ABSTRACT
Background
Assisted home dialysis (AHD) is an option to combine the benefits of home dialysis therapy with the needs of dialysis patients who are unable to perform self-treatment at home. While this method is growing in many countries worldwide, no data so far are reported for Germany.
Methods
A survey was designed to identify the barriers to the implementation of AHD with the focus on attitudes and beliefs concerning AHD. The survey was sent to all 2060 members of the Germany Society of Nephrology.
Results
The response rate was 14% of nephrologists (n = 286), representing 24% of all German centres. AHD was regarded as a highly meaningful option (>90% of all responding nephrologists). Fifty-five percent of the centres practice AHD (preferred peritoneal dialysis). The number of treated patients on AHD was small (77% of the centres treat no more than 10 patients). The nephrologists in centres that performed AHD were of older age and the number of dialysis patients treated in these centres was greater. AHD was offered in 57% of centres at chronic kidney disease Stage 4. Inadequate conventional dialysis and patient’s request were reasons for choosing AHD. Barriers for offering AHD were lack of reimbursement, shortage of staff, lack of expertise and lack of team motivation.
Conclusions
In the view of German nephrologists, AHD is a meaningful method to provide home dialysis care. Inadequate funding and a lack of qualified staff were identified as severe barriers to implementation of AHD. To overcome these barriers and to achieve a higher penetration of AHD, dedicated actions have to be considered. Further studies are needed to prove the AHD concept with regard to outcome effects and cost efficacy.
Keywords: assisted home dialysis, haemodialysis, outcome, peritoneal dialysis, reimbursement
Introduction
Home dialysis options worldwide are increasingly used, supported by the reported benefits of extended treatment schedules and patients’ well-being in self-care treatment [1]. Home dialysis is suggested to be a care system rather than a treatment [2]. Critical aspects in this system are patient information and decision making, expertise and motivation of the team of the caregivers and funding policy [2–5]. Taking into account the worldwide growth of the ageing population, older patients should not be excluded from the benefits of home dialysis [6–9]. Older chronic kidney disease (CKD) patients tend to present later for dialysis, have a higher number of comorbid conditions and are at higher risk of loss of cognitive function and most of them are dependent on professional care at home or in senior or nursing centres. These aspects are viewed by renal professionals as barriers to home dialysis treatment [9].
Assisted home dialysis (AHD) overcomes these barriers. AHD is suitable for (elderly) patients who are unable to perform treatment themselves [10]. This includes assistance by visiting caregivers (nurses and others) for several tasks to be performed at home [10]. To start and continue AHD with peritoneal dialysis (PD) is suggested to be a method of choice for the ageing dialysis population and provides a high quality of life [11–13]. In some cases, AHD closes the gap between active treatment to prolong life and the stage of palliative care where the main focus of treatment is best quality of life [12–14]. Remaining in the home setting is highly desired in the view of many affected patients [12].
AHD—PD and haemodialysis (HD)—has been initiated in many countries in Europe, the US, Canada and Australia [12]. In Germany, so far experience is limited and rarely published, although the age of the dialysis population has been continuously growing for 20 years, reaching a median age of 71 years in patients who started dialysis in 2014 [15]. While in some countries AHD is regularly reimbursed by public funding [16], in Germany, dialysis costs are reimbursed by a fixed payment per week with a small surcharge for PD and HD but no additional payment for professional assistance. For single cases where AHD is applied, special agreements need to be approved by the patient’s health care insurance.
Taking into account the financial obstacle and the lack of current data in Germany, this survey was designed to explore the current practice of AHD in renal care, nephrologists’ attitudes and presumed barriers for implementing AHD in routine dialysis care.
Materials and methods
Survey
The survey was developed with three sections adapted from the survey of Jayanti et al. [17]. The survey contains six questions on the structure of the dialysis center (Part 1), six questions on current practice patterns (Part 2) and two questions on presumed barriers to the implementation of AHD (Part 3). An option of additional remarks at the end of the questionnaire was provided. Parts 2 and 3 contained multiple choice questions with single or multiple answer options.
