Abstract
Introduction:
Posttraumatic stress disorder (PTSD) is a common and serious psychiatric illness. Exposure therapy is a type of cognitive behavioral therapy that is considered a first-line treatment option for PTSD. D-cycloserine (DCS) enhances fear extinction/exposure therapy in patients with various anxiety disorders, presumably via its N-methyl-D-aspartate receptor partial agonist effects. The aim of this paper is to review the published literature regarding the efficacy of DCS in the treatment of PTSD.
Methods:
A literature search for placebo-controlled trials assessing the use of DCS as the primary study drug in PTSD was conducted for trials published before June 2015 using PubMed, Ovid International Pharmaceutical Abstracts, and www.clinicaltrials.gov. The search terms were variations of “cycloserine” and “posttraumatic stress disorder.”
Results:
Seven clinical trials were analyzed, including 2 trials comparing DCS with placebo as add-on treatment to ongoing stable pharmacotherapy and 5 trials that compared DCS with placebo given prior to exposure therapy. D-cycloserine as adjunctive therapy showed no benefit in 1 trial and limited benefit in the other. As an enhancement of exposure therapy, DCS showed beneficial effects in 1 trial, detrimental effects in 1 trial, and inconclusive effects in 3 trials.
Discussion:
Current literature does not adequately support the use of DCS as adjunctive therapy without psychotherapy, but limitations of the 2 studies that exist make firm conclusions unfeasible. D-cycloserine might have a role in augmentation of exposure therapy. Future studies should consider receptor selectivity, administration time with respect to peak cerebrospinal fluid concentrations, number of exposure therapy sessions, and dose.
Keywords: posttraumatic stress disorder, D-cycloserine, pharmacotherapy
Introduction
Posttraumatic stress disorder (PTSD) is a psychiatric condition that can develop when an individual is exposed to actual or threatened death, serious injury, or sexual violence.1 Characteristic symptoms involve intrusion (eg, distressing memories, nightmares), avoidance (eg, avoiding reminders of the traumatic event), negative cognitions and mood (eg, blaming oneself, diminished interest in activities), and arousal and reactivity (eg, irritability, sleep disturbance).1 The disturbance must last for at least 1 month, and it must result in clinically significant distress or impairment in social, occupational, or other important areas of functioning.1 Posttraumatic stress disorder is quite common, as 12-month and lifetime prevalence rates in the United States are 3.5% and 8.7%, respectively.1 First-line treatment options in PTSD include pharmacotherapy or psychotherapy. Well-established pharmacotherapeutic options include selective serotonin reuptake inhibitors (eg, paroxetine, sertraline) and venlafaxine.2,3 Effective psychological treatments include trauma-focused cognitive behavioral therapy (CBT) and eye movement desensitization and reprocessing.2,3 Exposure therapy is a particularly effective type of CBT. Exposure therapy is designed to reduce the patient's distress through gradual confrontation of memories, emotions, and situations associated with the traumatic event.4,5 During therapy, exposure to the traumatic event can be accomplished through thoughts (imaginal exposure), actual situations (in vivo exposure), or computer-generated environments (virtual reality exposure).4,5
N-methyl-D-aspartate (NMDA) receptors are a subclass of glutamate receptors that are important in controlling synaptic plasticity and regulating memory.6 As such, NMDA receptor dysfunction has been implicated in the pathophysiology of a number of psychiatric and neurologic disease states.6 One important role for NMDA receptors is modulation of the fear extinction response.7 It has been demonstrated in animal models that blocking the NMDA receptor impairs extinction retention whereas enhancing the functioning of the NMDA receptor facilitates fear extinction.7 Enhancement of NMDA activity might therefore augment the effects of psychotherapy in various anxiety states as fear extinction is a key component of many types of psychotherapy.7
D-cycloserine (DCS) is an antibiotic that is approved for treatment of tuberculosis and urinary tract infections. DCS is a full agonist at GluN2C and a partial agonist at GluN2A, GluN2B, and GluN2D NMDA receptor subtypes.8 These NMDA receptor subtypes are differentially distributed throughout the central nervous system and play roles in both the formation and reinforcement of fear responses as well as fear extinction.9,10 D-cycloserine enhances fear extinction/exposure therapy in both animal models and humans with anxiety although its effects appear to decrease over repeated sessions.11 D-cycloserine has been used successfully in combination with CBT to treat patients with phobias, obsessive-compulsive disorder, and panic disorder.7 The aim of this article is to review the published literature regarding the efficacy of DCS in the treatment of PTSD.
