Abstract
Recent work has begun to shed light on the neural correlates and possible mechanisms of polygenic risk for schizophrenia. Here, we map a schizophrenia polygenic risk profile score (PRS) based on genome-wide association study significant loci onto variability in the activity and functional connectivity of a frontoparietal network supporting the manipulation versus maintenance of information during a numerical working memory (WM) task in healthy young adults (n = 99, mean age = 19.8). Our analyses revealed that higher PRS was associated with hypoactivity of the dorsolateral prefrontal cortex (dlPFC) during the manipulation but not maintenance of information in WM (r2 = .0576, P = .018). Post hoc analyses revealed that PRS-modulated dlPFC hypoactivity correlated with faster reaction times during WM manipulation (r2 = .0967, P = .002), and faster processing speed (r2 = .0967, P = .003) on a separate behavioral task. These PRS-associated patterns recapitulate dlPFC hypoactivity observed in patients with schizophrenia during central executive manipulation of information in WM on this task.
Keywords: genetic risk, prefrontal cortex, working memory, fMRI
Schizophrenia is a complex, heritable disorder wherein hundreds of common genetic polymorphisms are likely to each confer small effects on overall risk.1 A recent genome-wide association study (GWAS) identified 108 common risk alleles, which were subsequently used to generate a polygenic risk profile score (PRS) representing the cumulative impact of each individual locus on risk for developing schizophrenia.2 The majority (75%) of the loci comprising this schizophrenia polygenic risk are found in protein-coding genes, including notable targets of antipsychotic drugs as well as dopaminergic and glutamatergic signaling (eg, DRD2, GRM3). Other risk loci map onto genes harboring rare schizophrenia-related risk mutations including voltage-gated calcium channels and modulators of synaptic plasticity.3 There is further significant overlap between these genes and those associated with cognitive function.4 While the biological and functional implications of several of these genes is partially understood, the impact of cumulative genetic risk is still unclear.2,3,5 Identifying intermediate neurophysiological phenotypes through which these cumulative genetic effects may be conferred may help to better understand the functional role of these genetic variants.
A wealth of research has highlighted premorbid and persistent deficits in executive control as central to the etiology and pathophysiology of schizophrenia.2,6,7 In particular, working memory (WM), which represents a key component of executive control supported by synchronized activity within and between neuronal ensembles of the prefrontal cortex,8 has been found to be impaired in individuals with schizophrenia as well as in their unaffected siblings.9–11 These studies are consistent with the finding that deficits in WM broadly also have a genetic component and are associated with increased genetic risk.12
Analyses investigating patterns of brain activity during WM highlight both relatively exaggerated and diminished dorsolateral prefrontal cortex (dlPFC) activation, depending on the task demands and cortical subdivision of dlPFC.13,14 Postmortem analysis of dlPFC also reveals differences in gene and mRNA expression of some of the 108 GWAS schizophrenia risk loci.15 Thus, prefrontal dysfunction associated with important components of executive control, particularly WM supported by the dlPFC, may represent a core intermediate phenotype of schizophrenia and, as such, a putative systems-level mechanism through which genome-wide effects may be observed. However, discrepant findings of hypo- and hyper-frontality in schizophrenia patients with differing levels of functionality suggest the need for approaches that can (1) dissociate the various component processes within WM and (2) understand brain-wide effects.13,16–18
Here, we use an imaging genetics strategy to map genome-wide risk for schizophrenia onto prefrontal cortex neurophysiology. While deficits in schizophrenia are observed in WM encoding and maintenance, manipulation more selectively recruits dlPFC activity in comparison with WM maintenance alone.19–21 To help resolve the discrepant findings on dlPFC function in schizophrenia noted above, we focused on WM manipulation. Specifically, we examined blood oxygen level dependent functional magnetic resonance imaging (BOLD fMRI) activity and functional connectivity during the manipulation of information maintained in WM, which isolates a core computational function of the prefrontal cortex. Our analyses focus on data from healthy 18- to 22-year-old university students who, while not affected, are still within the age of risk for the development of schizophrenia.22 In fMRI studies of patients with schizophrenia, dlPFC engagement is relatively reduced, suggesting lower tuning or engagement while performing this task, a pattern that has been seen in some other studies of executive function in schizophrenia. We thus hypothesized that even in this healthy, relatively high-functioning cohort, higher polygenic risk would be associated with a similar pattern of dlPFC engagement at high levels of behavioral performance. We predicted that dlPFC hypoengagement would manifest in WM manipulation (above and beyond maintenance) and altered functional connectivity within the frontoparietal network, mirroring deficits observed in schizophrenia.
