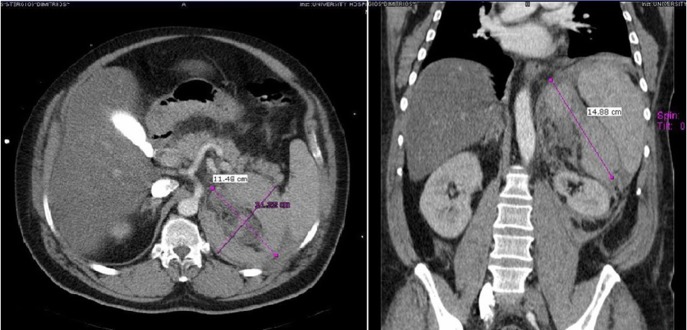
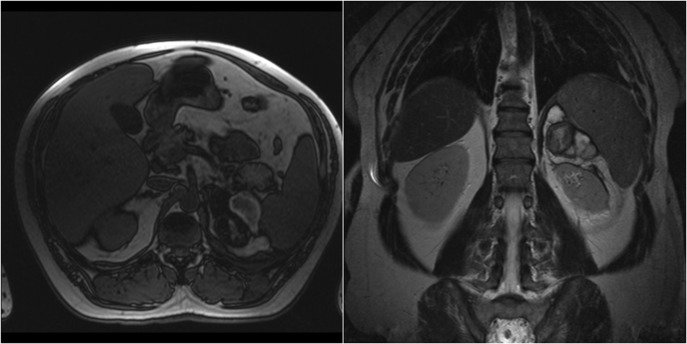
A 47-year-old male presented with, epigastric pain radiating to the back and profuse sweating. The patient was a smoker, obese and not on regular medication. Other than hypertension (BP: 210/120 mmHg), observations were normal. The abdomen was distended without rebound tenderness. Blood tests showed leukocytosis (WBC:12.000, 55% neutrophils), while hematocrit, platelets, coagulation profile and routine biochemistry were normal. Ultrasonography (US) was unremarkable, while computed tomography (CT) revealed a large retroperitoneal hematoma at the site of the left adrenal gland, with minimal contrast extravasation (Fig. 1). The patient was transferred to ICU, due to uncontrolled hypertension and suspicion of underlying pheochromocytoma, despite no obvious CT finding. The patient became hemodynamically unstable and Hct dropped to 35%. Angiography that followed showed no contrast extravasation. Further reduction of Hct to 22% necessitated transfusion with 2 units of blood. Subsequent CT showed stable hematoma without active bleeding. The patient was transferred to the ward after 8 days. Investigation for potential adrenal mass and post-hemorrhage adrenal insufficiency was commenced, requiring restricted diet for testing of urine vanillylmandelic acid (VMA). Blood cortisol and ACTH were normal, while investigation for exclusion of pheochromocytoma was inconclusive. Metaiodobenzylguanidine (MIBG) scan was negative. The patient was discharged with well-controlled blood pressure. On follow-up, urine VMA was reduced, while abdominal MRI, performed after 2 months, showed complete hematoma resolution and no underlying mass (Fig. 2).
Figure 1:
Case 1 presenting imaging (CT) showing a large left peri-adrenal hematoma.
Figure 2:
Case 1 follow-up MRI showing almost complete resolution of the hematoma and absence of underlying adrenal lesion.
CASE REPORT 2
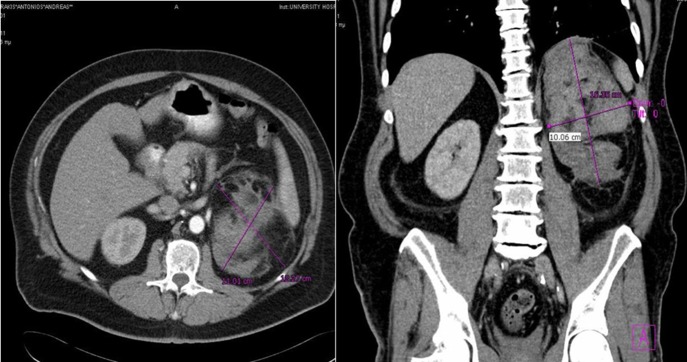
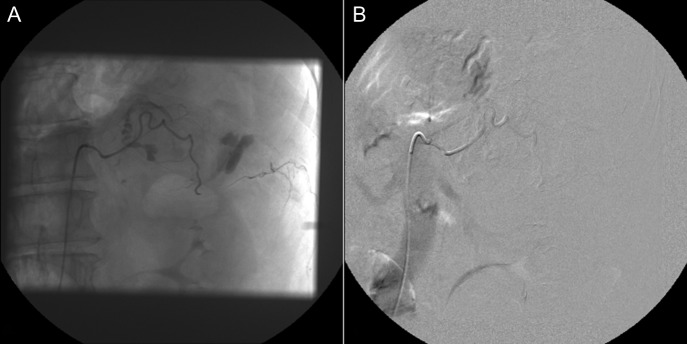
A 60-year-old male presented with pain at the left upper quadrant and left flank, radiating to the back. The patient had known hypertension and had suffered a myocardial infarction a year ago with stent insertion, now being on aspirin and clopidogrel. Upon admission all observations were normal. On examination, tenderness with guarding were noted at the left upper quadrant. Giordano sign was strongly elicited. Laboratory investigations were normal other than leukocytosis (WBC: 15.300, 89% neutrophils). US was unremarkable and CT scan revealed a retroperitoneal hematoma, at the site of the left adrenal gland, associated with extravasation of contrast (Fig. 3). The patient underwent angiography and active bleeding was confirmed from the middle adrenal artery which was successfully embolized microspheres (Fig. 4). The patient was transferred to ICU. He remained hemodynamically stable, without requiring blood transfusion. Four days later he was transferred to the ward. Pheochromocytoma was excluded with VMA, while ACTH and cortisol measurements ruled out adrenal insufficiency. Clopidogrel was permanently discontinued. The patient was discharged after 14 days and follow-up MRI confirmed hematoma resolution excluding an underlying mass.
