Abstract
Introduction:
Over the past decade, ketamine has been studied for major depressive disorder and bipolar depression. Ketamine is believed to exert its antidepressant properties through N-methyl-D-aspartate receptor antagonism.
Methods:
Study authors completed a literature review of seven randomized controlled trials of ketamine usage in major depressive disorder and bipolar depression.
Results:
Ketamine demonstrated a statistically significant improvement over placebo or midazolam in major depressive disorder. Ketamine also exhibited a statistically significant improvement over placebo in bipolar depression.
Discussion:
Ketamine has shown promise in quickly reducing symptoms in patients with treatment resistant depression and bipolar depression. Using ketamine may be helpful for patients that have exhausted other therapeutic options.
Keywords: ketamine, NMDA antagonist, depression, bipolar depression
Introduction
In the 1960s, ketamine was derived from phencyclidine and cyclohexamine and was found to have anesthetic and analgesic properties with possible dissociative and hallucinogenic effects. Ketamine is currently used in clinical settings to induce anesthesia during short procedures. It is also used for the treatment of postoperative, chronic cancer, and neuropathic pain as well as procedural sedation in the emergency department. Recently, ketamine has shown the potential to treat major depressive disorder (MDD) and bipolar depression.1
The noncompetitive inhibition of the N-methyl-D-aspartate glutaminergic receptors by ketamine has been linked to analgesic, dissociative, and neuroprotective effects. Ketamine can also interact with opioid receptors at high plasma concentrations, causing additional analgesic effects.2-5 Ketamine is thought to exert its antidepressant effects through N-methyl-D-aspartate receptor antagonism and possible inhibitory effects on the norepinephrine and serotonin transporter function.6,7 Combining all these effects, ketamine administration causes anesthesia, analgesia, tachycardia, increased blood pressure, impaired memory and cognitive function, and visual changes in a dose-dependent manner.8-10
Oral ketamine demonstrates poor oral bioavailability (17% to 20%); therefore, intravenous (IV) administration is the primary delivery route. When used for anesthesia, if can be given intravenously or intramuscularly; however, when used for pain management, it can also be given orally, epidurally, intrathecally, and intranasally.11,12 Ketamine is water soluble and has a short half-life of 1 to 3 hours.13 It is metabolized to dehydronorketamine, norketamine, and hydroxynorketamine.14 Norketamine is an active metabolite with one-third of the analgesic potency of ketamine. The hepatic enzymes responsible for ketamine's biotransformation are CYP3A4, 2B6, and 2C9.15
Since ketamine is predominantly administered through the IV route in the treatment of depression and bipolar depression, this may pose some challenges. However, its possible usage in patients holds significant promise. The purpose of this review is to summarize and critique data supporting the use of ketamine in depression and bipolar depression.
A study completed by Berman et al16 was the first double-blind placebo-controlled crossover trial to demonstrate rapid antidepressant effects of ketamine following a single dose (0.5 mg/kg infused over 40 minutes) in 7 patients. After this initial study, additional trials showed a similar effect in patients with unipolar and bipolar depression.17-20 Murrough et al21,22 further added to this small body of research by studying the effects of ketamine versus midazolam.