Methods
Participants
Neuroimaging and genomic data were available from a total of 139 non-Hispanic Caucasian participants who had successfully completed the ongoing Duke Neurogenetics Study between September 3, 2014 and October 13, 2016. We focus our current analyses on non-Hispanic Caucasian participants as this was the racial and ethnic demographic of the discovery sample from which the polygenic risk profile was identified.2 Informed consent was obtained from all participants before their participation as approved by the Duke University School of Medicine Institutional Review Board. General exclusion criteria included: (1) medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic disorder; (2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and (3) conditions affecting cerebral blood flow and metabolism (eg, hypertension). Thirty participants were excluded from further analyses because of a past or current DSM-IV Axis I or select Axis II (ie, borderline and antisocial personality) disorder as determined through clinical interview using the electronic MINI.23
Genotyping
All participants provided a saliva sample using Oragene DNA self-collection kits (DNA Genotek) customized for 23andMe (www.23andme.com). DNA isolation, extraction, and single nucleotide polymorphism (SNP) genotyping were performed through the National Genetics Institute (NGI), a CLIA-certified clinical laboratory and subsidiary of Laboratory Corporation of America. An Illumina HumanOmniExpress-24, with custom content was used to provide genome-wide SNP data.24–27
Polygenic Risk Profile Score
PRSs were calculated based on clumped SNPs provided by the Psychiatric Genomics Consortium (PGC).5 These genome wide independent SNPs (R2 < .1 within in 500 kbp region) were clumped using PLINK (http://pngu.mgh.harvard.edu/purcell/plink/). We calculated the PRS for each individual by summing the imputation probability of the reference allele of the clumped SNP weighted by the natural log of the odds ratio from PGC2 GWAS results,2 as shown in the following. We chose the PRS calculated based on loci meeting the most conservative genome-wide significance threshold (5 × 10−8), which comprises 107 SNPs in our dataset (supplementary figure 1).
where wi is the natural log of odds ratio from PGC2 GWAS for SNP i with wi = ln (ORi), and Pi is probability of reference allele for odds ratio in PGC2.
fMRI Paradigm
An event-related WM paradigm was used to elicit frontoparietal network activity during the manipulation of information in WM.28,29 Briefly, the paradigm included 10 trials for each of 6 different trial types, including 3 control conditions consisting only of a 3 s response phase, and 3 WM conditions consisting of a 0.5 s encoding phase followed by a 4 s maintenance interval and a 3 s response phase (supplementary figure 2).
In the maintenance (EC_RJ) condition, participants performed subtraction of 2 or 3 from one of 2 numbers during the brief encoding phase, then recalled the resulting 2 numbers and performed a numerical size judgment as instructed during the response phase after the maintenance interval. In the WM manipulation (E_RCJ) condition, the participants performed subtraction of 2 or 3 from one of the remembered numbers as after a delay, and then made the numerical size judgment. Control conditions were also included in which participants performed (1) a simple motor task (M) in response to a prompt, (2) a numerical size judgment (J), and (3) a numerical computation and size judgment task (CJ) in which they performed a numerical subtraction before size judgment. In each trial, numbers were single digits from 0 to 9; were equally balanced across 0–9, and equally likely to differ by either 2 or 3 units. Numerical computation and correct responses were counterbalanced for each trial type. Trials were interleaved with jittered rest intervals lasting 4–8.5 s for a total scan length of 11 min 48 s.
BOLD fMRI Data Acquisition
Each participant was scanned using 1 of 2 identical research-dedicated GE MR750 3T scanners equipped with high-power high-duty-cycle 50-mT/m gradients at 200 T/m/s slew rate and an 8-channel head coil for parallel imaging at high bandwidth up to 1 MHz at the Duke-UNC Brain Imaging and Analysis Center (additional details in supplementary methods).