The survey was developed in LimeSurvey [18] and mailed to all 2060 members of the German Society of Nephrology (DGfN). Survey participation was voluntary and respondents remained anonymous. In the invitation letter, the members of the society were asked to report on the current details and practice of their individual centre. Ethical approval and formal consensus process was not considered. Survey responses were obtained between 13 April and 30 May 2016. Two reminders were sent to encourage participation.
Statistical analysis
All statistical analyses were performed using R statistical software (version 3.3.1; R Project for Statistical Computing, Vienna, Austria) [19]. Descriptive univariate analysis was performed for each question of the survey. All results were rounded off to the nearest whole number.
Regarding bivariate statistics, the dependence between answers to a pair of questions was tested using Fisher’s exact test for count data. Tests were performed investigating the dependence between answers of centres offering or not offering AHD and for the different modalities of AHD (PD and HD).
Results
Demographics of the centres
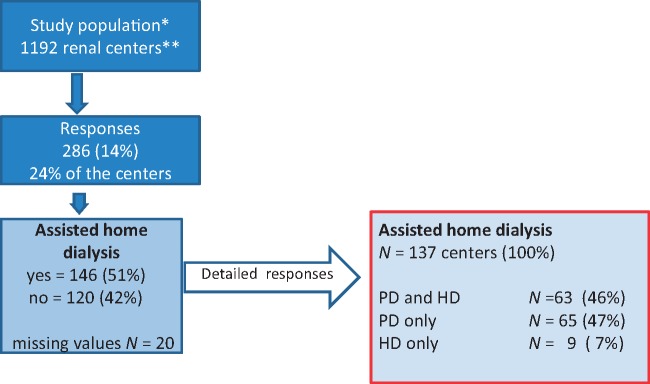
A total of 286 members of the 2060 contacted members answered the survey (14%). Of the 1192 contacted centres in Germany [20], 286 (24%) answered the survey (Figure 1). Response rates for the different questions were between 255 (89%) and 278 (97%) answers.
Fig. 1.
Overview of the study population and responses. *, Members of the German Society of Nephrology (DGfN) in April 2016 (N = 2060). **, ISN 2014 (KH4L-Report) [20].
Twenty-three percent of respondents were <45 years of age, 44% between 45 and 54 years and 33% were ≥55 years (Table 1). Sixteen percent of respondents have <50 patients in their overall dialysis program, 45% between 51 and 120 patients and 40% had >120 patients (Table 1).
Table 1.
Features of the study population
| Items | Number of responding centres (%) |
|---|---|
| Physician age (years) | 267 (100) |
| <45 | 62 (23) |
| 45–54 | 123 (44) |
| <55 | 82 (33) |
| Centre size (number of patients) | 275 (100) |
| <51 | 44 (16) |
| 51–120 | 122 (45) |
| >120 | 109 (40) |
| Patients treated by AHD | 266 (100) |
| None | 120 (45) |
| 1–10 | 113 (42) |
| >10 | 33 (13) |
| Questiona: ‘… which method is meaningful?’ | 256 (100) |
| PD | 239 (93) |
| HD | 175 (68) |
| None | 9 (4) |
Multiple answers possible.
Clinical practice patterns
Answers from all respondent centres provided details on assistance options as follows (multiple answers possible): preparation of dialysis machines (n = 150), connection/disconnection of the dialysis treatment (n = 218), documentation of treatment or patient data such as blood pressure and body weight (n = 104), surveillance of treatment (i.e. blood pressure and ultrafiltration control) and at home (n = 97).
In 137 centres that practiced AHD treatment, 46% offered both PD and HD, 47% PD only and 7% HD only (Table 2). Currently 42% of the centres treated 1–10 patients with AHD, 13% treated >10 patients and 45% treated no patients.
