Abstract
Introduction:
In January 2008 the US Food and Drug Administration issued a warning to healthcare professionals about the potential for an increased risk of suicidal thinking and behavior associated with antiepileptic drugs (AEDs). Given that AEDs are important for treating bipolar disorder (BD), a better understanding of suicide-related events is necessary.
Methods:
A PubMed search was performed using the following search terms: anticonvulsant OR valpro* OR carbamazepine OR lamotrigine OR oxcarbazepine OR topiramate AND bipolar AND suicid*. The objective was to identify published investigations reporting rate and/or risk data of suicide-related outcomes in BD patients treated with AED monotherapy.
Results:
The search identified 323 reviewable citations, with 13 of these studies (4.0%) being reviewed. Valproate was studied most often, and lithium treatment was frequently used as a reference group. Carbamazepine and lamotrigine had small treatment exposure durations. Suicide attempts and suicide deaths were studied the most; a few trials investigated suicidal thinking and/or hospitalizations for suicidal behavior. Suicide attempt rates occurred in the following order: no treatment > carbamazepine > valproate > lithium, while suicide death rates were: no treatment > valproate > lithium > carbamazepine. For valproate, the risk of suicide attempts and suicide death appeared higher than lithium, but lower than no treatment.
Discussion:
Investigating suicide-related events for AEDs in BD is difficult; more data are necessary for valproate, carbamazepine, and lamotrigine. An improved understanding of AED treatment and suicide-related events in BD may help pharmacists become more effective at supporting their patients with BD.
Keywords: valproate, carbamazepine, lamotrigine, bipolar disorder, suicide attempt, suicide completion
“Suicide is the anchor point on a continuum of suicidal thoughts and behaviors. This continuum is one that ranges from risk-taking behaviors at one end, extends through different degrees and types of suicidal thinking, and ends with suicide attempts and [death by] suicide. For some people, suicide is a sudden act; for others, it is a long-considered decision based on cumulative despair or dire circumstances. For many, it is both—a brash moment of action taken during a span of settled and suicidal hopelessness. The suffering of the suicidal person is private and inexpressible…”1
Introduction
Bipolar disorder (BD) is a complex mental illness that has a well-known association with suicidal ideation, suicide attempts, and death by suicide. The 12-month prevalence rates for the bipolar spectrum disorders have been reported to be 0.6% for Bipolar I, 0.8% for Bipolar II, and 1.4% for sub-threshold BD (lifetime prevalence rates are 1.0%, 1.1%, and 2.4%, respectively).2 The lifetime risk for attempting suicide in BD is up to 40%,3 with 3.9% of untreated BD individuals attempting suicide annually.4 Completed suicide in BD accounts for 15% to 20% of deaths,3 with 1.4% of untreated BD patients dying by suicide annually.4 It has been estimated5,6 that BD may account for as much as one quarter of all suicide deaths. Recently it was reported7 that there were 44 965 suicide deaths in 2016 and that suicide was the 10th leading cause of death in the United States that year. If one quarter of those dying by suicide had bipolar disorder, then it could mean an estimated 11 241 people with BD would have died from suicide in 2016.
In January 2008 the US Food and Drug Administration (FDA) issued a warning to health care professionals about the potential for an increased risk of suicidal thinking and behavior associated with antiepileptic drugs (AEDs). For its analysis, the FDA used data from 199 randomized clinical trials of 11 AEDs involving 43 892 study participants (27 863 [63.5%] exposed to an AED and 16 029 [36.5%] exposed to placebo).8 In the “Warnings and Precautions” section of AED product labels, it is stated that the risk of suicidality for those treated with AEDs during clinical trials was approximately twice that of placebo treatment (0.43% vs 0.24%; adjusted relative risk = 1.8, 95% confidence interval [CI]: 1.2, 2.7). During the clinical trials there were 4 suicide deaths in AED-treated patients and no suicide deaths in placebo-treated patients.8 One result from this labeling is that pharmacy practitioners acquired a professional responsibility to educate and support AED-treated patients about symptom changes that involve thoughts or behaviors centered on self-harm.