BOLD fMRI Data Preprocessing
Preprocessing was conducted using SPM8 (www.fil.ion.ucl.ac.uk/spm). Images for each participant were slice-time corrected, realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model (2 mm isotropic voxels) and smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter set at 6 mm FWHM. Next, the Artifact Detection Tool was used to generate regressors accounting for the possible confounding effects of volumes with large motion deflections (http://www.nitrc.org/projects/artifact_detect) (more details in supplementary methods). Of the 109 participants with available fMRI data, 5 participants had >5% outlier volumes and 1 participant’s data had a scanner artifact—these data were excluded from further analyses. Data for another 3 participants were excluded from further analyses for poor task performance reflected as less than 75% overall accuracy or 50% accuracy on any trial type. Genotype data were unavailable for 1 additional participant. Thus, our primary analyses were conducted in 99 participants.
BOLD fMRI Data Analysis
The general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was used to conduct fMRI data analyses. Following preprocessing, events were modeled for correctly performed trials for the response phase for each of the 6 trial types, and the maintenance and encoding (with and without computation modeled separately) phases for WM trials. Incorrect responses were also modeled as regressors of no interest.
A linear contrast employing the canonical hemodynamic response function was used to estimate main effects for the specific contrast of E_RCJ > EC_RJ for each individual in order to isolate the manipulation of information in WM above and beyond basic computation and maintenance of information across a delay. Individual contrast images for E_RCJ > EC_RJ were then used in second-level random effects models accounting for scan-to-scan and participant-to-participant variability to determine mean condition-specific regional responses using one-sample t-tests. Frontoparietal network activity associated with our contrast of interest was identified using a family-wise error (FWE) corrected whole-brain threshold of P <.05. An a priori region-of-interest (ROI) analysis was also conducted using bilateral Broadmann Areas 9 and 46 generated in the WFUPickAtlas toolbox for SPM8.30,31 This ROIs were used to investigate dlPFC activity specifically during our contrast of interest, once again using an FWE threshold of P <.05 across the ROIs. Mean single-subject BOLD voxel responses were then extracted from 5 mm spheres centered at left and right peak coordinates within the dlPFC ROI using the VOI tool in SPM8.32
In addition to the behavioral performance measures (ie, accuracy and reaction time) derived from our primary contrast of interest, we examined possible associations between PRS-modulated dlPFC activity during computation and neuropsychological measures of executive control including (1) digit span, which is a measure of WM capacity, (2) Paced Auditory Serial Addition Test (PASAT), which is a measure of attention and memory processing, and (3) the Symbol Digit Modalities Task (SDMT), which is a measure of attentional, visual, and motor processing speed.33,34
Linear Regressions of PRS onto Extracted BOLD Activity
Effects of polygenic PRS on right and left dlPFC activity as identified above were tested in IBM SPSS Statistics 24 using linear regression models covarying for 2 Caucasian-specific multidimensional scaling (MDS) ancestry markers, participant sex, and task accuracy on the E_RCJ condition.
Generalized Psycho-Physiological Interaction Analysis
The generalized psycho-physiological interaction (gPPI) toolbox35 was used to create a general linear model in SPM8 (http://www.fil.ion.ucl.ac.uk/spm) for examining task-dependent functional connectivity. We examined both left and right dlPFC connectivity using 5 mm spheres centered on the max voxels identified from the main effects of contrast (L: −42, 2, 32; R: 44, 10, 30) as seed regions. The event-related first-level gPPI model included regressors for the deconvolved fMRI time series from the seed region, each of the 10 task components, and the interactions of the seed time series and task regressors generated by the toolbox. Individual contrast images for E_RCJ > EC_RJ interaction terms were then entered into multiple linear regression models with PRS as the regressor of interest while covarying for 2 Caucasian-specific MDS ancestry markers, participant sex, and task accuracy on the E_RCJ condition. Correction for multiple comparisons in the PPI analyses was conducted using the Statistical nonParametric Mapping (SnPM) package (http://warwick.ac.uk/snpm) (supplemental methods). Because PPI analyses can be underpowered,36 we implemented a cluster-based testing approach. A cluster-forming threshold of P <.001 and FWE-corrected threshold of P <.05 were employed in these SnPM analyses to determine significant clusters in the PPI results.