Methods
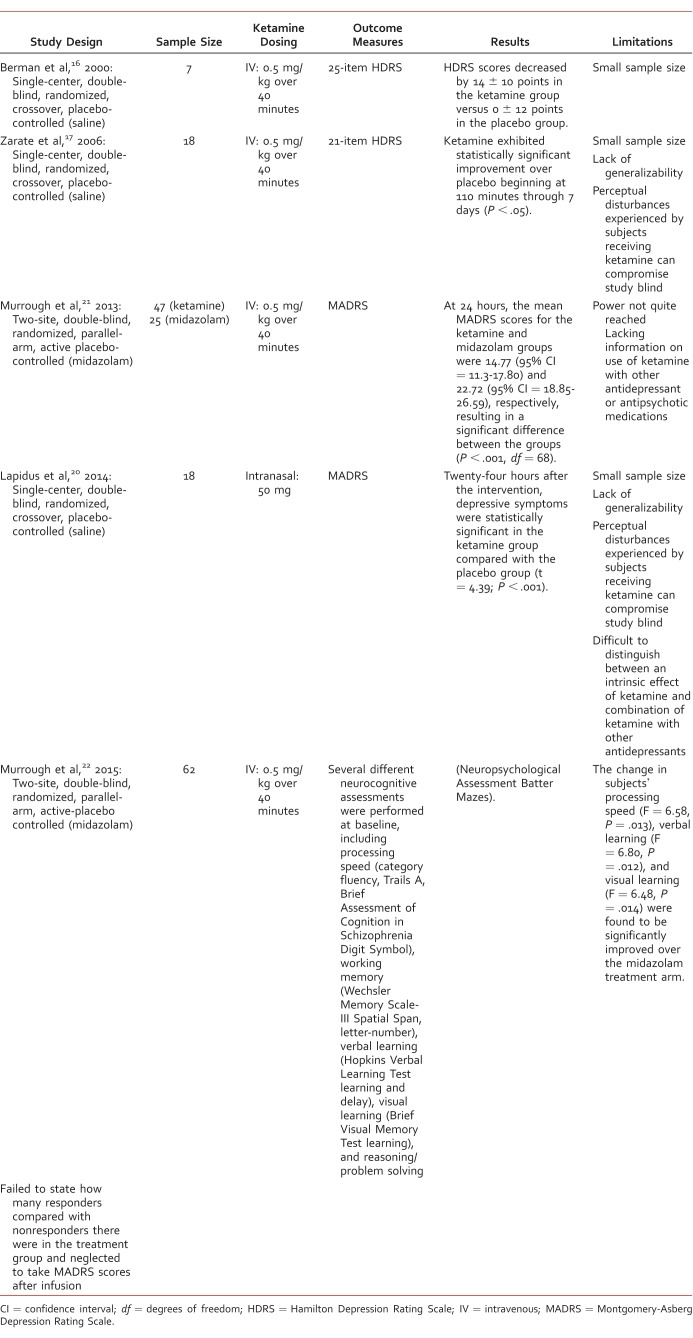
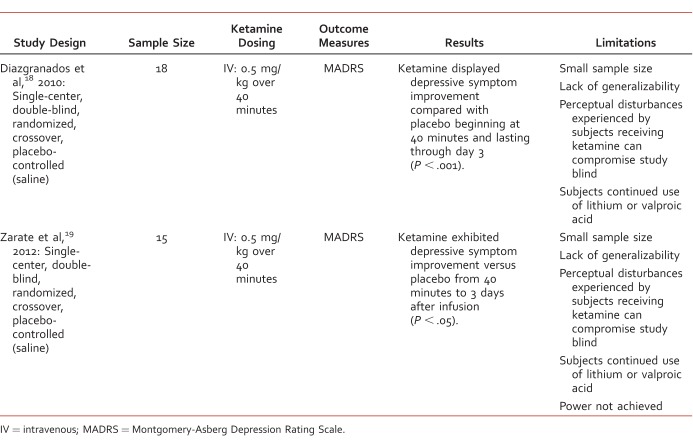
Study authors searched the EBSCO, PsycINFO, and MEDLINE databases through December 2015 using the key words “ketamine” and “depression” without limits on publication year. Studies were included if they were published in English in a peer-reviewed journal; were randomized controlled trials of ketamine; included subjects with the diagnosis of major depressive episode based on DSM III, IV, or V criteria; and if ketamine was administered 0.5 mg/kg IV or 50 mg intranasally. Seven studies met inclusion criteria for this analysis. Five evaluated the use of ketamine in MDD (Table 1) and 2 explored its use in bipolar depression (Table 2). Case reports and case series were excluded from this review. Study searches were performed by 2 authors (S.G., K.K.), and study selection was completed by 1 author (S.G.) and verified by another author (T.M.).
TABLE 1: .
Use of ketamine in major depressive disorder
TABLE 2: .
Use of ketamine in bipolar depression
Review of the Literature
Ketamine Versus Placebo
The objective of a single-setting, randomized, double-blind, crossover study by Zarate et al17 was to determine whether ketamine can achieve a rapid antidepressant response in 18 subjects with treatment-resistant unipolar depression. Inclusion criteria consisted of men and women aged 18- to 65-years-old who were inpatients with a diagnosis of MDD recurrent without psychotic features and who failed at least 2 previous adequate antidepressant trials. Subjects were required to have a score of 18 or higher on the 21-item Hamilton Depression Rating Scale at screening and before the first administration of either ketamine or placebo, be free of a comorbid substance use disorder for at least 3 months, have a negative urine toxicology screen, and have a 2-week medication free period before the start of the study. Exclusion criteria consisted of a DSM-IV diagnosis of bipolar disorder or a history of antidepressant or substance-induced mania or hypomania.
