Abstract
Introduction:
Medical cannabis (MC) is commonly claimed to be an effective treatment for chronic or refractory pain. With interest in MC in the United States growing, as evidenced by the 29 states and 3 US districts that now have public MC programs, the need for clinical evidence supporting this claim has never been greater.
Methods:
This was a retrospective, mirror-image study that investigated MC's effectiveness in patients suffering from chronic pain associated with qualifying conditions for MC in New York State. The primary outcome was to compare European Quality of Life 5 Dimension Questionnaire (EQ-5D) and Pain Quality Assessment Scale (PQAS) scores at baseline and 3 months post-therapy. The secondary outcomes included comparisons of monthly analgesic prescription costs and opioid consumption pre- and post-therapy. Tolerability was assessed by side effect incidence.
Results:
This investigation included 29 subjects. Quality of life and pain improved, measured by change in EQ-5D (Pre 36 – Post 64, P < .0001) and change in PQAS paroxysmal (Pre 6.76 – Post 2.04, P < .0001), surface (Pre 4.20 – Post 1.30, P < .0001), deep (Pre 5.87 – Post 2.03, P < .0001), unpleasant (Pre “miserable” – Post “annoying”, P < .0001). Adverse effects were reported in 10% of subjects.
Discussion:
After 3 months treatment, MC improved quality of life, reduced pain and opioid use, and lead to cost savings. Large randomized clinical trials are warranted to further evaluate the role of MC in the treatment of chronic pain.
Keywords: medical cannabis, chronic pain, quality of life, cost savings, analgesics, opioids, medical marijuana, opioid analgesics
Introduction
Pain is the most common reason individuals seek healthcare.1 Chronic pain (CP), defined as pain lasting longer than 3 months, has an estimated incidence in the United States higher than that of diabetes, cancer, and heart disease combined.2-4 Chronic pain is often complex and can result from both nociceptive and neuropathic pain mechanisms. Nociceptive pain is the nervous system's response to real or potential injury.5 Neuropathic pain involves changes to the nerves and an autonomous, maladaptive perception of pain, independent of real tissue damage. The lack of safe and effective treatment options for CP leaves both patients and providers stranded. First line therapy includes non-pharmacologic methods followed by non-opioid medications.6 If these methods fail to bring adequate relief or relief dwindles over time, patients are left with scarce options while suffering significant deterioration in quality of life.7 Despite the lack of evidence for sustained effectiveness, opioid analgesics have been increasingly prescribed for CP.8-10 As a result, inappropriate use of opioids has contributed to overdose deaths claiming more than 183 000 lives from 1999 to 2015.11
Evidence supporting medical cannabis (MC) as an effective treatment for chronic and refractory pain is expanding and drawing the attention of both advocates and critics alike.12-18 Two compounds present in cannabis, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), are responsible for a majority of the drug's pharmacologic effects. Delta-9-tetrahydrocannabinol, the primary psychoactive cannabinoid, acts as a partial agonist with high-affinity at 2 G-protein coupled receptors: cannabinoid receptors 1 and 2 (CB1 and CB2).19 Cannabidiol receptors are found in high concentrations in areas of the brain that modulate nociceptive pain in similar amounts and locations to that of opioid receptors.3 One such area is the periaqueductal gray.20,21 The periaqueductal gray is an important area of the brain for modulation of analgesia through descending pathways of pain.5 Cannabinoid receptor 1 is also densely expressed in the amygdala, an area of the brain that influences the emotional response to pain.22 This is of particular interest because increased emotional suffering is associated with CP.23,24 One imaging study25 confirmed THC's effect on the amygdala contributes to analgesia in humans. Cannabinoid receptor 2s are widespread and are associated with cells of the immune system. Delta-9-tetrahydrocannabinol may exert analgesic effects through both receptors, tempering perception of pain at CB1 and acting through anti-inflammatory mechanisms at CB2. Cannabidiol, another active compound in MC, acts on cannabinoid receptors indirectly and may have anxiolytic effects.26 Altering the ratio of THC to CBD changes MC's net effect because CBD can mitigate the psychoactive effects of THC. Although THC and CBD have been the primary focus of investigation for years, there are over 100 cannabinoids present in cannabis as well as aromatic terpenoids, which likely target receptors other than CB1 or CB2 and modulate psychopharmacology.27 Terpenoid profiles vary between strains of cannabis and are purported to influence the different effects of cannabis varietals such as indica or sativa.