Methods
Eligible study designs included English language placebo-controlled trials of DCS as the primary study drug in the treatment of posttraumatic stress disorder. A PubMed search was performed using MeSH terms “stress disorders, posttraumatic” and “cycloserine” and limited to “clinical trials” and a free text search “cycloserine and posttraumatic stress disorder.” An Ovid International Pharmaceutical Abstracts search was performed using search terms “cycloserine and posttraumatic stress disorder.” A search was performed at www.clinicaltrials.gov using search terms “posttraumatic stress disorder” and “cycloserine.”
Results
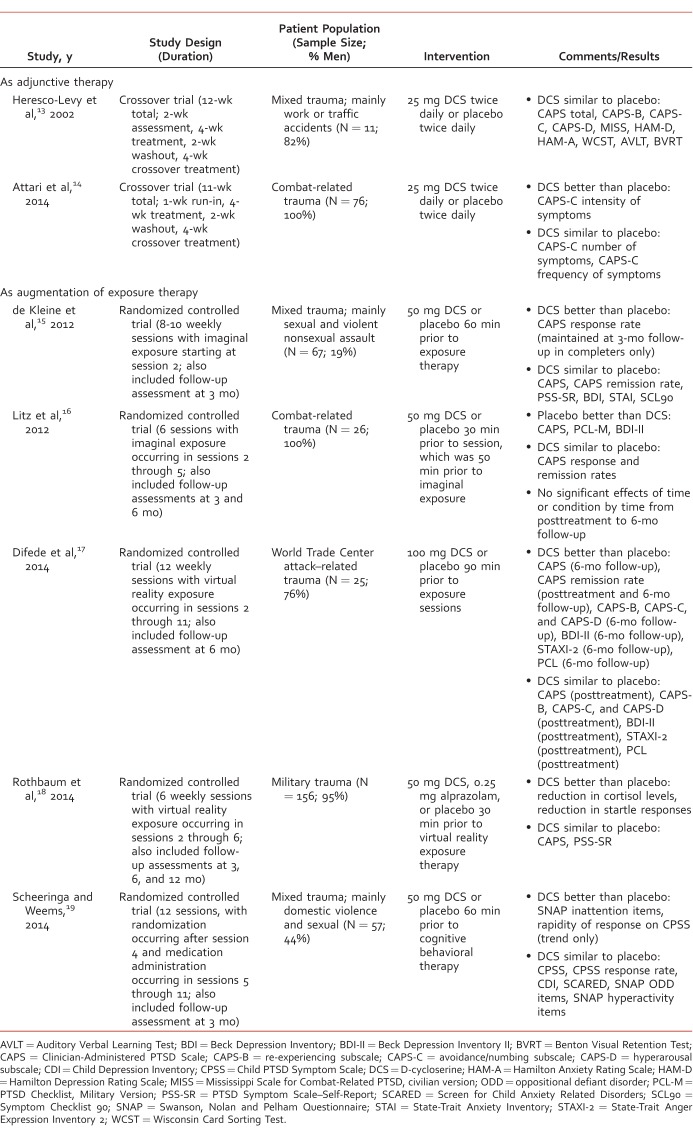
The search strategy yielded 8 published trials, but 1 trial12 was excluded from the review because DCS was administered along with another experimental medication, mifepristone. Thus, there were 7 published trials, including 2 studies that utilized DCS as adjunctive therapy13,14 and 5 studies that included DCS as augmentation of exposure therapy.15-19 These 7 studies are outlined in the Table.
Table:
Placebo-controlled trials involving D-cycloserine in the treatment of posttraumatic stress disorder
Adjunctive Therapy
Two published studies examined DCS as adjunctive therapy. In the first study,13 7 of 11 patients received ongoing stable psychotropic medication therapy whereas the other patients received no psychotropic medications. In the second study,14 all patients received ongoing stable psychotropic medication therapy, and 14 patients received marital therapy, family therapy, or individual cognitive therapy. Heresco-Levy et al13 found no statistically significant differences in treatment effects between DCS and placebo in terms of PTSD symptomatology, depression, anxiety, or neurocognitive parameters. Attari et al14 used a similar study design, but they focused on outcomes specifically relating to avoidance and numbing symptoms of PTSD. D-cycloserine treatment did not significantly influence the total number of symptoms or the frequency of symptoms although there was a nonsignificant trend in favor of DCS versus placebo (P = .083) as concerns the total number of symptoms. D-cycloserine treatment demonstrated a significant decrease versus placebo (P = .008) in regards to intensity of symptoms, but the analysis had to be limited to the first intervention period due to a significant carryover effect. Reported adverse effects were headache and nausea, and these did not differ significantly from placebo. DCS treatment was well tolerated in both trials, and there were no dropouts due to adverse effects.