The primary outcome measure was the changes in the Hamilton Depression Rating Scale scores from 60 minutes before infusion to 40, 80, 110, and 230 minutes after infusion. Rating scales were also administered 1, 2, 3, and 7 days after the infusion. Subjects were given ketamine 0.5 mg/kg intravenously infused over 40 minutes or 0.9% saline on 2 test days, 1 week apart. A fixed-effects linear model was used to examine differences between ketamine and placebo treatment from baseline to 7 days, and carryover effects were examined using a linear mixed model. Using only subjects who completed both phases of the study (14/18), the linear mixed model showed significant effects for drug (F = 58.24; P < .001), time (F = 9.48; P < .001), and drug and time (F = 4.15, P < .001). Ketamine exhibited statistically significant improvement over placebo beginning at 110 minutes through 7 days (P < .05). When assessing possible carryover effects, no effect for order was found (F = 1.54; P = .23). Adverse effects occurring more often in the ketamine group included perceptual disturbances, confusion, blood pressure increases, euphoria, dizziness, and increased libido. None of these were serious and most subsided within 80 minutes of the infusion.
Using a crossover design, each subject acted as his or her own control and between-patient variability was removed. Subjects were also required to have a 2-week medication-free interval before trial entrance so only the effects of ketamine would be exerted. Validated scales were used to measure and assess outcomes. The main limitations of this study included its small sample size and lack of generalizability to other forms of depression besides treatment-resistant unipolar depression. Also, the perceptual disturbances experienced by subjects receiving ketamine could have compromised the blinding of the study.
The objective of a single-setting, randomized, double-blind, crossover study by Diazgranados et al18 was to determine whether ketamine produces a rapid antidepressant response in 18 subjects with treatment-resistant bipolar depression. Inclusion criteria consisted of men and women aged 18 to 65 years who were inpatients with a diagnosis of bipolar I or II depression without psychotic features and had failed 1 previous adequate antidepressant trial and 1 prospective trial of either lithium or valproic acid while hospitalized. Subjects were required to have a current major depressive episode for at least 4 weeks, have a score of 20 or greater on the Montgomery-Asberg Depression Rating Scale (MADRS) at screening and before the first administration of either ketamine or placebo, be free of a comorbid substance-use disorder for at least 3 months, judged clinically not to be at serious risk of suicide, and medication free of any other psychotropic medication except lithium (serum lithium 0.6-1.2 mEq/L) or valproic acid (50-125 μg/mL) for at least 2 weeks before randomization (5 weeks for fluoxetine). Exclusion criteria consisted of any serious unstable medical condition, pregnancy or nursing, and previous treatment with ketamine.
The primary outcome measure was changes in the MADRS scores from 60 minutes before infusion to 40, 80, 110, and 230 minutes after infusion. Rating scales were also administered 1, 2, 3, 7, 10, and 14 days after the infusion. Subjects were given ketamine 0.5 mg/kg intravenously infused over 40 minutes or 0.9% saline on 2 test days, 2 weeks apart. A sample size of 19 was expected to reach 90% power with a 2-tailed test based on response rates at day 1. Linear mixed models with fixed, repeated-measures factors for time and treatment were used, and the primary analysis was intent to treat. Carryover effects were investigated by comparing the baseline values in each study phase within different analysis models. Using all available data in the intent-to-treat sample, the linear mixed model denoted a significant interaction between time and drug (F = 3.22; P < .001). Ketamine displayed depressive symptom improvement compared with placebo beginning at 40 minutes and lasting through day 3 (P < .001). Comparisons at days 7, 10, and 14 were not statistically significant (P = .21, P = .13, and P = .09, respectively). When examining potential carryover effects, no effect for order was established (F = 1.48; P = .24). Adverse effects occurring more frequently in the ketamine group included dissociation, feeling strange, dry mouth, tachycardia, and increased blood pressure. None of these were serious and most subsided within 80 minutes of the infusion.