As of July 2017, 29 states and 3 US districts have public MC programs.28 Medical cannabis products available in many state programs have experienced issues regarding variability in potency.29 The New York State (NYS) Department of Health's MC program is one of the most stringent to date.30 The state's legislation encompasses good manufacturing practices, reliable drug pedigree, and consistent product potency. Given the regulatory environment in NYS and the fact that opioid overdose deaths are reduced in states with public MC programs, we investigated MC's utility in the treatment of CP.31
Methods
Institutional review board approval was granted by Rochester Regional Health to conduct this retrospective, mirror-image study at a collaborative psychiatric private practice in Rochester, New York. Patients were enrolled if they (1) were referred to our clinic for CP; (2) qualified for MC use in NYS (Table 1); and (3) had yet to be issued a MC certificate. Patients were excluded if they (1) were under the age of 18 years; (2) had already undergone 3 months treatment with MC; or (3) were pregnant or breastfeeding. Eligible patients registered with the Department of Health, were prescribed MC by our physician, and picked up their prescription from a dispensary.
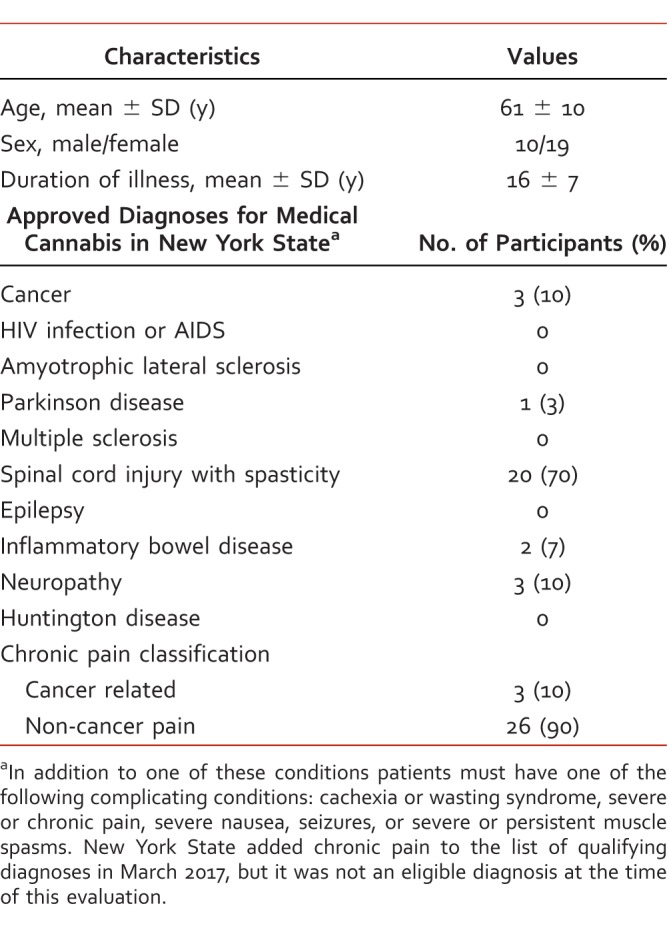
TABLE 1.
Summary of patient characteristics
The approach to therapy was based on the pharmacokinetics of the dosage forms. Oral absorption of MC is slow and produces peak plasma concentrations (Cmax) in 1 to 2 hours,32 whereas inhalation achieves Cmax in 3 to 10 minutes and was reasoned to be suitable for breakthrough pain. Subjects were treated with 10 mg MC capsules of THC and CBD in a 1:1 ratio, taken orally every 8 to 12 hours. If patients experienced breakthrough pain, they were prescribed a vapor pen inhaler of THC/CBD in a 20:1 ratio, 2 mg THC per 0.1 mg CBD. Patients were instructed to take 1 to 5 puffs every 15 minutes until relief was achieved and use every 4 to 6 hours as needed. Patients were prescribed a month's supply. A pharmacist administered the patient surveys at baseline and after 3 months therapy. Side effects were reported over the phone and were assessed at 1- and 3-month follow-up visits.