Augmentation of Exposure Therapy
DeKleine et al15 did not find an overall enhancement effect for DCS. There were no statistically significant differences in treatment effects between DCS and placebo on clinician- or self-measures of PTSD, depression, anxiety, or general psychopathology. However, patients who received DCS were more likely to achieve response (ie, Clinician-Administered PTSD Scale [CAPS] reduction ≥10 points) in both intent-to-treat analysis (63.6% versus 38.2%, χ21 = 4.32, P = .04) and completers analysis (87.5% versus 61.9%, χ21 = 3.97, P = .05) with the latter effect being maintained at 3-month follow-up (P = .04). There was no difference between treatments with respect to remission rate (ie, CAPS <20). A session-by-session analysis revealed that DCS treatment resulted in greater reduction in self-reported PTSD symptoms versus placebo treatment in patients with more severe pretreatment symptoms who required more sessions (ie, 8-10 sessions versus fewer than 8 sessions).
Litz et al16 found detrimental effects of DCS compared to placebo. Placebo was superior to DCS on the CAPS (P < .05); PTSD Checklist, Military Version (P < .01); and Beck Depression Inventory II (BDI-II; P < .05). Posttreatment, responder status was attained by 70% in the placebo group versus 30% in the DCS group, but this did not reach statistical significance. Three participants in the DCS group experienced clinically significant worsening of symptoms (ie, CAPS increase ≥10 points).
Difede et al17 found no significant differences between patients treated with DCS and placebo on measures of PTSD, depression, and anxiety at posttreatment except that the remission (ie, CAPS ≤20 and minimal/no impairment in functioning) rate was significantly greater in DCS-treated patients (46% versus 8%). However, the CAPS (P = .01), CAPS subscales (P < .05), BDI-II (P < .001), State-Trait Anger Expression Inventory 2 (P = .009), and PTSD Checklist (P = .008) were all improved with DCS treatment versus placebo at the 6-month follow-up assessment. Furthermore, the remission rates at 6-month follow-up were 69% versus 17% in DCS and placebo arms, respectively.
Rothbaum et al18 found no statistically significant differences in treatment effects between DCS and placebo on clinician- or self-rated PTSD symptoms at posttreatment or follow-up. However, an association between extinction learning and posttreatment scores on the CAPS (b = –2.19, confidence interval: –3.44 to –0.94, P = .001) and PTSD Symptom Scale–Self-Report (b = –0.82, confidence interval: –1.19 to 0.45, P = .001) was seen for the DCS group but not for the placebo group. D-cycloserine treatment resulted in improvement in two neurobiological biomarkers of PTSD. Salivary cortisol levels decreased (ie, from before to 15 minutes after scene presentation) more in the DCS group after treatment than in the placebo group (P < .05). Startle responses, which were measured via electromyography of the orbicularis oculi muscle contraction, were significantly reduced over time in the DCS group (P = .001) but not the placebo group.
Scheeringa and Weems19 published the only PTSD trial that examined the efficacy of DCS as augmentation of exposure therapy in pediatric patients. They found no significant treatment effects of DCS compared to placebo on symptoms relating to PTSD, depression, anxiety, hyperactivity, or oppositional defiant disorder. Completer analysis revealed that responder status (≥50% reduction on the Child PTSD Symptom Scale [CPSS]) was attained by 75% in the placebo group versus 52% in the DCS group, but this did not reach statistical significance. On the other hand, there were a few positive results for DCS, including a significant drug-by-time effect on inattention symptoms (P < .05) and a statistical trend (P = .056) toward a more rapid symptom recovery based on weekly CPSS ratings. Adverse effects included drowsiness, dizziness, irritability, headache, and dry mouth, but these did not differ significantly from placebo.