Since this trial was also a crossover design, its strengths and limitations are similar to those of the aforementioned study.17 However, an additional strength is the power calculation that was completed before the study and that 90% power was nearly reached on day 1 with strict inclusion criteria. A possible limitation is the subjects' continued use of lithium or valproic acid. A study by Zarate et al19 was a replication of the previous study by Diazgranados et al.18
The objective, inclusion and exclusion criteria, study design, statistical analysis, and outcome measures were the same. A sample size of 20 was expected to reach 80% power with a 2-tailed test. This study only enrolled 15 patients, so power was not achieved. Using all available data with the intent-to-treat sample, the linear mixed model showed a significant drug by time interaction (F = 5.94; P < .001). Ketamine exhibited depressive symptom improvement versus placebo from 40 minutes to 3 days after infusion (P < .05). Comparisons at days 7, 10, and 14 were not statistically significant (P = .34, P = .93, and P = .19, respectively). When studying possible carryover effects, no effect for order was established (P = .43). Adverse effects occurring more often in the ketamine group were dry mouth, dizziness, difficulty falling asleep, and flatulence.
A study by Lapidus et al20 was the first randomized controlled trial to determine the effects of intranasal ketamine. The study objective of this single-setting, randomized, double-blind crossover trial was to test the rapid antidepressant effect of one 50-mg dose of intranasal ketamine in patients with major depression. Inclusion criteria consisted of men and women aged 21 to 65 years with a primary diagnosis of MDD without psychotic features and who had failed at least 1 adequate antidepressant trial. Subjects were required to have a score of at least 30 or greater on the Inventory of Depressive Symptomatology – Clinician Rated and have a negative urine toxicology screen. Women of childbearing potential had to maintain adequate birth control for the study duration. All subjects were allowed to remain on stable doses of antidepressants and other psychotropic medications. Exclusion criteria consisted of any unstable medical condition, high risk of suicide, substance-use disorder within 6 months before the study, any psychotic disorder, bipolar disorder, developmental disorder, lifetime use disorder of ketamine or phencyclidine, and pregnancy.
The primary outcome measure was changes in the MADRS score 24 hours after administration of ketamine or placebo. Ratings were given 60 minutes before intervention as well as 40, 120, and 240 minutes after. Ratings were also administered 1, 2, 3, and 7 days after administration. Eighteen subjects were given 50 mg ketamine or 0.9% saline intranasally through an LMA MADgic mucosal atomization device on 2 test days, 1 week apart. A mixed-model approach was used to assess treatment, period, and carryover, and the primary analysis was a modified intent to treat. Twenty-four hours after the intervention, depressive symptoms were significantly improved in the ketamine group compared with the placebo group (t = 4.39; P < .001). The mean difference in MADRS score between ketamine and placebo was 7.6 ± 3.7 (95% confidence interval [CI] = 3.9-11.3). The repeated-measures mixed linear models analysis reported greater improvement with ketamine versus placebo over the 7 day follow-up period (F = 28.10; P < .001). Adverse effects occurring more frequently in the ketamine group were feeling strange, poor memory, and fatigue. None of these were serious and all subsided within 4 hours after the intervention.
A possible strength or limitation is the inclusion of subjects taking antidepressants and psychotropic medications during the study period. While this may render it difficult to distinguish between an intrinsic effect of ketamine and the combination of ketamine with other antidepressants, it may reflect clinical practice and the potential use of ketamine in treatment-resistant populations.
Ketamine Versus Midazolam
The small study size and lack of active control trials in previous studies investigating the use of ketamine for MDD inspired the study by Murrough et al.21 The objective of this 2-site, double-blind study was to test the rapid antidepressant efficacy of ketamine in treatment-resistant MDD using an active placebo-control condition. Inclusion criteria included the following: 21 to 80 years old, primary diagnosis of MDD, previously failed at least 2 therapeutic trials of antidepressant therapy, a history of at least 1 previous MDD episode, or a diagnosis of chronic MDD in addition to an Inventory of Depressive Symptomology test score >32 at baseline and 24 hours before infusion. Subjects were required to abstain from antidepressant and psychotropic medications for 1 to 4 weeks before infusion, and remain drug free until the conclusion of the study. Subjects were excluded if they had a lifetime history of a psychotic illness, bipolar disorder, alcohol or substance abuse in the past 2 years, an unstable medical condition, serious and imminent suicidal or homicidal ideation, a score <27 on the Mini Mental State Examination, or taking medications contraindicated with the study drug therapy. Seventy-three subjects were randomized to receive treatment with either ketamine or midazolam. Forty-eight received ketamine 0.5 mg/kg and 25 received midazolam 0.045 mg/kg both infused over 40 minutes. The primary outcome was a reduction in depression severity assessed via the MADRS 24 hours after infusion. A sample size of 72 randomly assigned in a 2:1 ratio (ketamine versus midazolam) would provide 80% power to detect a change in MADRS scores at 24 hours as a function of treatment.