Table 2.
Comparison of responses of centres that offer AHD by different modalities
| Characteristic | HD + PD | HD only | PD only | P-value |
|---|---|---|---|---|
| Number of centres (n = 137) | 63 (46%) | 9 (7%) | 65 (47%) | |
| Assistance useful in | ||||
| Preparing dialysis machines | 34 | 4 | 42 | n.s. |
| Connecting to device | 54 | 8 | 59 | n.s. |
| Documentation of treatment parameters | 20 | 1 | 33 | 0.02 |
| Surveillance at home | 27 | 2 | 24 | n.s. |
| Financial disadvantages to take on more patients | n.s. | |||
| Yes | 22 | 4 | 16 | |
| No | 24 | 1 | 24 | |
| Not sure | 17 | 4 | 25 | |
| Barriers | ||||
| Lack of adequate funding | 36 | 8 | 44 | 0.02 |
| Lack of professional staff | 25 | 4 | 42 | n.s. |
| Lack of expertise | 7 | 1 | 23 | 0.02 |
| Lack of motivation | 14 | 1 | 17 | n.s. |
| None of them | 20 | 0 | 6 | <0.01 |
| Time of offering AHD | n.s. | |||
| CKD Stage 4 routinely | 37 | 3 | 38 | |
| At stage of dialysis | 8 | 1 | 9 | |
| If dialysis is inadequate using conventional dialysis | 12 | 3 | 6 | |
| On patient’s request at any time | 6 | 2 | 11 | |
| Offering of AHD option | n.s. | |||
| To all patients | 19 | 1 | 10 | |
| Patients who request AHD | 21 | 3 | 21 | |
| All patients with impaired intellectual or physical capacity | 45 | 7 | 51 | |
n.s., not significant.
In the view of the respondents (n = 137), AHD should be offered to all patients complaining of the high burden of home dialysis (22%), at the request of the patient (33%) and to all home-treated patients with impaired capabilities (75%) [multiple answers possible (Table 2)].
AHD was routinely offered to all patients in CKD Stage 4 (57%), in patients who already undergo dialysis treatment (13%), in 15% if conventional dialysis is insufficient and in 14% when patients spcifically asked for AHD treatment (Table 2).
Nephrologists’ beliefs and attitudes towards assisted dialysis
AHD is suggested to be meaningful by 96% of the respondents. PD is the preferred method (93%). Nine (4%) respondents believed that AHD makes no sense at all (Table 1).
Most of the respondents (67%) work in an organization where new ideas are well appreciated (Table 3). Resistance to changeing established procedures or rules that limit the consideration of new possibilities were less frequent (11% and 20%) (Table 3).
Table 4.
Proposal for further actions to implement AHD as derived from results of the survey
| Area | Further actions |
|---|---|
| Scientific society | Provide recommendations, guidelines and standards. |
| Community | Change focus of treatment from conventional in-centre care to home treatment. |
| Health care payers | Establish regular and adequate funding for professional assistance. |
| Industry | Provide safe and easily usable devices. Implement telemedicine. |
| Renal units | Change motivation to home treatment. Encounter all key persons. Establish networks to realize support of patients in different settings (at home, nursing homes, hospital and community care). Train staff and caregivers for home care assistance. |
| Kidney patients | Get timely information on treatment options. Request for home dialysis and support options. Be trained according to personal needs. |
| Family, partners and caregivers | Support patient to start and continue treatment at home. Claim for adequate funding of assistance. |
| Researchers | Design studies to prove the concept and the impact of significant endpoints (morbidity, treatment-related complications, quality of life, cost-effectiveness, etc.). |
Table 3.
