Abstract
Introduction:
Valproic acid (VPA) and its derivatives are highly protein bound with free fraction increasing with dose and serum concentration. Consensus guidelines regarding dose adjustment for hypoalbuminemia are not available.
Methods:
A literature search was performed using PubMed to identify articles with the following key terms: “valproate,” “valproic acid,” “protein binding,” “albumin,” and “hypoalbuminemia.” We report our findings as well as 5 cases involving pharmacokinetic impact of hypoalbuminemia on valproate.
Results:
A previously published model for normalizing VPA serum concentration for hypoalbuminemia in patients with epilepsy was compared to results for 5 cases (4 female, 1 male) in which VPA was used for psychiatric illness. Only 1 of the cases had free serum concentrations in the range that would be expected with the model. Free concentrations ranged from 22% to 83% with no clear relationship to other factors (weight, age, serum creatinine, or dose). Female patients with similar albumin had higher free fractions than the 1 male patient.
Discussion:
Due to the variability in pharmacokinetic impact of hypoalbuminemia, it is important to monitor patients closely for signs of VPA toxicity in cases involving altered albumin levels. It would be prudent to use free serum VPA concentrations when patients experience fluctuations in albumin or have unexpected response to medication.
Keywords: valproate, valproic acid, hypoalbuminemia, albumin, protein binding
Background
Valproic acid (VPA) and its derivatives are available in many dosage forms. These range from parenteral injection to immediate and delayed-release oral formulations, which are marketed under multiple brand names. Although most VPA products are characterized by similar area-under-the-curve profiles, time to maximum concentration (tmax) and maximum concentration (Cmax) vary between products. The extended-release tablets have 10% to 20% lower bioavailability compared to other oral formulations. Valproic acid binds to serum proteins, primarily albumin, with a dose-dependent free fraction of 10% to 30% that may be higher in elderly patients. The unbound free fraction increases in a linear fashion as the dose and the serum concentration of VPA increases. In contrast, there is a nonlinear relationship between dose and total VPA serum concentration.1-3 The manufacturer recommends using caution when interpreting total levels in patients with hepatic disease and decreased albumin but provides no specific dosing recommendations. Renal failure may result in alterations in unbound clearance and protein binding; however, no specific dosing recommendations are available for renal impairment.1 Hermida and Tutor4 proposed an algorithm for correcting total VPA serum concentration based on albumin for patients 25 to 55 years of age with seizure disorders. The formula was CN = αHCH/αN (normalized VPA concentration = free fraction in hypoalbuminemia × total concentration/free fraction in normoalbuminemia). Their model demonstrated accuracy with total VPA concentrations less than 75 mcg/mL and albumin ranging from 1.8 to 4.2 g/dL. Free fraction based on their model ranged from 6.5% with albumin of 4.2 g/dL to 35.8% with albumin of 1.8 g/dL. Lampon and Tutor5 further evaluated this model to compare elderly and nonelderly patients with epilepsy and found that VPA clearance was decreased in elderly patients by nearly 40%. Clearance was not impacted by serum creatinine in either elderly or nonelderly groups, nor did results differ between male and female subjects.
Ideally, pharmacokinetic models could be used to make empiric decisions regarding VPA dosing in patients with acute or chronic changes in hepatic function. Manufacturer labeling does not provide any specific recommendations for these patients. The Hermida model includes only patients with seizure disorders rather than other indications for VPA. After unexpected treatment response, free fraction of VPA for 5 patients with hypoalbuminemia were compared to the Hermida and Tutor model.
Case Series: Impact of Hypoalbuminemia on VPA Pharmacokinetics
The psychiatry consult liaison service at our institution assists with psychiatric management of patients admitted to nonpsychiatric services. Consults commonly involve unexpected destabilization of psychiatric illness and/or unexpected response to psychotropic medication therapy in the setting of acute medical treatment. Alterations in serum albumin, serum creatinine, and liver enzymes are potential issues in this population that may impact medication management.