Results
Cohort Demographics and Behavioral Results
The 99 participants (57 females) included in our analyses of PRS associations with dlPFC activity and connectivity had an average age of 19.84 ± 1.22 years (range = 18–22). The PRSs for schizophrenia was relatively normally distributed (mean = −0.227 ± 0.2540 SD; skewness = −0.011, kurtosis = −0.177) across these participants (supplementary figure 3). The total accuracy across all trial types was 96.8 ± 3.49%, while on the E_RCJ condition of interest it was 92.0 ± 10.1% (table 1). PRS did not significantly correlate with either total accuracy (β = 0.007, P = .620) or accuracy for our condition of interest (β = −0.017, P = .684).
Table 1.
Demographic Characteristics and Task Performance of the Sample
| N | 99 | |
| Age | 19.84 (1.22)a | |
| Sex (female) | 57.6% | |
| PRS | −0.227 (0.254) | |
| Task condition (scanner) | Accuracy (%) | RT (ms) |
| E_RCJ (computation after maintenance) | 92.0 (10.1) | 1531 (273) |
| EC_RJ (computation before maintenance) | 97.8 (5.6) | 830 (143) |
| Total | 96.8 (3.49) | 1098 (143) |
| Additional tasks (outside scanner) | Score | |
| Digit span—normalized total | 12.5 (2.6) | |
| SDMT | 68.1 (10.2) | |
| PASAT | 44.3 (9.1) | |
PRS, polygenic risk profile score; SDMT, Symbol Digit Modalities Task; PASAT, Paced Auditory Serial Addition Test.
aMean (SD) reported for continuous measures.
Main Effects of Condition
First, we analyzed our contrast of interest (E_RCJ > EC_RJ) representing the manipulation of information maintained in WM, which manifested in a broad frontoparietal network (FPN) activation (figure 1; supplementary table 2). The distributed FPN activity included local maxima within bilateral superior parietal lobes, left inferior frontal gyrus (IFG) and left middle frontal gyrus (MFG), right insular cortex, occipital cortex, and thalamus. Within our a priori dlPFC ROIs, peak activations were observed in both hemispheres (x = −42, y = 2, z = 32; x = 44, y = 10, z = 30; figure 1; supplementary table 3). Single-subject BOLD values were extracted from 5 mm spheres centered on these peak voxels for subsequent analyses.
Fig. 1.
Activation patterns during computation trials allowing for the isolation of the manipulation of information maintained in working memory. Task activation on the E_RCJ > EC_RJ contrast (P < .05, FWE corrected) with superimposed dorsolateral prefrontal cortex peaks (5 mm spheres, black) from an a priori bilateral BA 9/46 anatomical regions of interest (supplementary table 3). Heat map and scale bar represent t-statistic from group-level general linear model for E_RCJ > EC_RJ contrast. Visualization constructed with BrainNet Viewer.37
Modulatory Effects of Polygenic Risk on dlPFC Activity
Analyses revealed that higher polygenic PRS was significantly negatively correlated with dlPFC activity (figure 2) in both left [r = −.240, t(93) = −2.413, P = .018] and right hemispheres [r = −.243, t(93) = −2.568, P = .012]. This association remained significant when including scanner as a covariate (P = .027; P = .032). The association of PRS with dlPFC activity was specific to the contrast of interest for computation and did not hold for peak coordinates from a contrast of WM maintenance > baseline. To confirm our focus on a PRS of 107 loci, we tested associations for more liberally derived scores, but these correlations were only significant for the top 2 most stringent PRS (supplementary figure 1). Thus, the analyses of dlPFC functional connectivity were conducted using the PRS comprised of only genome-wide significant loci (ie, P < 5 × 10−8).
Fig. 2.
Polygenic risk profile score (PRS) for schizophrenia modulates dorsolateral prefrontal cortex (dlPFC) activation during computation. Scatter plots of PRS versus (A) left and (B) right dlPFC BOLD from 5 mm spheres centered at peak voxel (L: −42, 2, 32; R: 44, 10, 30) during E_RCJ > EC_RJ contrast. Multiple regression was performed as described in “Methods” section. Shaded region bootstrapped 95% CI.