Comparison of features in centres with and without option of AHD
| AHD | Yes | No | P-value |
|---|---|---|---|
| Number of centres (n = 266) | 146 (55%) | 120 (45%) | |
| Age of the respondent (years) | 0.02 | ||
| <45 | 21 | 34 | |
| 45–54 | 74 | 46 | |
| >54 | 51 | 40 | |
| Prevalent patients treated (n) | <0.01 | ||
| <51 | 6 | 36 | |
| 51–120 | 67 | 53 | |
| >120 | 73 | 31 | |
| Fear of reimbursement problemsa | |||
| Yes | 42 | 43 | |
| No | 49 | 25 | |
| Not sure | 47 | 50 | 0.04 |
| Barriers (multiple answers possible) | |||
| Inadequate funding | 88 | 82 | 0.74 |
| Lack of staff | 71 | 62 | 0.41 |
| Lack of expertise | 31 | 40 | 0.15 |
| Lack of team motivation | 32 | 34 | 0.66 |
| None of them | 26 | 11 | 0.03 |
| Motivation to start new treatment optionsa | <0.01 | ||
| Very appreciated | 109 | 67 | |
| No because of resistance | 14 | 15 | |
| No because of restrictions and financial risks | 18 | 35 | |
| Not sure | 4 | 2 |
Missing responses.
Forty percent of respondents try to convince their patients to choose the dialysis modality that offers the best option, even if the patients are doubtful about it. Fifty-two percent do this sometimes, 6% never try to persuade their patients and 2% are not sure about it.
Barriers to assisted dialysis
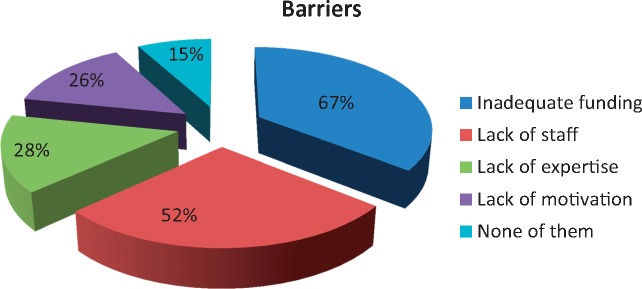
The ranking of barriers reported from centres (N = 255) was lack of funding [n = 170 (67%)], shortage of staff [n = 133 (52%)], lack of expertise or experience [n = 71 (28%)], lack of team motivation [n = 66 (26%)] and none of these [n = 37 (15%)] (Figure 2) (multiple answers possible).
Fig. 2.
Barriers to AHD (responses from n = 255 centres) (multiple answers possible).
The most important barriers to expanding further AHD treatment reported by centres (n = 137) who actively run assisted dialysis are lack of adequate funding [n = 88 (64%)], lack of professional staff [n = 71 (52%)], lack of expertise [n = 31 (23%)] and presumed demotivation [n = 32 (23%)] (multiple answers possible). Twenty-six (19%) stated none of these conditions (Table 2).
Centre differences
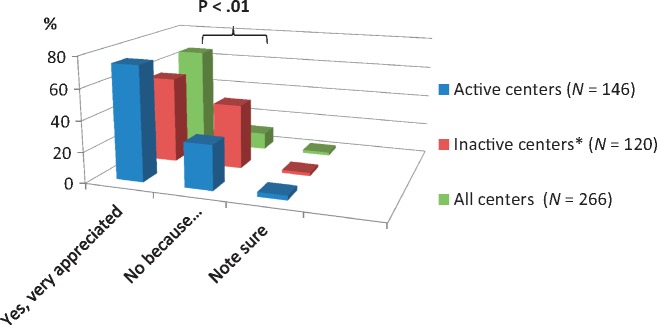
Between centres offering AHD and those who do not, significant differences are found in the following items (Table 3): respondents from active AHD centres are in the higher age group of >45 years (86% versus 71%; P < 0.05) and are caring for a greater number of dialysis patients (51–120 and >120 patients; P < 0.001). Suggestion of financial disadvantages was expressed in more centres that do not currently offer AHD (P < 0.05). Motivation to start new treatment options was reported higher in active AHD centres compared with centres without AHD. A total of 109 of 145 active centres (75%) versus 67 of 120 inactive centres (56%) ‘very appreciated’ new treatment options (Table 3 and Figure 3). In both groups, the proportion of demotivation by resistance of team members was similar while fear of restrictions and financial risks were expressed more in inactive centres (for detailed numbers, see Table 3). Smaller differences were found with regard to the offered type of AHD and practice patterns (Table 2). Lack of expertise was less likely in centres that offer both HD and PD, compared with centres that provide PD only (P < 0.05). No significant differences were found with regard to the method of the established treatment: centres that offer PD only compared with centres that offer HD only or both shared similar patterns in the offering and timing of AHD (Table 2)
Fig. 3.