A subset of the 11 AEDs implicated by FDA have been cornerstone treatments for BD. Thus, this FDA warning has potentially important implications on the clinical utility of those AEDs for the treatment of BD. However, since the time this warning was communicated, there have been questions raised by practitioners about the concept of suicide being an “adverse effect.” First, suicidal behavior that may occur during AED treatment in BD is not likely to be solely a receptor-based side effect as, for example, sedation or increased appetite might be for atypical antipsychotics with clinically relevant histamine-1 receptor antagonism. Rather, suicide is more likely to be a complex psychological construct determined by a convergence of multiple variables. Second, when considering the causal risks of suicidal behavior in BD, it is difficult to separate the contributions of the illness from those of its associated treatment(s), or any other non–illness-based risk factor. If there is a risk of suicidality with AED treatments in BD, it may be best conceptualized as being either a limitation of the therapeutic intervention, or perhaps treatment non-adherence. At this time, it is clear that significant gaps remain in our understanding of the potential for the pharmacologic provocation of suicide.9,10
Given the impact that suicide has on BD, and the potential for AEDs to at least be associated with suicidal behavior, it follows that pharmacists should understand what the published data is reporting. The goal of this review is to summarize the published research about the reported rates and risks of suicide for AED treatment in patients with BD.
Methods
A PubMed search for relevant papers was completed using the following search terms: anticonvulsant OR valpro* OR carbamazepine OR lamotrigine OR oxcarbazepine OR topiramate AND bipolar AND suicid*. No dates or timelines were imposed on the search so that the search yield could be optimized. Valproate, carbamazepine, and lamotrigine were included in the search because each have FDA-approved indications involving BD. Oxcarbazepine and topiramate were also included in the literature search given the local experiences of the authors engaging prescribers who may occasionally use these medications off-label to attempt mood stabilization treatment for certain individual patients with BD. The objective of the literature search was to identify published investigations with designs and methods that resulted in the reporting of rate and/or risk data of suicide-related outcomes in patients with BD treated with AED monotherapy. All pertinent articles were identified, and then papers that did not assess for the risk and/or rate of suicidal behavior in bipolar patients treated with AEDs were eliminated from review. After being identified, studies were analyzed to determine their suitability for final inclusion and comment. The definition of suicide-related outcomes included: suicidal ideation, hospitalizations related to suicidal behavior, attempted suicide, and/or completed suicide.
Results
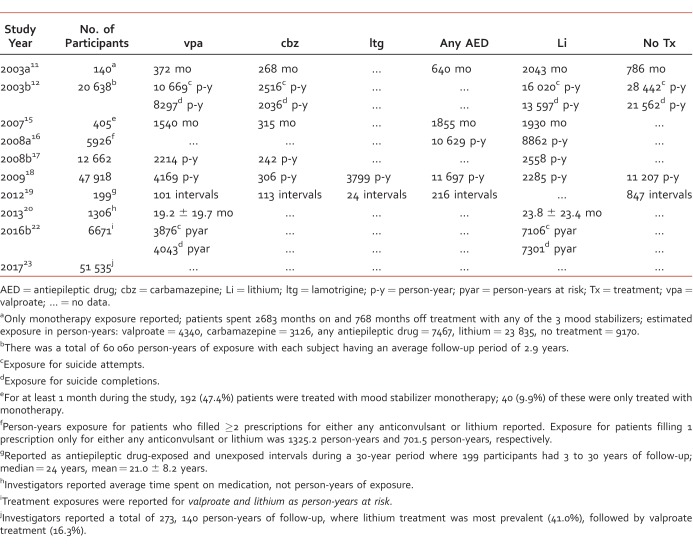
The literature search strategy generated a total of 323 reviewable citations, with 13 studies (4.0%)11-23 being selected for inclusion; all other citations were eliminated from review because they did not meet the primary objective. The selected investigations were either retrospective medical record reviews (n = 5)11,13-15,19 or retrospective database investigations (n = 8).12,16-18,20-23 Table 1 summarizes the characteristics of each study.
TABLE 1: .
Study characteristics
Most of the investigations selected were retrospective cohort studies (12/13; 92.3%).11-13,15-23 A total of 153 619 subjects (39.8% male) were studied and inclusion criteria for some investigations permitted additional diagnoses such as schizoaffective disorder bipolar type (5/13; 38.5%).11,13-15,20 The estimated number of subjects with diagnoses other than BD, however, was small (n = 323; 0.21% of all subjects). Among the AEDs of interest, valproate was the only AED included in all investigations. One trial16 grouped all patients receiving any AED treatment together into a single AED exposure group. Thus, individual study data specific to valproate, carbamazepine, or lamotrigine were available in 12/13 (92.3%),11-18,20-23 7/13 (53.8%),11-13,15,17,18,19,21 and 4/13 (30.8%)13,17,18,19 studies, respectively; lithium treatment arms were included in all but one investigation (92.3%).11-13,15-23 Regarding suicide-related outcomes, suicide attempts (11/13; 84.6%)11-13,15,17-23 and completed suicides (9/13; 69.2%)11-12,15-17,19,21-23 were the outcomes studied most frequently. A small number of studies also measured suicidal ideation13-14 and suicide-related hospitalizations.11,15 No study evaluated all 4 suicide-related outcomes. Specific diagnostic characteristics of study samples (ie, Bipolar I, Bipolar II) were provided for 38.5% of published investigations11,13-15,20 which represented 1.94% (n = 2979) of all study subjects. Of the studies providing diagnostic information, the majority of subjects had a Bipolar I diagnosis.