dlPFC Activity Modulates Reaction Time and Processing Speed
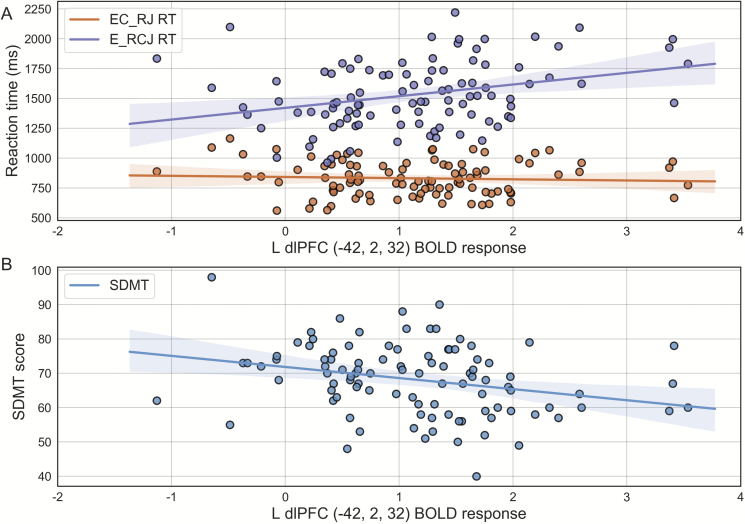
We next regressed the PRS-modulated, extracted BOLD values for manipulation of information in WM (E_RCJ > EC_RJ) onto reaction times for the computation-specific trial type (E_RCJ) for each participant. Left dlPFC PRS-modulated BOLD was positively correlated with E_RCJ reaction time [r = .311, t(92) = 3.106, P = .0025], but not EC_RJ reaction time [r = −.065, t(92) = −0.629, P = .531] or total reaction time [r = .157, t(92) = 1.530, P = .129] (figure 3a). These results held while controlling for task accuracy on the E_RCJ condition. This pattern was not observed for right dlPFC, which demonstrated no significant correlations with reaction time (P = .165; P = .176; P = .365).
Fig. 3.
Polygenic risk profile score (PRS)-modulated left dorsolateral prefrontal cortex (dlPFC) activity is associated with computation speed. (A) Scatter plot of left dlPFC BOLD response from 5 mm sphere centered at peak voxel (−42, 2, 32) during E_RCJ > EC_RJ contrast versus reaction time. There was a significant, positive correlation (r = .311, P = .0025) with mean reaction time on trials with computation following (E_RCJ) but not preceding (EC_RJ) WM maintenance. (B) dlPFC BOLD was further negatively correlated with Symbol Digit Modalities Task scores, which index processing speed, collected outside of the scanner (r = −.311, P = .0026). Shaded region bootstrapped 95% CI.
Additionally, we investigated whether such PRS-modulated measures of brain activity could predict measures of WM capacity or processing speed outside of the scanner. Again, left dlPFC BOLD was significantly correlated with SDMT scores [r = −.311, t(92) = −3.09, P = .0026] (figure 3b), with greater activity associated with lower scores (reflective of slower processing speed on the task). No significant correlations were observed between left dlPFC and the PASAT or digit span, or between right dlPFC and any measures (Ps > .05). For the above tests, we corrected for multiple comparisons using the method of Bonferroni (ie, P < .05/12 tests = .00417), and covaried for sex, 2 MDS Caucasian ancestry markers, scanner, and E_RCJ accuracy.
Exploratory Whole-Brain Analysis
To examine PRS-modulatory effects beyond the dlPFC, we conducted an exploratory post hoc whole-brain multiple regression analysis. This exploratory analysis revealed a negative correlation between PRS and activity within a cluster in the superior temporal gyrus (−66, −44, 12; FWE-corrected). However, these BOLD values did not correlate with E_RCJ or EC_RJ reaction time or with WM capacity and processing speed outside of the scanner.
Modulatory Effects of Polygenic Risk on dlPFC Connectivity
Finally, we sought to extend our results by investigating functional connectivity of the dlPFC. The task-defined left dlPFC ROI exhibited task connectivity during computation with anterior cingulate, insula, and visual cortex (figure 4A). Polygenic scores did not significantly modulate the observed task functional connectivity from our left dlPFC seed. Exploratory post hoc analyses, however, did reveal a significant PRS-modulated functional connectivity during WM maintenance (ie, maintenance > baseline). Specifically, higher PRS was associated with lower functional connectivity between the left dlPFC seed and both inferior parietal lobe and left cerebellum Crus I (figures 4B and 4C). PRS-modulated functional connectivity patterns were not significantly correlated with task performance or any of the neuropsychological measures.
Fig. 4.