Motivation to start new treatment options by centres (details see text). *, Centres with no patients on assisted dialysis.
Discussion
The primary goal of this survey was to gain information on the attitudes, beliefs and practices of nephrologists towards AHD in Germany. More than 90% of all respondents stated that AHD is a meaningful option. Fifty-five percent of the centres that responded to the survey practice this option with a small number of patients (75% with 1–10 patients). The preferred method is assisted PD, while about half of the centres offered both assisted PD and HD (Table 2). The majority of respondents (62%) consider AHD in those patients with limited intellectual or physical function. The timing of offering AHD varies: the majority of centres that responded to the survey provide this option at Stage 4 CKD (57%). A smaller group of centres (15%) considered this for patients when conventional dialysis is inadequate and the efficacy of treatment must be increased. Fourteen percent of the centres offered AD only at the request of dialysis patients (and their families) (Table 2).
The attitude of nephrologists concerning home dialysis is inconsistent [21]. Home dialysis is generally accepted to be a good treatment [1, 2]. But a gap between attitudes and reality remains: in Germany, only a small proportion of the total end-stage renal disease population is treated with home dialysis (HD ∼1%, PD 4–5%) [15]. Thus the positive attitude towards AHD in this report and the result that 55% of the responding members of the society actively practised this treatment is a surprise and strong indication of by reporting bias. No further data are published so far to control for this assumption.
In the survey, AHD is offered more in centres with physicians in the higher age group (Table 3). This could be explained by a greater level of expertise and experience in renal care. Furthermore, AHD seems to be offered more in centres with a larger dialysis population. Together with the significant finding that active AHD treatment is more prevalent in centres with highly motivated staff (Table 3), specific centre factors may exist that influence practice patterns. In contrast, a quarter of centres who do not offer AHD report a lower proportion of staff who are motivated to start new treatment options for different reasons (Table 3). More experienced physicians with highly motivated staff in bigger centres, as described in other reports [22], seems to be a prerequisite for AHD. Especially in the elderly, individualization of dialysis modalities with the option of AHD needs a motivated team, time and a consistent approach of shared decision making [23–26].
Only half of the centres in this report who practice AHD offered AHD before dialysis initiation (Table 2). Together with the attitude of offering AHD only on a patient's request seems to contradict the process of timely information and shared decision making. Results from different reports indicate that a fully informed (older) patient and pre-dialysis care significantly influence outcome parameters [23–26].
Both centres that offer AHD and those that do not suffer from a lack of qualified staff (Table 3). Staff qualification is critical in Germany: 20 years ago, two-thirds of nurses reported a shortage of registered nurses to provide high-quality care [27]. Due to demographic changes in the German population, today this situation is more aggravated. While professional staff in nursing homes can be qualified for AHD—in most cases for cycler PD—trained nurses for home care are very rare. Although an oversupply of physicians is reported in some German regions [28], the situation in nephrology is quite similar to the situation in nursing care. Thus a shortage of professionals may influence the proliferation of innovation in patient care like AHD.