The primary outcome was change in European Quality of Life 5 Dimension Questionnaire (EQ-5D) and Pain Quality Assessment Scale (PQAS) from baseline to 3 months after MC therapy. Pain Quality Assessment Scale was evaluated using a previously published method for factor analysis.33 Secondary outcomes included change from baseline in opioid consumption and analgesic prescription costs. Opioid consumption was assessed by calculating each patient's daily dose of opioid medications and converting to morphine equivalents.34 Opioid medications were decreased according to guidelines set forth by the Centers for Disease Control and Prevention6 pending patient response to therapy. Prescription costs were evaluated by a cost minimization analysis from the payer's perspective. Analgesic prescription costs were calculated at baseline, extrapolated 3 months back (Pre), and compared to analgesics and MC prescription costs incurred 3 months after therapy (Post). Average wholesale prices were used to estimate analgesic prescription costs. Medical cannabis prescription costs were obtained from corresponding dispensaries. Descriptive statistics were used to describe the demographic data and clinical characteristics. A paired t-test was used to compare each individual to him/herself from baseline to 3 months post-therapy. Service utilization and safety were analyzed using the Kruskal-Wallis test for analysis of variance, and a Wilcoxon signed rank test was used for non-parametric data.
Results
Twenty-nine patients were included in this investigation. Average subject age (mean ± SD) was 61 ± 10 years, and 65% of the sample were female (10 male, 19 female). Diagnoses approved for medical use included: spinal tissue damage (n = 20, 70%), neuropathies (n = 3, 10%), cancer (n = 3, 10%), irritable bowel disease (n = 2, 7%), and Parkinson disease (n = 1, 3%). Three patients in our sample suffered from cancer-related CP and 26 (90%) suffered from chronic non-cancer pain. The average duration of illness was 16 ± 7 years. Patient characteristics are summarized in Table 1. Quality of life EQ-5D scores (range 0 to 100) improved from 36.08 ± 19.85 at baseline to 64.43 ± 19.15 after 3 months treatment (P < .0001). Pain quality measured by PQAS factor analysis also responded favorably. Paroxysmal pain decreased from 6.76 to 2.04 (P < .0001); surface from 4.20 to 1.30 (P < .0001); deep from 5.87 to 2.03 (P < .0001); and unpleasant rating declined from “miserable” to “annoying” after 3 months therapy. Pain Quality Assessment Scale criteria grouping for factor analysis are available in Table 2, and changes in specific PQAS criteria are listed in Table 3. Opioid consumption was reduced from 79.94 (range 0 to 450) to 19.65 (range 0 to 150) morphine equivalents per day (P < .05). Monthly analgesic prescription costs decreased from $354.70 Pre (range $0 to $1838) to $241.10 Post (range $0 to $477; P < .05). Adverse events reported at 1 month included dry mouth (n = 6, 21%), dizziness (n = 1, 3%), and increased appetite (n = 1, 3%). After 3 months of therapy, dry mouth (n = 3, 10%) was the only adverse event reported.
TABLE 2.