Figure 3:
Case 2 presenting imaging (CT) showing a large left peri-adrenal hematoma.
Figure 4:
Selective adrenal artery angiography showing active extravasation from the middle adrenal artery (A) and subsequent successful embolization (B).
DISCUSSION
The pathophysiology of adrenal gland’s bleeding propensity has not been clarified. Clues lie behind vascular architecture [1]. After entering the adrenal surface, arterial branches transit abruptly to capillaries, forming the ‘adrenal dam’ [6] and medullary sinusoids drain the capillary plexus, forming the adrenal vein [1]. This pattern [6] and the eccentric venous smooth muscle arrangement result in turbulent flow, creating bleeding predisposition. Physiologic factors including reduced capillary resistance and stress-induced levels of catecholamines and ACTH, result in vasoconstriction, platelet aggregation, reperfusion and bleeding, especially in the capillaries situated within the distal corticomedullary junction [6].
Spontaneous AH, represents a rare condition [1, 7–9]. It includes cases associated with adrenal masses [4], pregnancy [5] (due to cortex hypertrophy and hyperplasia) and idiopathic cases [6]. Trauma, resulting in adrenal vein thrombosis, hypertensive crisis and corticotropin therapy, have been reported [1]. Risk factors in this report included hypertension in the first case, while the second patient had no known predisposing factors other than antiplatelet medication. This association has been previously reported [4]. Patients with AH, present with abdominal pain [1, 4–6]. However, the majority present insidiously [1]. Diagnosis is established by CT or MRI [4]. An adrenal hematoma has a heterogeneous, high density appearance on CT, while in the acute setting, the presence of an underlying adrenal mass cannot be excluded [4]. MRI is the most accurate imaging technique and differentiates subacute from chronic hemorrhage [4]. In both our cases, diagnosis was made with CT, while MRI confirmed hematoma resolution and absence of mass.
Assessment of hemodynamic stability [4, 5], exclusion of adrenal tumor (especially a hormonally active lesion) [4] and assessment for adrenal insufficiency, are of cardinal importance [1].
Cases refractory to transfusion, require embolization. Emergency laparotomy may be required in the absence of interventional radiology facilities [4]. Both our patients underwent angiography with one requiring embolization.
Exclusion of an adrenal tumor in the acute setting is challenging [4] and delayed MRI is usually required [5]. Hormonal evaluation, especially for glucocorticoid and catecholamine excess rules out a hormonally active tumor [4]. Surgery with an underlying pheochromocytoma may prove catastrophic, with a 45% mortality [4]. Pheochromocytomas cause a 4-fold increase in serum catecholamines [4]. If serum cortisol is increased, ACTH differentiates stress from a functional tumor [4]. In the absence of preoperative investigation, appropriate intraoperative management with alpha and beta blockers and stress dose glucocorticoids is required [4]. Planned adrenalectomy is the treatment of choice for patients with functional adrenal lesions [4]. Adrenal insufficiency is rare after unilateral AH [4]. Hypotension, hyponatraemia, hypoglycemia and hyperkalemia may indicate adrenal insufficiency, while serum cortisol helps in diagnosis. A value >34 μg/dL excludes it, while <15 μg/dL confirms it [10]. In conclusion, spontaneous AH is a rare, underestimated, potentially lethal surgical emergency. Raised suspicion and a multidisciplinary approach, for both resuscitation and definitive management, are essential for a successful outcome.
CONFLICT OF INTEREST
All authors have no conflicts of interest and nothing to disclose.
FUNDING
None.
REFERENCES
- 1. Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 2001;76:161–8. [DOI] [PubMed] [Google Scholar]
- 2. Moore MA, Biggs PJ. Unilateral adrenal hemorrhage: an unusual presentation. South Med J 1985;78:989–92. [DOI] [PubMed] [Google Scholar]
- 3. Cardwell MS. Spontaneous adrenal hemorrhage in pregnancy. A case report. J Reprod Med 1988;33:233–5. [PubMed] [Google Scholar]
- 4. Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg 2012;36:75–82. [DOI] [PubMed] [Google Scholar]
- 5. Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: a review of the literature and a case report of successful conservative management. Obstet Gynecol Surv 2005;60:191–5. [DOI] [PubMed] [Google Scholar]
- 6. Dhawan N, Bodukam VK, Thakur K, Singh A, Jenkins D, Bahl J. Idiopathic bilateral adrenal hemorrhage in a 63-year-old male: a case report and review of the literature. Case Rep Urol 2015;2015:503638 doi:10.1155/2015/503638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P. Spontaneous unilateral adrenal hemorrhage: computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol 1995;154:1647–51. [DOI] [PubMed] [Google Scholar]
- 8. Kobayashi T, Uenoyama S, Miura K, Takehara Y. Idiopathic unilateral adrenal hematoma: report of a case. Surg Today 2004;34:279–82. [DOI] [PubMed] [Google Scholar]
- 9. Kawashima A, Sandler CM, Ernst RD, Takahashi N, Roubidoux MA, Goldman SM, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics 1999;19:949–63. [DOI] [PubMed] [Google Scholar]
- 10. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med 2003;139:194–204. [DOI] [PubMed] [Google Scholar]