Treatment Exposure
Table 2 provides details about the duration study subjects spent exposed to mood stabilizer treatment. Investigators did not employ uniform methods for quantifying treatment exposure. Approximately half of studies with available data reported treatment exposure in terms of “person-years”; the remaining studies reported treatment exposure in terms of total months of exposure, average months of participation, or duration of follow-up during a given time period. Three investigations did not report treatment exposure.13,14,21
TABLE 2: .
Treatment exposure
From all mood stabilizers evaluated, lithium treatment appeared to have the largest total treatment exposure (>34 408 person-years), and valproate had the largest treatment exposure for all AEDs studied (>18 556 person-years). Carbamazepine and lamotrigine had substantially lower exposure times than valproate or lithium. In general, investigators did not evaluate the doses, serum concentrations, or treatment adherence of any AED or mood stabilizer being studied. Most investigations evaluated treatment exposures to a single mood stabilizer, not mood stabilizer combinations. Single mood stabilizer treatment was often not as sole drug therapy, but rather as part of a multi-drug regimen that could have included any other psychotropic medication.
Antiepileptic Drugs and Suicidal Ideation
Two studies13,14 evaluated AED-treated BD patients for suicidal thinking. Born et al13 evaluated suicidal thinking by identifying patients who had a positive suicidal thinking item score on the Inventory of Depressive Symptoms – Clinician Version rating scale during the period of review. The mean time of participation for patients was 13.3 ± 12.2 months (142.1 person-years), and AED mood stabilizers were compared to lithium. Positive rating scale scores were identified in 38/128 (29.7%; mean age = 45 ± 16.3 years) patients, and there were 99 positive ratings from all evaluated time points. Positive scores were identified in 17 lithium-treated patients (44.7%), 12 valproate-treated patients (31.6%), 6 carbamazepine-treated patients (15.8%), and 9 lamotrigine treated patients (23.7%). When compared to lithium, there were no significant differences in risk for suicidal thinking between treatments: lamotrigine relative risk = 0.85 (P = .17), valproate relative risk = 1.16 (P = .14), and carbamazepine relative risk = 1.54 (P = .14).
Goldberg et al14 performed a cross-sectional evaluation of the first 1000 patients who entered the Systematic Treatment Enhancement Program for Bipolar Disorder trial for their psychotropic medication use and suicidal thinking at study entry. The investigators identified patients who had a positive suicidal thinking item score on the Affective Disorders Evaluation rating scale. Study participants had a mean age of 40.6 ± 2.7 years and were mostly white (92%) and female (59%). These participants predominantly had Bipolar I disorder (71%) and were mostly euthymic (61%) at study entry. Positive Affective Disorder Evaluation ratings were identified for 211 (21%) patients, with suicidal thinking being significantly more likely to have been occurring in patients experiencing depressed or mixed episodes than manic, hypomanic, or euthymic episodes (P < .001). For these patients, the rates of suicidal thinking were not significantly different in patients who were treated with lithium vs patients who were not taking lithium (22% vs 26%). There were similar findings for those taking vs not taking valproate (20% vs 22%). Significant associations were identified between the presence of suicidal thinking and severity of illness, current depressive episode, severity of depressive episode, history of a suicide attempt, and the male sex. Use of lithium (P = .048), but not valproate (P = .330), was significantly associated with suicidal thinking.
Antiepileptic Drugs and Hospitalizations for Suicidal Behavior
Two studies11,15 investigated BD patients treated with AEDs for hospitalizations related to suicidal thinking or behavior. Yerevanian et al11 reported the results of a retrospective medical record review of 140 patients with BD who had been treated continuously for a minimum of 6 months. Patients included in the study were required to have a DSM diagnosis of BD (I or II), cyclothymia, or schizoaffective disorder, bipolar type. From the investigation it was reported that during valproate and carbamazepine monotherapy, patients were exposed for a total of 372 and 268 months, respectively. Each treatment had 2 hospitalizations for suicidal thinking or behavior, yielding rates of 6.45 and 8.96 per 100 person-years, respectively. Lithium treatment exposure occurred during a total of 2043 months, and patients had 15 hospitalizations for a rate of 8.81 per 100 person-years. There was no significant difference in these rates among the 3 treatments (P = .91).