General and polygenic risk profile score (PRS)-modulated left dorsolateral prefrontal cortex (dlPFC) functional connectivity during working memory maintenance and computation. (A) Left dlPFC regions of interest exhibited task connectivity [generalized psycho-physiological interaction (generalized psycho-physiological interaction)] during computation with anterior cingulate, insula, and visual cortex (supplementary table 4). Heat map and scale bar represent t-statistic from group-level gPPI for E_RCJ > EC_RJ contrast. Critically, PRS did not modulate this pattern of functional connectivity. PRS-modulated negative functional connectivity between the left dorsolateral prefrontal cortex regions of interest and (B) left parietal cortex (peak voxel: −36, −62, 56), and (C) cerebellum (peak voxel: −22, −72, −36). See supplementary table 5 for more details. Heat map and scale bar represent t-statistic from group-level generalized psycho-physiological interaction for maintenance > baseline contrast. Cluster significance established via nonparametric testing (see “Methods” section).
Discussion
Here, we demonstrate that genome-wide risk for schizophrenia is associated with altered patterns of prefrontal activity during the manipulation of information maintained in WM (ie, computation), as well as with altered prefrontal connectivity during the maintenance of information in WM. Remarkably, while performance on this task in our healthy university students is considerably higher than in an earlier study of patients with schizophrenia, the association of higher genetic risk as reflected in PRS was analogous. Higher polygenic risk was associated with lower bilateral dlPFC activity during the manipulation of information in WM, and this relative hypoactivity in left dlPFC was associated with enhanced computation processing speed during the task and better attentional-motor performance on the SDMT task outside the scanner. Notably, lower dlPFC activity during WM manipulation aligns with observations in patients with schizophrenia,20 but also appears to contrast with other studies associating risk for schizophrenia with prefrontal inefficiency or poorly tuned dlPFC engagement during executive cognition tasks.
The observed pattern of PRS-modulated dlPFC hypoactivity reflected in faster computational and attentional-motor processing suggests that higher PRS within the range observed in our healthy participants may be linked to more efficient prefrontal functioning, which does not seem to apply to the association of this pattern with patient performance. In other words, the same pattern of dlPFC engagement in the context of genetic risk but markedly differing performance may suggest alternative physiological mechanisms underlying a similar pattern of engagement. Although dlPFC inefficiency is often observed in schizophrenia,14 prior research has found that individual candidate genes may have similar or differential functional effects in unaffected individuals and those with schizophrenia. For example, schizophrenia risk alleles in NRG1, ERBB4, and AKT1 are associated with a similar pattern of cognitive deficits and inefficient prefrontal cortex neurophysiology in both unaffected and affected individuals.38 However, risk alleles in other genes such as SCN2A and COMT, have been associated with cognitive and prefrontal dysfunction in affected individuals but improved cognitive and prefrontal function in unaffected individuals.39–41 These studies, however, did not employ the same executive function task as in our study, and this may complicate direct comparisons of results.
The low mean PRS of our sample (supplementary figure 3) further speaks to the potential of an observed dissociable genetic influence of this profile. That is, PRS may confer a cognitive benefit as the risk score approaches the mean, but begins to have detrimental effects as scores move above the mean and risk accumulates. Notably, an inverted-U shaped relationship is commonly observed between dlPFC function and WM, and recent research has found a flattening of this inverted-U function in schizophrenia associated with increasing WM load.42 Because we did not alter WM load, our results cannot speak to an inverted U-curve, specifically. Nonetheless, these prior findings are relevant by suggesting that PRS-modulated dlPFC activity may mirror the lower activity observed in schizophrenia patient, especially during WM manipulation.20
Several recent studies investigating schizophrenia PRS derived from thousands of SNPs found that higher risk was associated with lower, more inefficient, prefrontal activity as a function of higher WM load.14,43,44 Here, however, we provide evidence linking PRS to lower, but potentially more efficient, dlPFC activity during WM in high-functioning healthy participants. Additionally, our results build on these previous finding in several important ways. First, our task allowed for the precise mapping of polygenic risk onto a core component of executive function, the manipulation of information maintained in WM,19 and maintenance alone. Second, we focus on schizophrenia PRS derived from 107 SNPs with described biological relevance,2 which allows for our observed systems-level effects of increasing risk to be examined with regard to underlying cellular- and molecular-level effects. Third, we demonstrate associations between increasing polygenic risk and the function of the dlPFC, which is specifically abnormal in schizophrenia across molecular-, cellular-, and systems-levels of analysis.15,16
Finally, our analysis included the investigation of functional connectivity, revealing differential connectivity patterns during manipulation and maintenance with increasing polygenic risk. A recent study reported differences in resting-state functional connectivity, especially between the dlPFC and anterior insula, as a function of PRS.45 In our task-related connectivity analyses, PRS significantly modulated dlPFC connectivity during WM maintenance but not manipulation. Future research may help reconcile these diverging connectivity results from the canonical inverted U-curve pattern of dlPFC activity by manipulating WM load. For example, dlPFC activity may desynchronize with other brain regions near the peak of the inverted U-curve, leading to potential overcompensation in the BOLD signal. However, it should be noted that our observed patterns of PRS-modulated dlPFC connectivity did not correlate with our performance or neuropsychological measures. Such correlations may likewise emerge when manipulating WM load.