Pain Quality Assessment Scale factor analysis, baseline and 3 months after medical cannabis therapy; scores range 0 to 10
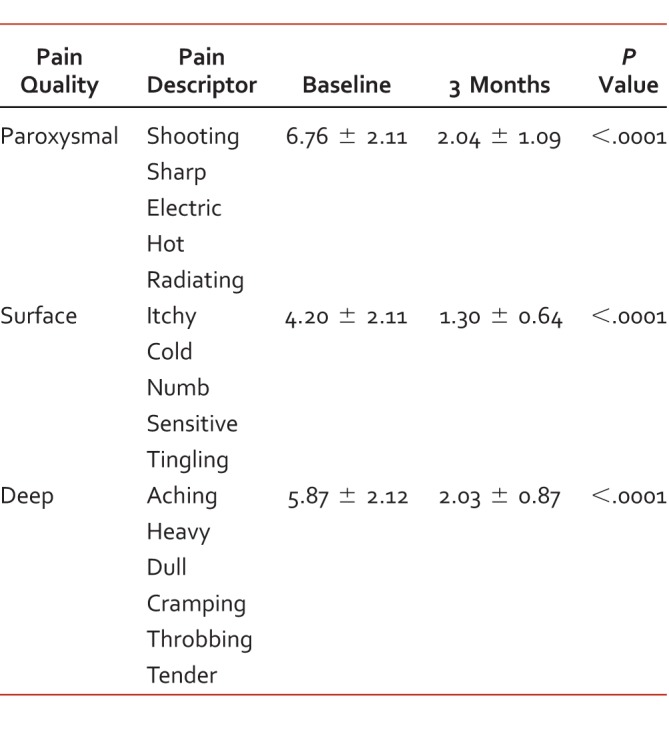
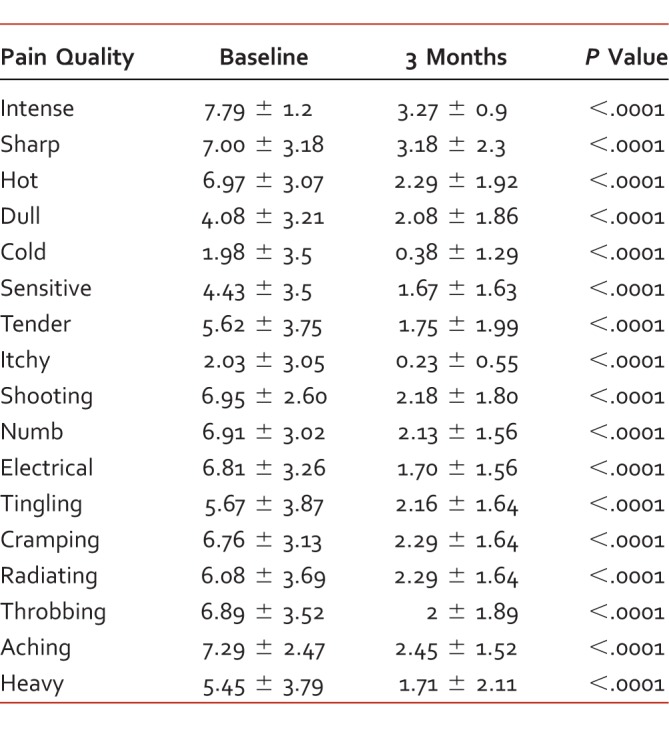
TABLE 3.
Pain Quality Assessment Scale criteria; scores range 0 to 10
Discussion
In this retrospective, mirror-image study, MC improved quality of life and pain outcomes in patients with CP. These results are consistent with previous reports demonstrating MC's effectiveness in neuropathic pain.14,35-39 Our results provide new data on MC use in treating CP associated with spinal tissue injuries (n = 20, 70%). Past investigations in subjects with spinal tissue injury have focused on MC's effects on spasticity and overactive bladder.40-42 Smoked cannabis refers to direct ignition of the cannabis plant and inhalation of the smoke from combustion,43 whereas vaporization is a method of delivering inhaled MC while decreasing the number of toxic byproducts produced by combustion. Our study utilized only non-smokeable dosage forms of MC. This is contrary to other studies investigating MC for pain indications that used smoked MC cigarettes.13,35,36,39 An important question concerning MC analgesia is whether this treatment is capable of providing enough relief to reduce, or even replace, opioid use in chronic settings. Preclinical evidence suggests cannabinoids increase the analgesic effect of opioids, thus requiring a lower dose to achieve relief.15,44-46 However, human studies demonstrating opioid sparing effects of MC are limited.47,48 One large controlled study reported this effect in a fairly large sample utilizing smoked MC, however, a reduction in opioid use did not achieve statistical significance.13 Alternatively, our study demonstrated a significantly reduced opioid consumption with MC despite the small sample size. Additionally, we observed a decrease in monthly analgesic prescription costs, due to a reduction in the number of oral opioids and fentanyl patches used.