In a second report, Yerevanian et al15 reported findings from a similarly designed study of 405 BD patients. The investigators only analyzed monotherapy treatment periods of lithium, valproate, or carbamazepine. From the investigation it was reported that during valproate and carbamazepine monotherapy, patients were exposed for a total of 1540 and 315 months, respectively. Valproate treatment had 6 hospitalizations, and carbamazepine had 1 hospitalization related to suicidal thinking or behavior, yielding rates of 4.67 and 3.80 per 100 person-years, respectively. Lithium treatment exposure totaled 1930 months, and patients had 4 hospitalizations for a rate of 2.49 per 100 person-years. There was no significant difference in these rates among the 3 treatments (P = .61).
Antiepileptic Drugs and Suicide Attempts
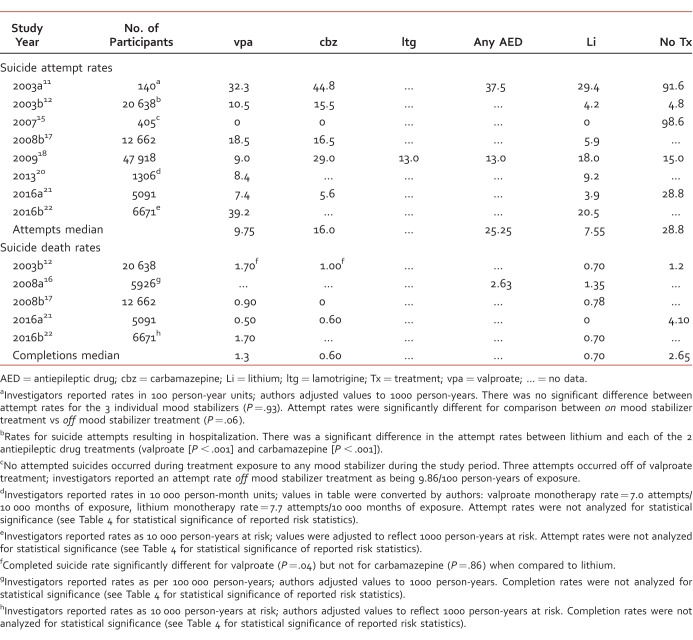
For studies with available data, investigators reported 1473 suicide attempts for subjects treated with valproate, 79 suicide attempts for carbamazepine, and 2219 suicide attempts for lithium.11-12,15,17-23 Table 3 summarizes the studies reporting suicide attempt rates for individual treatments. In most investigations, estimated suicide attempt rates for AED treatments were numerically greater than for respective lithium treatment rates. Valproate treatment had suicide attempt rates ranging from 7.4 to 39.2 per 1000 person-years, carbamazepine treatment ranged from 5.6 to 44.8 attempts per 1000 person-years, and lithium treatment ranged from 3.9 to 29.4 attempts per 1000 person-years. Rates reported for subjects receiving no mood stabilizer treatment ranged from 4.8 to 98.6 attempts per 1000 person-years. One investigation reported the suicide attempt rate for lamotrigine as being 13.0 per 1000 person-years (50 attempts during 3799 person-years).
TABLE 3: .
Suicide attempt and death rate estimates (per 1000 person-years)
Only 2 investigations compared the suicide attempt rates associated with individual mood stabilizer treatment for statistical significance, and the results were conflicting. Yerevanian et al11 reported that suicide attempt rates for valproate, carbamazepine, and lithium in 140 subjects were not significantly different from each other (P = .93). However, Goodwin et al12 (n = 20 638 subjects) reported that the suicide attempt rates related to both valproate and carbamazepine were significantly higher than for lithium (P < .001).
Two investigations18,20 reported suicide attempt rates for lithium that were numerically, but not significantly, higher than for valproate (18.0 vs 9.0 per 1000 person-years and 9.2 vs 8.4 per 1000 person-years). Neither trial compared these rates against each other for statistical significance. In the only trial providing an estimate of suicide attempt rates for lamotrigine, lithium's rate was higher than lamotrigine's (18.0 vs 13.0 per 1000 person-years, respectively).18
The highest suicide attempt rate reported for AEDs was from investigators who conducted a retrospective cohort study in the United Kingdom. Using a 19-year period, the investigators collected patient data from primary care electronic health records in order to compare rates of suicide attempts in patients prescribed lithium, valproate, olanzapine, or quetiapine (n = 6671). Valproate-treated patients had an attempt rate of 39.2 per 1000 person-years, and lithium treatment had an attempt rate of 20.5 per 1000 person-years. The investigators reported that the number of suicides was too low to show any differences by individual medications.