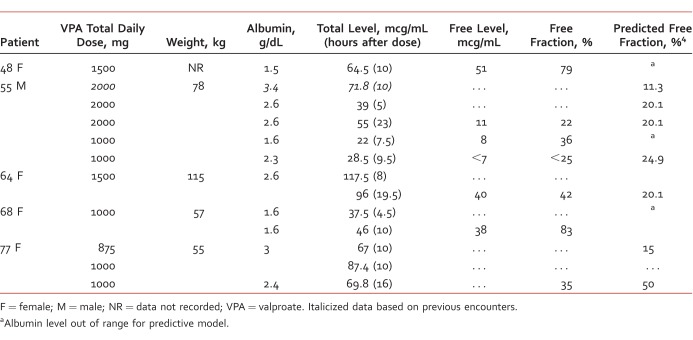
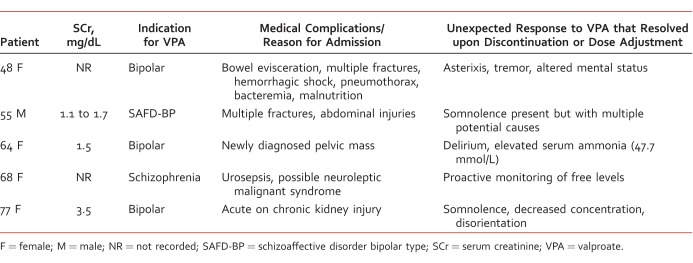
Table 1 summarizes data for 5 cases involving both unexpected clinical response and VPA serum concentrations in 5 medically compromised patients. All 5 patients had different medical complications and unexpected responses to VPA (Table 2). Three of the 5 patients had an albumin level lower than the cutoff for the Hermida predictive model (1.8 g/dL to 4.2 g/dL); 2 of whom had significantly elevated free fraction (79% and 83%, respectively). Two patients had free fractions 2-fold higher than predicted by the model (42.1% versus 20.1% expected and 40% versus 23.2% expected). The 55-year-old male patient was the only one who had free concentrations consistent with the model.
TABLE 1:
Dose and serum concentrations of VPA (total and free)
TABLE 2:
Indication for VPA, comorbid medical complications and response to VPA
Three of the 5 patients had documented elevation in serum creatinine. Timing of levels ranged from 4.5 hours to 23 hours after the previous dose.
Discussion
Retrospective case reviews have inherent limitations, including lack of complete dosing history (eg, prior to admission), potential drug interactions with concomitant medications, and interpatient variability in collection of serum samples for laboratory testing (eg, serum creatinine not recorded for all patients). Although trough VPA levels are the most accurate for therapeutic drug monitoring, 4 of 13 total VPA levels were not troughs, generally drawn early for concerns of toxicity. Although trough VPA levels are the most accurate for therapeutic drug monitoring, timing of levels should not impact free fraction results, which is the focus of these cases.
Although the Hermida model was used as a comparator, not all patients fit the criteria for the model. Three of the 13 total VPA serum concentrations (in 2 of 5 patients) were outside the range of the Hermida model. Four of the 11 albumin results (in 3 of 5 patients) were also outside of the Hermida model. Limitations of the predictive model were also highlighted with these cases. It is common practice to use VPA at levels above 75 mcg/mL for bipolar spectrum disorders, which is outside the range of the model.6
Conclusions
There are no specific recommendations for adjusting VPA dosing in cases of hypoalbuminemia. These 5 cases demonstrate the importance of considering baseline albumin as well as new onset hypoalbuminemia when dosing VPA. Patients with albumin less than 2 g/dL appear to be at high risk of developing significantly elevated free fractions that do not fit previous predictive models. Additionally, patients who have target VPA levels greater than 75 mcg/mL do not fit previous models and may be at higher risk of developing toxicity. It would be prudent to assess carefully for signs of toxicity as well as use free VPA serum concentration for therapeutic monitoring in patients with clinically significant hypoalbuminemia. Further research would be helpful in validating or developing predictive models in these patients.
References
- 1. Depakene® [package insert]. Abbott Park (IL): Abbott Laboratories; 2009. [Google Scholar]
- 2. Perucca E. . Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002; 16 10: 695- 714. DOI: 10.2165/00023210-200216100-00004. PubMed PMID: 12269862. [DOI] [PubMed] [Google Scholar]
- 3. Gram L, Flachs H, Würtz-Jørgensen A, Parnas J, Andersen B. . Sodium valproate, serum level and clinical effect in epilepsy: a controlled study. 1979; 20 3: 303- 11. DOI: 10.1111/j.1528-1157.1979.tb04808.x. PubMed PMID: 376307. [DOI] [PubMed] [Google Scholar]
- 4. Hermida J, Tutor JC. . A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005; 97 4: 489- 93. DOI: 10.1254/jphs.FPE04007X. PubMed PMID: 15840952. [DOI] [PubMed] [Google Scholar]
- 5. Lampon N, Tutor JC. . Apparent clearance of valproic acid in elderly epileptic patients: estimation of the confounding effect of albumin concentration. Ups J Med Sci. 2012; 117 1: 41- 6. DOI: 10.3109/03009734.2011.640412. PubMed PMID: 22206465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirschfeld RM, Baker JD, Wozniak P, Tracy K, Sommerville KW. . The safety and early efficacy of oral-loaded divalproex versus standard-titration divalproex, lithium, olanzapine, and placebo in the treatment of acute mania associated with bipolar disorder. J Clin Psychiatry 2003; 64 7: 841- 6. PubMed PMID: 12934987. [DOI] [PubMed] [Google Scholar]