In spite of a national opioid epidemic and sparse evidence supporting long-term use, opioids are still used to treat CP conditions.8-10 Owing to their nature of rapidly developing tolerance, 1 in 4 people treated in the primary care setting with opioids for chronic non-cancer pain develop opioid use disorder.49-52 Clearly, there is a need to transition many patients maintained on chronic opioids to safer, more effective therapies. All but 3 (n = 26) of our patients completely discontinued use of their opioids, and the remaining patients reduced their doses by approximately 75%. These findings coupled with the fact that opioid overdose deaths decrease in states with MC legislation, raise the question of whether MC is a safe alternative to opioids for CP management.31
This study was limited by its retrospective nature, small sample size, lack of blinding, and potential bias. The small sample size and limited diagnoses in our study limits the generalization of our findings. Patients were not screened for cannabinoid metabolites and other drugs at baseline, which increases the likelihood of selection bias in our sample. Our cost minimization analysis was limited by the use of average wholesale prices. Maximum allowable cost would have more accurately reflected prescription costs from the payer's perspective.
Future studies of MC should assess drug interactions and side effects, monitoring parameters and dose response. Federal rescheduling of cannabis will facilitate and encourage further research. Large controlled trials are warranted to further evaluate the role of MC in treatment of CP.
Footnotes
Disclosures: None
References
- 1. American Pain Society. Pain assessment and treatment in the managed care environment. A position statement from the American Pain Society. Case Manager. 2000; 11 5: 50- 3. PubMed PMID: 11942280. [DOI] [PubMed] [Google Scholar]
- 2. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986; 3: S1- 226. PubMed PMID: 3461421. [PubMed] [Google Scholar]
- 3. Abrams DI, Guzman M. . Cannabis in cancer care. Clin Pharmacol Ther. 2015; 97 6: 575- 86. DOI: 10.1002/cpt.108. PubMed PMID: 25777363. [DOI] [PubMed] [Google Scholar]
- 4. National Center for Health Statistics (US). Health, United States, 2006: With chartbook on trends in the health of Americans. Hyattsville (MD): National Center for Health Statistics (US); 2006 Nov. Report No.: 2006-1232. PubMed PMID: 20698067. [PubMed] [Google Scholar]
- 5. Edmund S, Higgins MD. . Pain. The neuroscience of clinical psychiatry: the pathophysiology of behavior and mental illness. 2nd ed. Alphen aan den Rijn (Netherlands): Wolters Kluwer; 2013. p 132. [Google Scholar]
- 6. Dowell D, Haegerich TM, Chou R. . CDC guideline for prescribing opioids for chronic pain—United States. JAMA. 2016; 315 15: 1624- 45. DOI: 10.1001/jama.2016.1464. PubMed PMID: 26977696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker N, Thomsen AB, Olsen AK, Sjøgren P, Bech P, Eriksen J. . [Pain epidemiology and health-related quality of life in patients with chronic non-malignant pain]. Ugeskr Laeger. 1998; 160 47: 6816- 9. PubMed PMID: 9835791. [PubMed] [Google Scholar]
- 8. Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009; 18 12: 1166- 75. DOI: 10.1002/pds.1833. PubMed PMID: 19718704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010; (1):CD006605. DOI: 10.1002/14651858.CD006605.pub2. PubMed PMID: 20091598. [DOI] [PMC free article] [PubMed]
- 10. Guy GP Jr, Zhang K, Bohm MK, Losby J, Lewis B, Young R, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017; 66 26: 697- 704. DOI: 10.15585/mmwr.mm6626a4. PubMed PMID: 28683056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volkow ND, McLellan AT. . Opioid abuse in chronic pain–Misconceptions and mitigation strategies. N Engl J Med. 2016; 374 13: 1253- 63. DOI: 10.1056/NEJMra1507771. PubMed PMID: 27028915. [DOI] [PubMed] [Google Scholar]