The lowest suicide attempt rate reported for AEDs (valproate = 7.4 attempts per 1000 person-years, and carbamazepine = 5.6 attempts per 1000 person-years) were from a study with a methodology that focused on each subject's final prescription received 30 days before the study endpoint, which was defined as either reaching the end of the study period without a suicide event, or experiencing a suicide event.21 Tsai et al21 were interested in studying the short-term antisuicidal effects of each mood stabilizing agent (valproate, carbamazepine, or lithium). This methodology differs from the other studies which largely quantified suicide attempt rates during a subject's exposure to a studied mood stabilizer. Comparisons for AED-treated subjects were made against subjects who had not received mood stabilizer treatment within the 30 days prior to study end-point, not against lithium-treated subjects who had a rate of 3.9 attempts per 1000 person-years.
Antiepileptic Drugs and Death by Suicide
For studies with available data,11-12,15-17,19-23 investigators reported 131 suicide deaths for subjects treated with valproate, 5 suicide deaths for carbamazepine, and 259 suicide deaths for lithium. Table 3 also summarizes the studies that reported death by suicide rates for individual mood stabilizing treatments. Ranges for suicide completion rates for each treatment were 0.50 to 1.70 per 1000 person-years for valproate, 0.00 to 1.00 per 1000 person-years for carbamazepine, and 0.00 to 1.35 per 1000 person-years for lithium. Lamotrigine did not have data reported for an estimated suicide completion rate, and subjects exposed to no mood stabilizer treatment had rates ranging from 1.20 to 4.10 per 1000 person-years. One investigation12 compared the suicide completion rate between lithium and AEDs and found that the rate for valproate was significantly higher (P = .04) and that the rate for carbamazepine was not significantly different from lithium (P = .86).
The lowest suicide completion rates reported for AEDs (valproate = 0.5 completions per 1000 person-years, and carbamazepine = 0.6 completions per 1000 person-years) were reported by Tsai et al.21 Comparisons for AED-treated subjects were made against subjects who had not received mood stabilizer treatment within the 30 days prior to study end-point, not against lithium-treated subjects who had a rate of 0 completions per 1000 person-years.
The highest suicide completion rate reported for AEDs was again by investigators conducting an electronic health records cohort study in the United Kingdom. Valproate-treated patients had a completion rate of 1.7 per 1000 person-years, and lithium treatment had a rate of 0.7 per 1000 person-years. The investigators reported that the number of suicides was too small to show any differences.
Risk for Suicide Attempts and Suicide Deaths
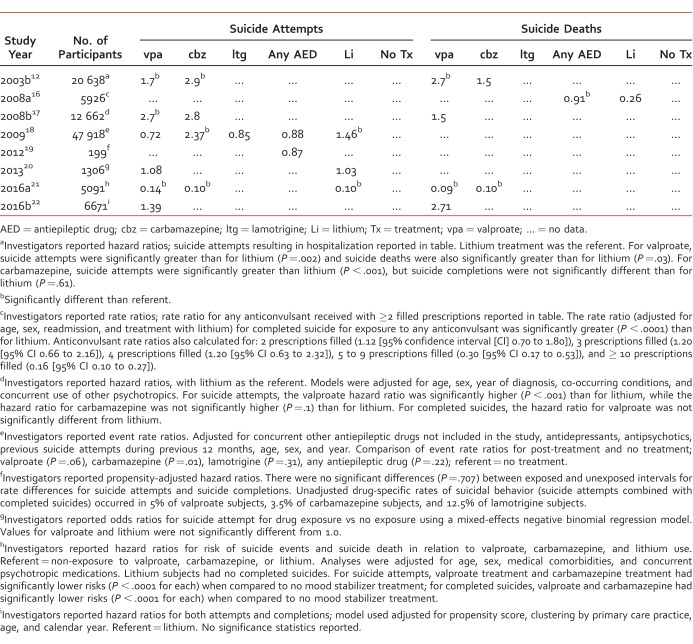
A total of 9 studies12,16-23 reported risk measurements for suicide attempts and/or suicide completions (Table 4), with more studies reporting risk data for suicide attempts. The risk statistic most commonly reported by investigators was the hazard ratio.12,17,19,21-23
TABLE 4: .