Reimbursement of AHD is a second barrier reported in this survey. Home dialysis itself is considered a cost-saving method compared with in-centre dialysis [3], although consideration of hidden costs should be taken into account [29]. For AHD, additional costs for staff assistance are to be calculated. Funding issues are identified in many reports as barriers to extend home dialysis [2–4]. Costs and reimbursement for (PD) home assistance vary widely in Europe and Canada [16]. AHD is suggested as a cost-effective treatment [3] not exceeding the costs of in-centre dialysis [16]. Arbitrary calculations from individual cases in our institution result in cost savings of ∼€13 000/case/year, mostly resulting from avoiding transport to in-centre treatment.
Qualification of professional staff along with adequate funding remain key issues in the proliferation of AHD. ‘Home dialysis first’ [6] provokes a cultural change from the prevalent system of standard in-centre dialysis to a more patient-focused approach [29]. Motivation of the renal team for new approaches in clinical practice remains a challenge [22, 30].
Limitations of our study may result from reporting bias of centres that advocate and successfully practice home dialysis. Further studies are needed to prove the concept of AHD.
So far, AHD seems to be an emerging field of renal replacement therapy in Germany. AHD is an option for patients whose quality of life is benefitted by home treatment [13, 28]. Life situations in these patients may be different, including patients who need more effective treatment or palliative care [13, 30–33]. Results from this survey indicate further actions in different areas to obtain sufficient information on the effects of a broader use of AHD (Table 4).
Conflict of interest statement
W.P. is a consultant on the project ‘Network Assisted Dialysis’ (PMC-Pflege Managed Care, Berlin) and received honoraries for lectures from FMC-Fresenius Medical Care, Germany. No conflicts of interest were stated by the other authors.
References
- 1. Palmer SC, Palmer AR, Craig JC. et al. Home versus in-centre haemodialysis for end-stage kidney disease. Cochrane Database Syst Rev 2014; 11: CD009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piccoli GB, Salomone M.. Home hemodialysis: a system and not only a treatment [letter]. Kidney Int 2010; 78: 819. [DOI] [PubMed] [Google Scholar]
- 3. Walker RC, Howard K, Morton RL.. Home hemodialysis: a comprehensive review of patient-centered and economic considerations. ClinicoEcon Outcomes Res 2017; 9: 149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Komenda P, Copland M, Makwana J. et al. The cost of starting and maintaining a large home hemodialysis program. Kidney Int 2010; 77: 1039–1045 [DOI] [PubMed] [Google Scholar]
- 5. Robinson BM, Akizawa T, Jager KJ. et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016; 388: 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thodis ED, Oreopoulos DG.. Home dialysis first: a new paradigm for new patients. J Nephrol 2011; 24: 398–404 [DOI] [PubMed] [Google Scholar]
- 7. Prakash S, O’Hare AM.. Interacting of aging and CKD. Semin Nephrol 2009; 29: 497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tonelli M, Riella M.. Chronic kidney disease and the ageing population. Nephrol Clin Pract 2014; 128: 319–322 [DOI] [PubMed] [Google Scholar]
- 9. Tong A, Palmer S, Manns B. et al. Clinicians beliefs and attitudes about hemodialysis: a multinational interview study. BMJ Open 2012; 2: e002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dimkovic N, Aggarwal V, Khan S. et al. Assisted peritoneal dialysis: what is it and who does it involve? Adv Perit Dial 2009; 25: 165–170 [PubMed] [Google Scholar]
- 11. Dimkovic N, Oreopoulos DG.. Assisted peritoneal dialysis as a method of choice for elderly with end-stage renal disease. Int J Urol 2008; 40: 1143–1150 [DOI] [PubMed] [Google Scholar]
- 12. Brown EA, Johansson L.. Dialysis options for end-stage renal disease in older people. Nephron Clin Pract 2011: 119: c10–c13 [DOI] [PubMed] [Google Scholar]