Discussion
D-cycloserine as adjunctive therapy to current, stable therapy with psychotropic medication showed no benefit in 1 trial13 and only limited benefit in a second trial.14 Given that the proposed mechanism of DCS is to enhance fear extinction,20,21 it is perhaps unsurprising that DCS in the absence of psychotherapy would yield these results. However, there are several important points that need to be made in this regard. First, this approach has been inadequately studied. The trial by Heresco-Levy et al13 had a very limited sample size, and the trial by Attari et al14 only examined outcomes on avoidance and numbing symptoms. Second, the trial by Attari et al14 did demonstrate at least some favorable findings in a cluster of symptoms that has characteristically been considered more difficult to treat. Third, the use of twice-daily dosing in both trials may have attenuated the effects of DCS. In animal models, tachyphylaxis for cognitive effects develops rapidly with repeated dosing of DCS, and once-weekly administration in patients with schizophrenia has demonstrated benefit for negative symptoms and memory consolidation.22 Thus, although current literature does not support the use of DCS in this manner, larger trials with broader outcome measures and use of intermittent dosing could clarify the role of DCS as adjunctive therapy.
As an enhancement of exposure therapy, DCS showed beneficial effects in 1 trial,17 detrimental effects in 1 trial,16 and inconclusive effects in 3 trials.15,18,19 Therefore, DCS might have a role in augmentation of exposure therapy. Due to the varied results seen in these trials, a closer examination of their features could provide several opportunities for design optimization in future trials. Some factors that should be considered include the effect of receptor subtypes on treatment response, the pharmacokinetic parameters that govern cerebrospinal fluid concentrations, number of therapy sessions, and dose. Another consideration is that recent studies in anxiety states suggest that the quality of exposure therapy session is crucial in the success of DCS as evidenced by improvement with successful exposure therapy plus DCS and detriment with unsuccessful exposure therapy plus DCS.23,24
N-methyl-D-aspartate receptors are tetrameric, mixed ion channels (ie, Ca2+, Na+, and K+) that are comprised of 2 glycine binding subunits referred to as GluN1 and two glutamate binding subunits, referred to as GluN2. Both the glycine subunits and glutamate subunits need to be occupied by the agonist in order for the channel to be activated. There are multiple splice variants of the glycine binding subunits and 4 gene products of the glutamate subunits referred to as GluN2A, GluN2B, GluN2C, and GluN2D. D-cycloserine binds to all of the glycine subunits but can convey different receptor conformations in that it displays full agonist activity at receptors containing GluN2C and partial agonism at GluN2A, GluN2B, and GluN2D subtypes.25 Importantly, these NMDA receptor subtypes are differentially distributed throughout the central nervous system and play different roles in acquisition and extinction of fear responses. The GluN2A receptor may be involved in the initial formation and stabilization of the fear response whereas the GluN2B and GluN2C receptors are both involved in extinction of fear responses. N-methyl-D-aspartate receptors require the coagonists of glycine and glutamate to be fully activated, suggesting that DCS may interact with different receptors at different times depending upon when glutamate is released in various brain regions. Better pharmacological tools may soon be on the way to better differentiate the roles of various NMDA receptors in PTSD. A group has recently reported the synthesis and activity of a series of compounds that are selective allosteric modulators of the GluN2C receptor that has been implicated in extinguishing fear responses.26 This may be a novel new drug class for use in PTSD and other anxiety disorders.
One key difference in the PTSD trials was the administration time with respect to exposure therapy. The peak cerebrospinal fluid concentration is reported to be 2 hours after administration,27 and in the trial that showed benefit of DCS,17 the drug was administered 90 minutes prior to exposure therapy. Therefore, the drug concentration would peak in the middle of the 1-hour exposure therapy session. Alternatively, when DCS was administered 30 to 60 minutes prior to exposure therapy,15,16,18,19 the peak concentrations were not being reached until after the exposure therapy session was completed. Another important pharmacokinetic consideration is that the time to peak concentration can be prolonged by high-fat meals,28 which was not addressed by any of the trials that were analyzed. These differences in administration may be relevant in this situation, considering the peak cerebrospinal fluid concentration would ideally match the time of the exposure therapy session.
Therapeutic outcomes may have been affected by the number of exposure therapy sessions. Addition of DCS was expected to speed improvement based on past trials with social anxiety disorder29 and obsessive compulsive disorder30,31; however, instead of speeding the response to therapy, DCS might strengthen the response over time in PTSD. The positive trial used 10 sessions with DCS,17 the negative trial used only 4 sessions with DCS,16 and the inconclusive trials used 5 to 9 sessions with DCS.15,18,19
The last major difference between these trials was dosing. Four of the trials15,16,18,19 used a dose of 50 mg DCS prior to exposure therapy, which was consistent with the dose that has been commonly used in trials for social anxiety disorder, panic disorder, and agoraphobia.29,32-34 The trial that showed the clearest benefit17 used a dose of 100 mg. The higher dosing of DCS showed no significant increase in adverse effects, so it may be reasonable to try this dose in future trials.