Risk for suicide attempts and deaths
With respect to hazard ratios for suicide attempts, 4 investigations12,17,19,22 used lithium as the reference group, while 2 investigations21,23 used no treatment, or non-exposure, as the reference group. Using lithium as the reference group, hazard ratios ranged between 1.39 (95% CI: 1.09, 1.78) and 2.7 (95% CI unavailable) for valproate, and from 2.8 (95% CI: 1.9, 4.4) and 2.9 (95% CI unavailable) for carbamazepine.12,17,22 The United Kingdom study22 reported that there were too few suicide attempts to perform significance testing; the Leon et al19 study reported a hazard ratio for suicidal behavior (attempts plus completions) related to exposure to any AED and reported no significant difference between exposure and non-exposure. Tsai et al21 reported suicide attempt hazard ratios for valproate, carbamazepine, and lithium as each being significantly lower (P < .0001 for each) than for non-exposure. While Song et al23 reported that the hazard ratio (attempts plus completions) for valproate in comparison with non-exposure was 1.02 (95% CI: 0.89, 1.15) and that it was significantly different (P = .038) than the hazard ratio for lithium compared with non-exposure (P = .86 [95% CI: 0.78, 0.95]).
Of the remaining 3 studies16,18,20 evaluating suicide attempts, just 2 investigations18,20 reported risk estimates. Gibbons et al18 reported event rate ratios for valproate, carbamazepine, lamotrigine, and lithium using non-exposure as the comparison. Both carbamazepine and lithium's event rate ratios were significantly greater. Ahearn et al20 reported suicide attempt rate odds ratios (OR) for valproate and lithium that were not significantly different from non-exposure.
Risk data reported for suicide deaths were reported in 5 studies.12,16,17,21,22 Hazard ratios were again the most common risk measurement calculated.12,17,21,22 Three of these studies12,17,22 used lithium as the reference group, and 1 study21 used non-exposure as the reference group. Compared to lithium, hazard ratios for valproate ranged between 1.5 (95% CI: 1.1, 6.3) and 2.7 (95% CI: 0.75, –9.80),12,17,22 and for carbamazepine it was 1.5 (95% CI: 0.13, 7.0).12 Søndergård et al16 reported significantly higher rate ratios for treatment with any AED compared with lithium treatment (0.91 vs 0.26; P < .0001), and Tsai et al21 reported hazard ratios for completed suicides that were significantly lower for valproate (hazard ratio = .09; P < .0001) and lithium when compared with non-exposure.
Discussion
Despite suicide being the 10th leading cause of death in the United States, from a research perspective it is a rare event (44 193 annual deaths in a population of >300 million).7,24 Further, studying suicide-related outcomes for AED treatments in patients with BD is a complex task because of the number of variables and risk factors that should be accounted for in any such analysis. Ideally, research on this topic should involve millions of at-risk individuals over the course of many years.
In the FDA report8 most subjects were exposed to either topiramate (27% of subjects) or pregabalin (24%), neither of which are approved for the treatment of BD. Closer inspection of the data gives additional perspective about valproate, carbamazepine, or lamotrigine with respect to suicidality. In the case of valproate, there were a total of 14 trials (7% of all included studies) involving 1327 (4.8%) exposed subjects. Of those valproate-treated subjects, most (1285; 96.8%) were enrolled in a study for a psychiatric indication (specific indications not provided). Valproate exposure was associated with 11 suicidal behavior / ideation events, which generated an OR of 0.72 (95% CI: 0.29, 1.84). The OR for the group of 11 AEDs was 1.80 (95% CI: 1.24, 2.66). The AEDs with the highest ORs included pregabalin (OR = 1.52), lamotrigine (OR = 1.78), zonisamide (OR = 1.96), levetiracetam (OR = 2.43), and topiramate (OR = 2.57). Further, there were 2 AEDs whose CI did not include a value of 1: lamotrigine (adjusted OR = 2.08 [95% CI: 1.03, 4.40]) and topiramate (adjusted OR = 2.53 [95% CI: 1.21, 5.85]). Using this expanded data from the FDA report, it does not appear that valproate was associated with an increased risk of suicide-related outcomes; however, lamotrigine does appear to have been associated with an increased risk. Carbamazepine had an OR of 0.65 (95% CI: 0.08, 4.42), and thus also did not appear to be associated with an increased risk of suicidality.
An important observation regarding the investigations selected for this review relates to the methodologic variability that investigators used to study this important treatment issue. Included in this variability was (1) an inconsistency in the studied outcomes; (2) a lack of detail with respect to the diagnosed type of BD; (3) an estimated 1.5:1 ratio of female-to-male subjects studied; (4) a lack of detail with respect to suicidality risk factors; (5) very little monitoring of mood stabilizer treatment adherence; and (6) variability in how treatment exposure was measured. Collectively, this means that data and results from these investigations should be interpreted cautiously and with a mindset that more research is necessary to become more certain about the impact that valproate, carbamazepine, and lamotrigine have on suicidality in BD. Further, it appears that some consensus is needed concerning the methods that investigators use to study this issue.