- 12. Hill KP. . Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015; 313 24: 2474- 83. DOI: 10.1001/jama.2015.6199. PubMed PMID: 26103031. [DOI] [PubMed] [Google Scholar]
- 13. Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain. 2016; 32 12: 1036- 43. DOI: 10.1097/AJP.0000000000000364. PubMed PMID: 26889611. [DOI] [PubMed] [Google Scholar]
- 14. Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. . Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013; 14 2: 136- 48. DOI: 10.1016/j.jpain.2012.10.009. PubMed PMID: 23237736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walker JM, Huang SM. . Cannabinoid analgesia. Pharmacol Ther. 2002; 95 2: 127- 35. PubMed PMID: 12182960. [DOI] [PubMed] [Google Scholar]
- 16. Eisenberg E, Ogintz M, Almog S. . The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: a phase 1a study. J Pain Palliat Care Pharmacother. 2014; 28 3: 216- 25. DOI: 10.3109/15360288.2014.941130. PubMed PMID: 25118789. [DOI] [PubMed] [Google Scholar]
- 17. Lynch ME, Ware MA. . Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol. 2015; 10 2: 293- 301. DOI: 10.1007/s11481-015-9600-6. PubMed PMID: 25796592. [DOI] [PubMed] [Google Scholar]
- 18. Boychuk DG, Goddard G, Mauro G, Orellana MF. . The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015; 29 1: 7- 14. DOI: 10.11607/ofph.1274. PubMed PMID: 25635955. [DOI] [PubMed] [Google Scholar]
- 19. Pertwee RG. . The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol, and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008; 153 2: 199- 215. DOI: 10.1038/sj.bjp.0707442. PubMed PMID: 17828291; PubMed Central PMCID: PMC2219532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saito VM, Moreira FA. . Cannabinoids, anxiety, and the periaqueductal gray. Psychol Neurosci. 2010; 3 1: 39- 42. DOI: 10.3922/j.psns.2010.1.004. [Google Scholar]
- 21. Palazzo E, Luongo L, de Novellis V, Rossi F, Maione S. . The role of cannabinoid receptors in the descending modulation of pain. Pharmaceuticals (Basel). 2010; 3 8: 2661- 73. DOI: 10.3390/ph3082661. PubMed PMID: 27713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neugebauer V. . Amygdala pain mechanisms. Handb Exp Pharmacol. 2015; 227: 261- 84. DOI: 10.1007/978-3-662-46450-2_13. PubMed PMID: 25846623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. . The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007; 133 4: 581- 624. DOI: 10.1037/0033-2909.133.4.581. PubMed PMID: 17592957. [DOI] [PubMed] [Google Scholar]
- 24. Shuchang H, Mingwei H, Hongxiao J, Si W, Xing Y, Antonius D, et al. Emotional and neurobehavioural status in chronic pain patients. Pain Res Manag. 2011; 16 1: 41- 3. PubMed PMID: 21369540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee MC, Ploner M, Wiech K, Bingel U, Wanigasekera V, Brooks J, et al. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain. 2013; 154 1: 124- 34. DOI: 10.1016/j.pain.2012.09.017. PubMed PMID: 23273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, Machado S, Arias-Carrion O, Crippa JA, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets. 2014; 13 6: 953- 60. PubMed PMID: 24923339. [DOI] [PubMed] [Google Scholar]
- 27. Russo EB. Taming THC:. potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011; 163 7: 1344- 64. DOI: 10.1111/j.1476-5381.2011.01238.x. PubMed PMID: 21749363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. State medical marijuana laws [Internet]. Denver: National Conference of State Legislatures; c2017 [updated 2017 Jul 7; cited 2017 Sep 4]. Available from: http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
- 29. Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. . Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015; 313 24: 2491- 3. DOI: 10.1001/jama.2015.6613. PubMed PMID: 26103034. [DOI] [PubMed] [Google Scholar]
- 30. Medical Use of Marihuana, Title 5A Article 33 Pub. L. Health No. 3360-9 (2013).