Forty-seven subjects from the ketamine group and 25 from the midazolam group were included in the modified intention-to-treat analysis. Baseline characteristics were similar between the 2 groups. At 24 hours, the mean MADRS scores for the ketamine and midazolam groups were 14.77 (95% CI = 11.3-17.80) and 22.72 (95% CI = 18.85-26.59), respectively, resulting in a significant difference between the groups (P < .001, degrees of freedom [df] = 68). When adjusted for baseline and site, there was a significant difference between MADRS scores in both groups (95% CI = 3.20-12.7) without a difference between sites (P = .43, df = 1.70). Subject's scores were studied at 1, 2, 3, and 7 days after infusion to evaluate durability of ketamine's effects. Analysis failed to show changes over time as a function of treatment (F = 5.93, df = 1,202, P < .58), but showed main effects for time (F = 7.62, df = 1,202, P < .006) and treatment (F = 5.93, df = 1,202, P < .02). The study showed that over time, subjects in the ketamine group had lower MADRS scores (mean 16.93; 95% CI = 14.03-19.82) than the midazolam group (mean = 23.19; 95% CI = 19.02-27.34; t = 2.33, df = 202, P < .02). The positive effects of ketamine were lost starting around day 3 after infusion. At day 7, the positive effects on depression shown through the MADRS scale no longer showed significant differences between groups. Adverse effects lasting up to 4 hours and occurring more frequently in the ketamine group were dizziness, blurred vision, headache, nausea or vomiting, dry mouth, poor coordination, poor concentration, and restlessness. Of the subjects receiving ketamine, 17% reported significant dissociative symptoms after infusion which subsided within 2 hours.
Murrough et al21 were successful in creating a strong study design that maximized internal and external validity. Study investigators did so by standardizing infusion and monitoring procedures, using blinding in the study and having an effective control that resulted in similar side effects at the time of and 24 hours after infusion. Using a nonactive drug placebo would undermine the integrity of the study as ketamine produces behavioral effects that would not be present using nondrug placebo. Limitations of this trial include not quite reaching power and lacking information on use of ketamine with other antidepressant or antipsychotic medications that may be used to manage MDD. Many patients with MDD may also have other concurrent mental illnesses, and many of these conditions were excluded from the study.
Another study by Murrough et al22 assessed cognitive functioning after a single dose of ketamine. Study investigators theorized that neurocognitive performance would not be reduced 7 days after ketamine administration; however, the primary outcome measure was a 50% reduction in MADRS score relative to baseline. This study was similar in sites and design to the previous study.21 Sixty-two subjects underwent randomization to receive either a single IV infusion of ketamine 0.5 mg/kg (n = 43) or midazolam 0.045 mg/kg (n = 19) over 40 minutes under double-blind conditions. Several different neurocognitive assessments were performed at baseline, including processing speed (category fluency, Trails A, Brief Assessment of Cognition in Schizophrenia Digit Symbol), working memory (Wechsler Memory Scale-III Spatial Span, letter-number), verbal learning (Hopkins Verbal Learning Test learning and delay), visual learning (Brief Visual Memory Test learning), and reasoning/problem solving (Neuropsychological Assessment Batter Mazes).
The effects of time, treatment condition, and antidepressant response were analyzed using analysis of variance models and logistic regression. The change in subjects' processing speed (F = 6.58; P = .013), verbal learning (F = 6.80; P = .012), and visual learning (F = 6.48; P = .014) were found to be significantly improved over the midazolam treatment arm. There were no changes in working memory or reasoning. It was determined through linear regression that ketamine responders had significantly slower processing speed at baseline (T score = 43.37 ± 8.78) compared with ketamine nonresponders (T score = 49.24 ± 10.1; F = 4.36; P = .043).
The study of Murrough et al22 was well designed and had a fairly large sample size for this subject matter and strict inclusion and exclusion criteria. Another strength was the use of an active placebo to control for expectation bias. The authors used appropriate statistical analyses; however, they failed to state how many responders compared with nonresponders there were in the treatment group and neglected to take MADRS scores after infusion.