By analyzing the data and knowledge that we have, perhaps the scope can be narrowed for future research. Major considerations for future research include receptor specificity, timing of DCS administration, number of exposure therapy sessions, and dose. Ideally, future trials would administer DCS to ensure the peak cerebrospinal fluid concentration peaks during exposure therapy, provide 10 or more exposure therapy sessions with DCS, and use a dose of 100 mg.
References
- 1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Ed. Arlington (VA): The Association; 2013. [Google Scholar]
- 2. Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014; 28 5: 403- 39. DOI: 10.1177/0269881114525674. PubMed PMID: 24713617. [DOI] [PubMed] [Google Scholar]
- 3. Department of Veterans Affairs/Department of Defense Clinical Practice Guideline for Management of Post-Traumatic Stress [Internet]. c 2010. [cited 2015 Sept 21]. Available from: http://www.healthquality.va.gov/guidelines/MH/ptsd/.
- 4. Prolonged Exposure Therapy for PTSD [Internet]. Center for Deployment Psychology; c2013 [cited 2014 Jul 25]. Available from: http://www.deploymentpsych.org/treatments/prolonged-exposure-therapy-ptsd-pe
- 5. Virtual reality exposure therapy to battle PTSD [Internet]. National Center for Telehealth and Technology; c2012 [cited 2014 Jul 24]. Available from: http://www.t2.health.mil/video/virtual-reality-exposure-therapy=battle=ptsd
- 6. Zhou Q, Sheng M. . NMDA receptors in nervous system diseases. Neuropharmacology. 2013; 74: 69- 75. DOI: 10.1016/j.neuropharm.2013.03.030. PubMed PMID: 23583930. [DOI] [PubMed] [Google Scholar]
- 7. Davis M. . NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci. 2011; 13 4: 463- 74. PubMed PMID: 22275851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheinin A, Shavit S, Benveniste M. . Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001; 41 2: 151- 8. PubMed PMID: 11489451. [DOI] [PubMed] [Google Scholar]
- 9. Dalton GL, Wu DC, Wang YT, Floresco SB, Phillips AG. . NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology. 2012; 62 2: 797- 806. DOI: 10.1016/j.neuropharm.2011.09.001. PubMed PMID: 21925518. [DOI] [PubMed] [Google Scholar]
- 10. Ogden KK, Khatri A, Traynelis SF, Heldt SA. . Potentiation of GluN2C/D NMDA receptor subtypes in the amygdala facilitates the retention of fear and extinction learning in mice. Neuropsychopharmacology. 2014; 39 3: 625- 37. DOI: 10.1038/npp.2013.241. PubMed PMID: 24008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Norberg MM, Krystal JH, Tolin DF. . A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008; 63 12: 1118- 26. DOI: 10.1016/j.biopsych.2008.01.012. PubMed PMID: 18313643. [DOI] [PubMed] [Google Scholar]
- 12. Wood NE, Rosasco ML, Suris AM, Spring JD, Marin MF, Lasko NB, et al. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Res. 2015; 225 1-2: 31- 9. DOI: 10.1016/j.psychres.2014.09.005. PubMed PMID: 25441015. [DOI] [PubMed] [Google Scholar]
- 13. Heresco-Levy U, Kremer I, Javitt DC, Goichman R, Reshef A, Blanaru M, et al. Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharm. 2002; 5 4: 301- 7. DOI: 10.1017/S1461145702003061. PubMed PMID: 12466030. [DOI] [PubMed] [Google Scholar]
- 14. Attari A, Rajabi F, Maracy MR. . D-cycloserine for treatment of numbing and avoidance in chronic post traumatic stress disorder: a randomized, double blind, clinical trial. J Res Med Sci. 2014; 19 7: 592- 8. PubMed PMID: 25364356. [PMC free article] [PubMed] [Google Scholar]
- 15. de Kleine RA, Hendriks G-J, Kusters WJC, Broekman TG, van Minnen A. . A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012; 71 11: 962- 8. DOI: 10.1016/j.biopsych.2012.02.033. PubMed PMID: 22480663. [DOI] [PubMed] [Google Scholar]
- 16. Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, et al. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012; 46 9: 1184- 90. DOI: 10.1016/j.jpsychires.2012.05.006. PubMed PMID: 22694905. [DOI] [PubMed] [Google Scholar]