Our work, of course, is not without limitations. Notably, our study participants represented a somewhat narrow range of PRS, although our mean PRS value below zero is not a reflection of this restricted range, but rather that computation of the natural log of the OR is negative as the reference allele is typically protective. Future work that includes at risk individuals, cases and unaffected siblings, could compare results across the broader spectrum of polygenic risk. Similarly, performance on our fMRI task was generally high and within a somewhat narrow range. While we were still able to identify a significant association between dlPFC activity and computation speed, greater sensitivity may be gained by employing a range of cognitive tasks for isolating high and low-performing individuals, as well as by testing parametric modulation of WM load.42 We also would note that our finding of significant effects only for the PRS calculated from GWAS significant loci and those near statistical significance is somewhat unexpected. Risk probability for schizophrenia based on PRS tends to increase until reaching an asymptote based on loci that are only 10−2. This observation has been generally interpreted to reflect that many true positive risk associated genes are of lesser effect size and contained within these less significant loci. Our failure to observe association in our imaging paradigm with PRS based on these less significant loci may suggest that our paradigm uncovers only a restricted component of genetic risk at the level of prefrontal executive function and that other tasks will reveal additional and potentially independent effects. This is further reflected in the limited variability in dlPFC activity (~6%) accounted for by PRS in our analyses. However, this should be considered nontrivial given the high functioning nature of our healthy participants.
These limitations notwithstanding, our results provide initial evidence that genome-wide significant common polymorphisms conferring risk for schizophrenia are associated with altered dlPFC activity and functional connectivity during the manipulation and maintenance of information in WM. As such, they highlight the importance of this genome-wide collective risk on molecular and cellular signaling pathways that modulate FPN activity supporting executive control. Of the 108 GWAS identified risk variants, 20 were recently linked with altered gene expression in postmortem dlPFC,2,15 which is consistent with the large number of these risk loci falling within protein coding regions including glutamatergic and dopaminergic genes of known importance in executive control and FPN function. Our observed neural circuit-level effects may, furthermore, represent a differential effect of genome-wide risk for schizophrenia on prefrontal physiology and a window through which behavioral outcomes can be studied in cognitively high-functioning individuals.46
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
M.A.S. is supported by an NSF Graduate Research Fellowship. The Duke Neurogenetics Study was supported by Duke University and National Institutes of Health (NIH; R01DA033369). A.R.H. is further supported by NIH (R01AG049789).
Supplementary Material
Acknowledgment
We thank all lab members for their assistance in conducting the Duke Neurogenetics Study.