In this review of 13 investigations11-23 that studied the rates and risk of suicide-related events associated with AED treatment in BD, there were 153 619 subjects. The AED studied most frequently was valproate, but its treatment exposure duration was only approximately half that of lithium, which was the most common reference group. Carbamazepine and lamotrigine had exposure times that were much smaller than valproate, and so for these FDA-approved AEDs for BD it is difficult to make any conclusions about suicide-related event rates and risk. The most commonly studied suicide-related events were deaths by suicide and suicide attempts. Median suicide attempt rates occurred in the following order: lithium < valproate < carbamazepine < no treatment; median suicide death rates were: carbamazepine < lithium < valproate < no treatment. For valproate, suicide attempts occurred 70%,12 170%,17 and 39%22 more often than for lithium. For risk of suicide death, valproate was reported to be associated with 170%,12 50%,17 and 171%22 more than lithium. When compared to no treatment however, valproate was associated with 28%18 and 86%21 fewer suicide attempts, and 91%21 fewer suicide deaths.
While it is encouraging that there are published studies examining the rates and risk of suicide-related events with the AEDs that are FDA-approved for BD, data gaps remain. It is clear from this review that the best data available for any single AED is for valproate, a commonly used mood stabilizer. Compared with no treatment, valproate appears to be associated with a treatment benefit regarding suicide attempts and completions, though more data replicating this outcome is needed. Compared to lithium however, valproate appears to be associated with a higher risk of suicide attempts and completions. Lamotrigine, another commonly used AED for BD, requires much more investigation related to suicide-related events, especially given the reported potential risk in the FDA report. Carbamazepine, perhaps the least used AED for BD, has comparable amounts of data to valproate, but the rates and risks data reported were generated from exposure durations that were substantially lower than for valproate or lithium. This supports the need for more suicide-related events data for carbamazepine as well.
Footnotes
Disclosures: An earlier version of this manuscript was presented as a poster at the 2014 Annual Meeting of the College of Psychiatric and Neurologic Pharmacists in Phoenix, AZ.
References
- 1. Jamison KR. . Suicide and bipolar disorder. J Clin Psychiatry. 2000; 61 Suppl 9: 47- 51. PubMed PMID: 10826661. [PubMed] [Google Scholar]
- 2. Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007; 64 5: 543- 52. DOI: 10.1001/archpsyc.64.5.543. PubMed PMID: 17485606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simon GE, Hunkeler E, Fireman B, Lee JY, Savarino J. . Risk of suicide attempt and suicide death in patients treated for bipolar disorder. Bipolar Disord. 2007; 9 5: 526- 30. DOI: 10.1111/j.1399-5618.2007.00408.x. PubMed PMID: 17680924. [DOI] [PubMed] [Google Scholar]
- 4. Baldessarini RJ, Pompili M, Tondo L. . Suicide in bipolar disorder: risks and management. CNS Spectr. 2006; 11 6: 465- 71. PubMed PMID: 16816785. [DOI] [PubMed] [Google Scholar]
- 5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013. [Google Scholar]
- 6. Huber RS, Coon H, Kim N, Renshaw PF, Kondo DG. . Altitude is a risk factor for completed suicide in bipolar disorder. Med Hypotheses. 2014; 82 3: 377- 81. DOI: 10.1016/j.mehy.2014.01.006. PubMed PMID: 24495565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. USA Suicide: 2016 Official Final Data [Internet]. Washington: American Association of Suicidology; c2017. [updated 2018 Jan 9; cited 2017 Dec 24]. Available from: http://www.suicidology.org/Portals/14/docs/Resources/FactSheets/2016/2016datapgsv1b.pdf?ver=2018-01-15-211057-387 [Google Scholar]
- 8. Statistical review and evaluation: antiepileptic drugs and suicidality [Internet]. Silver Spring (MD): US Food and Drug Administration; c2008. [updated 2008 May 23; cited 2017 Apr 10]. Available from: https://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4372b1-01-FDA.pdf [Google Scholar]