- 17. Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, et al. D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology. 2014; 39 5: 1052- 8. DOI: 10.1038/npp.2013.317. PubMed PMID: 24217129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan war veterans. Am J Psychiatry. 2014; 171 6: 640- 8. DOI: 10.1176/appi.ajp.2014.13121625. PubMed PMID: 24743802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheeringa MS, Weems CF. . Randomized placebo-controlled D-cycloserine with cognitive behavior therapy for pediatric posttraumatic stress. J Child Adolesc Psychopharmacol. 2014; 24 2: 69- 77. DOI: 10.1089/cap.2013.0106. PubMed PMID: 24506079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson R. . Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learn Mem. 2004; 11 5: 510- 6. DOI: 10.1101/lm.78204. PubMed PMID: 15466301. [DOI] [PubMed] [Google Scholar]
- 21. Walker DL, Ressler KJ, Lu K-T, Davis M. . Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002; 22 6: 2343- 51. PubMed PMID: 11896173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goff DC. . D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull. 2012; 38 5: 936- 41. DOI: 10.1093/schbul/sbs012. PubMed PMID: 22368237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, et al. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res. 2013; 47 10: 1455- 61. DOI: 10.1016/j.jpsychires.2013.06.020. PubMed PMID: 23870811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, et al. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biol Psychiatry. 2013; 73 11: 1054- 8. DOI: 10.1016/j.biopsych.2012.12.009. PubMed PMID: 23332511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, et al. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010; 30 7: 2741- 54. DOI: 10.1523/JNEUROSCI.5390-09.2010. PubMed PMID: 20164358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zimmerman SS, Khatri A, Garnier-Amblard EC, Mullasseril P, Kurtkaya NL, Gyoneva S, et al. Design, synthesis, and structure–activity relationship of a novel series of GluN2C-selective potentiators. J Med Chem. 2014; 57 6: 2334- 56. DOI: 10.1021/jm401695d. PubMed PMID: 24512267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baron H, Epstein IG, Mulinos MG, Nair KG. . Absorption, distribution and excretion of Cycloserine in man. Antibiot Annu. 1955-6; 3: 136- 40. PubMed PMID: 13355257. [PubMed] [Google Scholar]
- 28. Zhu M, Nix DE, Adam RD, Childs JM, Peloquin CA. . Pharmacokinetics of cycloserine under fasting conditions and with high-fat meal, orange juice, and antacids. Pharmacotherapy. 2001; 21 8: 891- 97. DOI: 10.1592/phco.21.11.891.34524. PubMed PMID: 11718495. [DOI] [PubMed] [Google Scholar]
- 29. Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006; 63 3: 298- 304. DOI: 10.1001/archpsyc.63.3.298. PubMed PMID: 16520435. [DOI] [PubMed] [Google Scholar]
- 30. Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008; 165 3: 335- 41. DOI: 10.1176/appi.ajp.2007.07050776. PubMed PMID: 18245177. [DOI] [PubMed] [Google Scholar]
- 31. Chasson GS, Buhlmann U, Tolin DF, Rao SR, Reese HE, Rowley T, et al. Need for speed: evaluating slopes of OCD recovery in behavior therapy enhanced with D-cycloserine. Behav Res Ther. 2010; 48 7: 675- 79. DOI: 10.1016/j.brat.2010.03.007. PubMed PMID: 20362975. [DOI] [PubMed] [Google Scholar]
- 32. Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008; 63 6: 544- 9. DOI: 10.1016/j.biopsych.2007.11.011. PubMed PMID: 18179785. [DOI] [PubMed] [Google Scholar]
- 33. Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of D-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010; 67 4: 365- 70. DOI: 10.1016/j.biopsych.2009.07.036. PubMed PMID: 19811776. [DOI] [PubMed] [Google Scholar]
- 34. Siegmund A, Golfels F, Finck C, Halisch A, Räth D, Plag J, et al. D-cycloserine does not improve but might slightly speed up the outcome of in-vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. J Psychiatr Res. 2011; 45 8: 1042- 7. DOI: 10.1016/j.jpsychires.2011.01.020. [DOI] [PubMed] [Google Scholar]