Discussion
Ketamine has shown promise in quickly reducing symptoms in patients with treatment-resistant depression and bipolar depression. Several small clinical trials have shown that a single subanesthetic dose of ketamine, 0.5mg/kg infused over 40 minutes, produces a quick antidepressant response within 2 to 4 hours of administration.23 This antidepressant effect may reach the highest impact 24 hours after infusion and last up to 7 days. A meta-analysis by Romeo et al24 analyzed ketamine's efficacy in depression at day 1, day 2, days 3-4, day 7, and day 14 compared with placebo in treatment-resistant depression. Ketamine demonstrated a statistically significant antidepressant effect compared with placebo from day 1 through day 7. Analyses were repeated to determine if there were any efficacy differences between unipolar and bipolar depression. When including only participants with unipolar depression, ketamine's efficacy was not affected from day 1 through day 7. Patients with bipolar depression, however, only saw efficacy through day 4.
Ketamine offers many advantages to patients suffering from treatment-resistant depression and bipolar depression. These include a novel mechanism of action, a fast antidepressant effect, and the absence of some known antidepressant adverse effects (weight changes, sexual dysfunction).23 Although ketamine works quickly, its antidepressant effects appear to be temporary (lasting a few days to a week) after a single infusion. Ketamine also has its own adverse effect profile along with abuse potential. Reported adverse effects include psychotic and dissociative effects, blood pressure and heart rate fluctuations, blurry vision, and drowsiness.23 Another risk to consider is the current unregulated practice of ketamine prescribing, dispensing, and monitoring. Patients may perform an Internet search to locate ketamine prescribers and compounding pharmacies that will allow them to receive intranasal and sublingual formulations of ketamine in the mail.25 Thus, the selection of ketamine for patients will depend on a thorough review of the risks and benefits to each individual.
Using ketamine appears to be a helpful option for patients who have exhausted other pharmacotherapeutic avenues. Current data suggest that a single infusion of ketamine is appropriate for patients with treatment resistant unipolar or bipolar depression without psychotic features who lack substance-use disorders. However, larger, multisite, and more generalizable trials should be done to further supplement and enhance the available evidence. These studies should explore the use of ketamine given in multiple infusions for treatment-resistant depression or bipolar depression and examine the utility of ketamine in depressed patients with suicidality. Also, as it does not seem reasonable to completely withdraw antidepressant or mood-stabilizing medication for weeks in clinical practice, studies analyzing ketamine as adjunctive treatment to these agents in patients with a partial response to medications alone seems logical. Lastly, the authors of this manuscript recommend further research regarding ketamine administration frequency to elicit best outcomes for patients.
References
- 1. Li JH, Vicknasingam B, Cheung YW, Zhou W, Nurhidayat AW, Jarlais DC, et al. To use or not to use: an update on licit and illicit ketamine use. Subst Abuse Rehabil. 2011; 2: 11- 20. DOI: 10.2147/SAR.S15458. PubMed PMID: 24474851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bräu ME, Sander F, Vogel W, Hempelmann G. . Blocking mechanisms of ketamine and its enantiomers in enzymatically demyelinated peripheral nerve as revealed by single-channel experiments. Anesthesiology. 1997; 86 2: 394- 404. PubMed PMID: 9054257. [DOI] [PubMed] [Google Scholar]
- 3. Duque JC, Oleskovicz N, Guirro ECBP, Valadão CAA, Soares VE. . Relative potency of ketamine and S(+)-ketamine in dogs. J Vet Pharmacol Ther. 2008; 31 4: 344- 8. DOI: 10.1111/j.1365-2885.2008.00965.x. PubMed PMID: 18638295. [DOI] [PubMed] [Google Scholar]
- 4. Wolff K, Winstock AR. . Ketamine: from medicine to misuse. CNS Drugs. 2006; 20 3: 199- 218. PubMed PMID: 16529526. [DOI] [PubMed] [Google Scholar]
- 5. Hirota K, Okawa H, Appadu BL, Grandy DK, Devi LA, Lambert DG. . Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology. 1999; 90 1: 174- 82. PubMed PMID: 9915326. [DOI] [PubMed] [Google Scholar]