- 9. Lavigne JE, Mathews J, Knox KL. . Post-market surveillance for suicide: the case of gabapentin. J Pharm HSR. 2010; 1: 47- 51. [Google Scholar]
- 10. Lavigne JE. . Suicidal ideation and behavior as adverse events of prescribed medications: an update for pharmacists. J Am Pharm Assoc (2003). 2016; 56 2: 203- 6. DOI: 10.1016/j.japh.2015.12.011. PubMed PMID: 27000172. [DOI] [PubMed] [Google Scholar]
- 11. Yerevanian BI, Koek RJ, Mintz J. . Lithium, anticonvulsants and suicidal behavior in bipolar disorder. J Affect Disord. 2003; 73 3: 223- 8. PubMed PMID: 12547290. [DOI] [PubMed] [Google Scholar]
- 12. Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D. . Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003; 290 11: 1467- 73. DOI: 10.1001/jama.290.11.1467. PubMed PMID: 13129986. [DOI] [PubMed] [Google Scholar]
- 13. Born C, Dittmann S, Post RM, Grunze H. . Newer prophylactic agents for bipolar disorder and their influence on suicidality. Arch Suicide Res. 2005; 9 3: 301- 6. DOI: 10.1080/13811110590929541. PubMed PMID: 16020172. [DOI] [PubMed] [Google Scholar]
- 14. Goldberg JF, Allen MH, Miklowitz DA, Bowden CL, Endick CJ, Chessick CA, et al. Suicide ideation and pharmacotherapy among STEP-BD patients. Psychiatr Serv. 2005; 56 12: 1534- 40. DOI: 10.1176/appi.ps.56.12.1534. PubMed PMID: 16339615. [DOI] [PubMed] [Google Scholar]
- 15. Yerevanian BI, Koek RJ, Mintz J. . Bipolar pharmacotherapy and suicidal behavior. Part I: lithium, divalproex and carbamazepine. J Affect Disord. 2007; 103 1-3: 5- 11. DOI: 10.1016/j.jad.2007.05.019. PubMed PMID: 17628692. [DOI] [PubMed] [Google Scholar]
- 16. Søndergård L, Lopez AG, Andersen PK, Kessing LV. . Mood-stabilizing pharmacological treatment in bipolar disorders and risk of suicide. Bipolar Disord. 2008; 10 1: 87- 94. DOI: 10.1111/j.1399-5618.2008.00464.x. PubMed PMID: 18199245. [DOI] [PubMed] [Google Scholar]
- 17. Collins JC, McFarland BH. . Divalproex, lithium and suicide among Medicaid patients with bipolar disorder. J Affect Disord. 2008; 107 1-3: 23- 8. DOI: 10.1016/j.jad.2007.07.014. PubMed PMID: 17707087. [DOI] [PubMed] [Google Scholar]
- 18. Gibbons RD, Hur K, Brown CH, Mann JJ. . Relationship between antiepileptic drugs and suicide attempts in patients with bipolar disorder. Arch Gen Psychiatry. 2009; 66 12: 1354- 60. DOI: 10.1001/archgenpsychiatry.2009.159. PubMed PMID: 19996040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leon AC, Solomon DA, Li C, Fiedorowicz JG, Coryell WH, Endicott J, et al. Antiepileptic drugs for bipolar disorder and the risk of suicidal behavior: a 30-year observational study. Am J Psychiatry. 2012; 169 3: 285- 91. DOI: 10.1176/appi.ajp.2011.11060948. PubMed PMID: 22193537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahearn EP, Chen P, Hertzberg M, Cornette M, Suvalsky L, Cooley-Olson D, et al. Suicide attempts in veterans with bipolar disorder during treatment with lithium, divalproex, and atypical antipsychotics. J Affect Disord. 2013; 145 1: 77- 82. DOI: 10.1016/j.jad.2012.07.015. PubMed PMID: 22871534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai C-J, Cheng C, Chou P-H, Lin C-H, McInnis MG, Chang C-L, et al. The rapid suicide protection of mood stabilizers on patients with bipolar disorder: a nationwide observational cohort study in Taiwan. J Affect Disord. 2016; 196: 71- 7. DOI: 10.1016/j.jad.2016.02.014. PubMed PMID: 26919054. [DOI] [PubMed] [Google Scholar]
- 22. Hayes JF, Pitman A, Marston L, Walters K, Geddes JR, King M, et al. Self-harm, unintentional injury, and suicide in bipolar disorder during maintenance mood stabilizer treatment: a UK population-based electronic health records study. JAMA Psychiatry. 2016; 73 6: 630- 7. DOI: 10.1001/jamapsychiatry.2016.0432. PubMed PMID: 27167638. [DOI] [PubMed] [Google Scholar]
- 23. Song J, Sjölander A, Joas E, Bergen SE, Runeson B, Larsson H, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017; 174 8: 795- 802. DOI: 10.1176/appi.ajp.2017.16050542. PubMed PMID: 28595491. [DOI] [PubMed] [Google Scholar]
- 24. US and World Population Clock [Internet]. Suitland (MD): c2017. [updated 2017 Dec 12; cited 2017 Dec 19]. Available from: https://www.census.gov/popclock/. [Google Scholar]