References
- 1. Kavanagh DH, Tansey KE, O’Donovan MC, Owen MJ. Schizophrenia genetics: emerging themes for a complex disorder. Mol Psychiatry. 2015;20:72–76. [DOI] [PubMed] [Google Scholar]
- 2. Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hubbard L, Tansey KE, Rai D, et al. Evidence of common genetic overlap between schizophrenia and cognition. Schizophr Bull. 2016;42:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. [DOI] [PubMed] [Google Scholar]
- 8. D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol. 2015;66:115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldberg TE, Torrey EF, Gold JM, et al. Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. Schizophr Res. 1995;17:77–84. [DOI] [PubMed] [Google Scholar]
- 10. Brahmbhatt SB, Haut K, Csernansky JG, Barch DM. Neural correlates of verbal and nonverbal working memory deficits in individuals with schizophrenia and their high-risk siblings. Schizophr Res. 2006;87:191–204. [DOI] [PubMed] [Google Scholar]
- 11. Barrantes-Vidal N, Aguilera M, Campanera S, et al. Working memory in siblings of schizophrenia patients. Schizophr Res. 2007;95:70–75. [DOI] [PubMed] [Google Scholar]
- 12. Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res. 2009;108:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walton E, Geisler D, Lee PH, et al. Prefrontal inefficiency is associated with polygenic risk for schizophrenia. Schizophr Bull. 2014;40:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fromer M, Roussos P, Sieberts SK, et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19:1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Callicott JH, Sust S, Tan HY, et al. Prefrontal cortex inefficiency during working memory in unaffected siblings of patients with schizophrenia: A replication. Neuropsychopharmacolgy. 2005;30:S120–S120. [Google Scholar]
- 17. Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cannon TD, Glahn DC, Kim J, et al. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62:1071–1080. [DOI] [PubMed] [Google Scholar]
- 19. D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. [DOI] [PubMed] [Google Scholar]
- 20. Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry. 2005;162:1849–1858. [DOI] [PubMed] [Google Scholar]
- 21. Anticevic A, Repovs G, Barch DM. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr Bull. 2013;39:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37:504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur Psychiat. 1997;12:224–231. [Google Scholar]
- 24. Eriksson N, Macpherson JM, Tung JY, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6:e1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Do CB, Tung JY, Dorfman E, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 2011;7:e1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tung JY, Do CB, Hinds DA, et al. Efficient replication of over 180 genetic associations with self-reported medical data. PLoS One. 2011;6:e23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu YN, Shmygelska A, Tran D, Eriksson N, Tung JY, Hinds DA. GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nat Commun. 2016;7:10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan HY, Chen Q, Goldberg TE, et al. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scult MA, Knodt AR, Swartz JR, Brigidi BD, Hariri AR. Thinking and feeling: individual differences in habitual emotion regulation and stress-related mood are associated with prefrontal executive control. Clin Psychol Sci. 2017;5:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 31. Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. [DOI] [PubMed] [Google Scholar]
- 32. Tong YX, Chen Q, Nichols TE, et al. Seeking optimal region-of-interest (ROI) single-value summary measures for fMRI studies in imaging genetics. PLoS One. 2016;11:e0151391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheridan LK, Fitzgerald HE, Adams KM, et al. Normative symbol digit modalities test performance in a community-based sample. Arch Clin Neuropsychol. 2006;21:23–28. [DOI] [PubMed] [Google Scholar]
- 34. Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch Clin Neuropsychol. 2006;21:53–76. [DOI] [PubMed] [Google Scholar]
- 35. McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicodemus KK, Law AJ, Radulescu E, et al. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry. 2010;67:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickinson D, Straub RE, Trampush JW, et al. Differential effects of common variants in SCN2A on general cognitive ability, brain physiology, and messenger RNA expression in schizophrenia cases and control individuals. JAMA Psychiatry. 2014;71:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scult MA, Trampush JW, Zheng F, et al. A common polymorphism in SCN2A predicts general cognitive ability through effects on PFC physiology. J Cogn Neurosci. 2015;27:1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsuzaka CT, Christofolini D, Ota VK, et al. Catechol-O-methyltransferase (COMT) polymorphisms modulate working memory in individuals with schizophrenia and healthy controls. Rev Bras Psiquiatr. 2017. doi: 10.1590/1516-4446-2016-1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Snellenberg JX, Girgis RR, Horga G, et al. Mechanisms of working memory impairment in schizophrenia. Biol Psychiatry. 2016;80:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kauppi K, Westlye LT, Tesli M, et al. Polygenic risk for schizophrenia associated with working memory-related prefrontal brain activation in patients with schizophrenia and healthy controls. Schizophr Bull. 2015;41:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whalley HC, Hall L, Romaniuk L, et al. Impact of cross-disorder polygenic risk on frontal brain activation with specific effect of schizophrenia risk. Schizophr Res. 2015;161:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang T, Zhang X, Li A, et al. Polygenic risk for five psychiatric disorders and cross-disorder and disorder-specific neural connectivity in two independent populations. Neuroimage Clin. 2017;14:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.