- 6. Skolnick P, Popik P, Trullas R. . Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009; 30 11: 563- 9. DOI: 10.1016/j.tips.2009.09.002. PubMed PMID: 19837463. [DOI] [PubMed] [Google Scholar]
- 7. Zhao Y, Sun L. . Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J Clin Neurosci. 2008; 15 11: 1264- 9. DOI: 10.1016/j.jocn.2007.11.007. PubMed PMID: 18815045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pal HR, Berry N, Kumar R, Ray R. . Ketamine dependence. Anaesth Intensive Care. 2002; 30 3: 382- 4. PubMed PMID: 12075653. [DOI] [PubMed] [Google Scholar]
- 9. Curran HV, Cognitive Morgan C., . dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction. 2000; 95 4: 575- 90. PubMed PMID: 10829333. [DOI] [PubMed] [Google Scholar]
- 10. Weiner AL, Vieira L, McKay CA, Bayer MJ. . Ketamine abusers presenting to the emergency department: a case series. J Emerg Med. 2000; 18 4: 447- 51. PubMed PMID: 10802423. [DOI] [PubMed] [Google Scholar]
- 11. Walker AK. . Intramuscular ketamine in a developing country. Experience in the British Solomon Islands. Anaesthesia. 1972; 27 4: 408- 14. PubMed PMID: 4634748. [DOI] [PubMed] [Google Scholar]
- 12. Hocking G, Cousins MJ. . Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003; 97 6: 1730- 9. PubMed PMID: 14633551. [DOI] [PubMed] [Google Scholar]
- 13. White PF, Way WL, Trevor AJ. . Ketamine—its pharmacology and therapeutic uses. Anesthesiology. 1982; 56 2: 119- 36. PubMed PMID: 6892475. [DOI] [PubMed] [Google Scholar]
- 14. Cheng PS, Fu CY, Lee CH, Liu C, Chien CS. . GC-MS quantification of ketamine, norketamine, and dehydronorketamine in urine specimens and comparative study using ELISA as the preliminary test methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 852 1-2: 443- 9. DOI: 10.1016/j.jchromb.2007.02.005. PubMed PMID: 17339137. [DOI] [PubMed] [Google Scholar]
- 15. Hijazi Y, Boulieu R. . Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2002; 30 7: 853- 8. PubMed PMID: 12065445. [DOI] [PubMed] [Google Scholar]
- 16. Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000; 47 4: 351- 4. DOI: 10.1016/S0006-3223(99)00230-9. PubMed PMID: 10686270. [DOI] [PubMed] [Google Scholar]
- 17. Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006; 63 8: 856- 64. DOI: 10.1001/archpsyc.63.8.856. PubMed PMID: 16894061. [DOI] [PubMed] [Google Scholar]
- 18. Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010; 67 8: 793- 802. DOI: 10.1001/archgenpsychiatry.2010.90. PubMed PMID: 20679587; PubMed Central PMCID: PMC3000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012; 71 11: 939- 46. DOI: 10.1016/j.biopsych.2011.12.010. PubMed PMID: 22297150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lapidus KAB, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014; 76 12: 970- 6. DOI: 10.1016/j.biopsych.2014.03.026. PubMed PMID: 24821196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013; 170 10: 1134- 42. DOI: 10.1176/appi.ajp.2013.13030392. PubMed PMID: 23982301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murrough JW, Burdick KE, Levitch CF, Perez AM, Brallier JW, Chang LC, et al. Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment-resistant depression: a randomized controlled trial. Neuropsychopharmacology. 2015; 40 5: 1084- 90. DOI: 10.1038/npp.2014.298. PubMed PMID: 25374095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pradhan B, Parikh T, Makani R, Sahoo M. . Ketamine, transcranial magnetic stimulation, and depression specific yoga and mindfulness based cognitive therapy in management of treatment resistant depression: review and some data on efficacy. Depress Res Treat. 2015; 2015: 842817 DOI: 10.1155/2015/842817. PubMed PMID: 26509083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romeo B, Choucha W, Fossati P, Rotge J-Y. . Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015; 230 2: 682- 8. DOI: 10.1016/j.psychres.2015.10.032. PubMed PMID: 26548981. [DOI] [PubMed] [Google Scholar]
- 25. Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, et al. Potential risks of poorly monitored ketamine use in depression treatment. Am J Psychiatry. 2016; 173 3: 215- 8. DOI: 10.1176/appi.ajp.2015.15081082. PubMed PMID: 26926127. [DOI] [PubMed] [Google Scholar]