- 31. Bachhuber MA, Saloner B, Cunningham CO, Barry CL. . Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med. 2014; 174 10: 1668- 73. DOI: 10.1001/jamainternmed.2014.4005. PubMed PMID: 25154332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grotenhermen F. . Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003; 42 4: 327- 60. DOI: 10.2165/00003088-200342040-00003. PubMed PMID: 12648025. [DOI] [PubMed] [Google Scholar]
- 33. Victor TW, Jensen MP, Gammaitoni AR, Gould EM, White RE, Galer BS. . The dimensions of pain quality: Factor analysis of the Pain Quality Assessment Scale. Clin J Pain. 2008; 24 6: 550- 5. DOI: 10.1097/AJP.0b013e31816b1058. PubMed PMID: 18574365. [DOI] [PubMed] [Google Scholar]
- 34. Opioid Morphine Equivalent Conversion Factors [Internet]. Centers for Medicare and Medicaid Services ; c2014 [updated 2017 Aug 1; cited 2017 Sep 4]. Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-March-2015.pdf
- 35. Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007; 68 7: 515- 21. DOI: 10.1212/01.wnl.0000253187.66183.9c. PubMed PMID: 17296917. [DOI] [PubMed] [Google Scholar]
- 36. Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009; 34 3: 672- 80. DOI: 10.1038/npp.2008.120. PubMed PMID: 18688212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013; 260 4: 984- 97. DOI: 10.1007/s00415-012-6739-4. PubMed PMID: 23180178. [DOI] [PubMed] [Google Scholar]
- 38. Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014; 18 7: 999- 1012. DOI: 10.1002/j.1532-2149.2013.00445.x. PubMed PMID: 24420962. [DOI] [PubMed] [Google Scholar]
- 39. Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008; 9 6: 506- 21. DOI: 10.1016/j.jpain.2007.12.010. PubMed PMID: 18403272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hagenbach U, Gafoor N, Brenneisen R, . editors Clinical investigation of delta-9-tetrahydrocannabinol (THC) as an alternative therapy for overactive bladders in spinal cord injury patients. Cologne, Germany: Congress on Cannabis and Cannabinoids; 2001.
- 41. Hagenbach U, Luz S, Ghafoor N, Berger JM, Grotenhermen F, Brenneisen R, et al. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord. 2007; 45 8: 551- 62. DOI: 10.1038/sj.sc.3101982. PubMed PMID: 17043680. [DOI] [PubMed] [Google Scholar]
- 42. Malec J, Harvey RF, Cayner JJ. . Cannabis effect on spasticity in spinal cord injury. Arch Phys Med Rehabil. 1982; 63 3: 116- 8. PubMed PMID: 6978699. [PubMed] [Google Scholar]
- 43. Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. . Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. 2007; 82 5: 572- 8. DOI: 10.1038/sj.clpt.6100200. PubMed PMID: 17429350. [DOI] [PubMed] [Google Scholar]
- 44. Shapira M, Gafni M, Sarne Y. . Long-term interactions between opioid and cannabinoid agonists at the cellular level: cross-desensitization and downregulation. Brain Res. 2003; 960 1: 190- 200. PubMed PMID: 12505672. [DOI] [PubMed] [Google Scholar]
- 45. Cichewicz DL, Martin ZL, Smith FL, Welch SP. . Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther. 1999; 289 2: 859- 67. PubMed PMID: 10215664. [PubMed] [Google Scholar]
- 46. Cichewicz DL. . Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004; 74 11: 1317- 24. PubMed PMID: 14706563. [DOI] [PubMed] [Google Scholar]
- 47. Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, et al. Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology. 2017; 42 9: 1752- 65. DOI: 10.1038/npp.2017.51. PubMed PMID: 28327548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meng H, Hanlon JG, Katznelson R, Ghanekar A, McGilvray I, Clarke H. . The prescription of medical cannabis by a transitional pain service to wean a patient with complex pain from opioid use following liver transplantation: a case report. Can J Anaesth. 2016; 63 3: 307- 10. DOI: 10.1007/s12630-015-0525-6. PubMed PMID: 26507533. [DOI] [PubMed] [Google Scholar]
- 49. Dumas EO, Pollack GM. . Opioid tolerance development: a pharmacokinetic/pharmacodynamic perspective. AAPS J. 2008; 10 4: 537- 51. DOI: 10.1208/s12248-008-9056-1. PubMed PMID: 18989788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. . Opioid use behaviors, mental health and pain–development of a typology of chronic pain patients. Drug Alcohol Depend. 2009; 104 1-2: 34- 42. DOI: 10.1016/j.drugalcdep.2009.03.021. PubMed PMID: 19473786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. . Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain. 2007; 8 7: 573- 82. DOI: 10.1016/j.jpain.2007.02.432. PubMed PMID: 17499555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boscarino JA, Rukstalis M, Hoffman SN, Han JJ, Erlich PM, Gerhard GS, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010; 105 10: 1776- 82. DOI: 10.1111/j.1360-0443.2010.03052.x. PubMed PMID: 20712819. [DOI] [PubMed] [Google Scholar]