- 13. Iyasere OU, Brown EA, Johansson L. et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialyis. Clin J Am Soc Nephrol 2016; 11: 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aydede SK, Komenda P, Djurdjev O. et al. Chronic kidney disease and support provided by home care services: a systematic review. BMC Nephrol 2014; 15: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MNC. Jahresbericht zur Qualität in der Dialyse. https://www.g-ba.de/downloads/17-98-4166/2016-09-15_QSD-RL_MNC-Jahresbericht-2015_Einleitung-und-Jahresbericht.pdf (13 March 2017, date last accessed)
- 16. Dratwa M. Cost of home assistance for peritoneal dialysis: results of a European survey. Kidney Int 2008; 73: S72–S75 [DOI] [PubMed] [Google Scholar]
- 17. Jayanti A, Morris J, Stenvinkel P. et al. Home hemodialysis: beliefs, attitudes, and practice patterns. Hemodial Int 2014; 18: 767–776 [DOI] [PubMed] [Google Scholar]
- 18. LimeSurvey Project Team/LimeSurvey. LimeSurvey: An Open Source Survey Tool LimeSurvey Project, Hamburg, Germany, 2015. http://www.limesurvey.org (14 March 2017, date last accessed)
- 19. R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing, 2013. http://www.R-project.org
- 20.Kidney Health for Life (KH4L). Chronic Kidney Disease Multinational Inventory (March 31, 2014). https://www.theisn.org/images/Initiatives/KH4L_-_CKD_Multinational_Inventory.pdf) (14 March 2017, date last accessed)
- 21. Fluck RJ, Fouque D, Lockridge RS Jr.. Nephrologists’ perspectives on dialysis treatment: results of an international survey. BMC Nephrol 2014; 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishani A, Slinin Y, Greer N. et al. Comparative effectiveness of home-based kidney dialysis versus in-center or outpatient kidney dialysis locations—a systematic review. VA ESP Project No. 09-009. Washington, DC: Department of Veterans Affairs, 2015 [PubMed]
- 23. Kooman JP, Cornelis T, van der Sande FM. et al. Renal replacement therapy in geriatric end-stage renal disease patients. A clinical approach. Blood Purif 2012; 33: 171–176 [DOI] [PubMed] [Google Scholar]
- 24. Chanouzas D, Ng KP, Fallouh B. et al. What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Tranplant 2012; 27: 1542–1547 [DOI] [PubMed] [Google Scholar]
- 25. Morton RL, Tong A, Howard K. et al. The view of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ 2010; 340: c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fischer MJ, Stroupe KT, Kaufman JS. et al. Predialysis nephrology care and dialysis-related health outcomes among older adults initiating dialysis. BMC Nephrol 2016; 17: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aiken LH, Clarke SP, Sloane DM. et al. Nurses’ reports on hospital care in five countries. Health Aff 2001; 20: 43–53 [DOI] [PubMed] [Google Scholar]
- 28. Kabene SM, Orchard C, Howard JM. et al. The importance of human resources management in health care: a global context. Hum Resour Health 2006; 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laplante S, Krepel H, Simons B. et al. Offering assisted peritoneal dialysis is a cost-effective alternative to the current care pathway in frail elderly patients. Int J Healthc Manag 2013; 6: 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown EA, Wilkie M.. Assisted peritoneal dialysis as an alternative to in-center hemodialysis. Clin J Am Soc Nephrol 2016; 11: 1522–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown EA. How to address barriers to peritoneal dialysis in the elderly. Perit Dial Int 2011; 31: S83–S85 [DOI] [PubMed] [Google Scholar]
- 32. Querido S, Branco PQ, Costa E. et al. Results in assisted peritoneal dialysis: a ten years experience. Int J Nephrol 2015; 2015: 712539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown EA, Finkelstein FO, Ivasere OU. et al. Peritoneal or hemodialysis for the frail elderly patient, the choice of 2 evils? Kidney Int 2017; 91: 294–303 [DOI] [PubMed] [Google Scholar]