Abstract
The complete mitochondrial genome (mitogenome) of Ugyops sp. (Hemiptera: Delphacidae) was sequenced, making it the first determined mitogenome from the subfamily Asiracinae, the basal clade of the family Delphacidae. The mitogenome was 15,259 bp in length with A + T content of 77.65% and contained 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs), two ribosomal RNA genes (rRNAs), and a control region. The gene order was identical with that of the ancestral insect. The nucleotide composition analysis indicated that the whole mitogenome was strongly A-skewed (0.288) and highly C-skewed (−0.270). For PCGs on the J-strand, the AT skew was positive, and the GC skew was negative. All PCGs started with canonical ATN codons, except for cox1 and nad5, which used CTG and GTG as start codon, respectively. All tRNAs could fold into typical cloverleaf secondary structures, with the exception of trnS1 (AGN), in which the dihydrouridine arm was reduced to a simple loop. The control region included a poly-T stretch downstream of the small rRNA gene (rrnS), a subregion of higher A + T content and tandemly repeated sequence near trnI. The mitogenome of Ugyops sp. could be very helpful in exploring the diversity and evolution of mitogenomes in Delphacidae.
Keywords: Ugyops sp, mitochondrial genome, gene arrangement, control region
The insect mitochondrial genome (mitogenome) generally encodes 37 genes, including 13 protein-coding genes (PCGs), 22 transfer RNA (tRNA) genes, and two ribosomal RNA (rRNA) genes (Boore 1999). These genes are typically arranged on a compact circular genome in the range of 15–18 kb (Cameron 2014a). In addition, there are some noncoding elements, with the largest one termed the control region regulating the transcription and replication of the mitogenome (Clayton 1982, 1992; Taanman 1999). The control region, alternatively called the A + T-rich region, is characterized by high A + T content and the occurrence of tandem repeat units (Zhang and Hewitt 1997).
The prevalent use of insect mitogenomes is phylogenetic analysis. Mitochondrial phylogenomics studies on the Hemiptera are extensive. The suborder Heteroptera has the largest number of published complete mitogenomes in Hemiptera (Song et al. 2016). Mitogenome sequencing is of much smaller scale within the suborder Auchenorrhyncha, especially within the infraorder Fulgoromorpha. Currently, only 11 complete mitogenomes have been sequenced in the superfamily Fulgoroidea (= Fulgoromorpha) (Hua et al. 2009; Song and Liang 2009; Song et al. 2010, 2012; Zhang et al. 2013, 2014, 2016a; Huang and Qin 2018a,b), including five species of Delphacidae: Changeondelphax velitchkovskyi, Laodelphax striatellus, Nilaparvata lugens, Peregrinus maidis, and Sogatella furcifera. Moreover, gene rearrangements are known for these species, with two clusters trnW-trnC-trnY and trnT-trnP-nad6 undertaking conversion to trnC-trnW-trnY and nad6-trnP-trnT, respectively (Zhang et al. 2013, 2014).
The family Delphacidae is the most diverse and cosmopolitan group of the superfamily Fulgoroidea, with approximately 2,100 described species, of which the vast majority (80%) belong to the most species-rich subfamily Delphacinae (Urban et al. 2010, Huang et al. 2017). Species of Delphacidae feed on the phloem tissues of host plants, and a variety of species are economically significant pests of many important crops, such as rice and maize. Delphacid feeding causes serious yield losses of crops directly, but they are also vectors of phytoplasma, or viral plant pathogens (Wilson 2005). Approximately 30 delphacid species transmit plant viruses (Wilson 2005, Hogenhout et al. 2008).
The Ugyops Guérin-Méneville is an Oriental delphacid genus with 101 known species and is placed in the tribe Ugyopini of the subfamily Asiracinae (Fennah 1979, Bourgoin 2018). Phylogenetic analysis has shown that Asiracinae is not monophyletic and Ugyopini represents the earliest lineage in Delphacidae (Asche 1985, 1990; Emeljanov 1996). Comprehensive phylogenetic reconstruction of Delphacidae, combining nucleotide sequence and morphological characters, also indicated that Ugyopini (represented by two species of the genus Ugyops) was one of the most basal groups (Urban et al. 2010). The number of complete or nearly complete mitogenomes is slightly increasing in Auchenorrhyncha. However, relatively little is known about the mitogenomes from the tribe Ugyopini or the subfamily Asiracinae. In the present study, the complete mitogenome of Ugyops sp. was sequenced. This is the first representative mitogenome reported in the subfamily Asiracinae. Nucleotide composition, gene order, and other features were compared between Ugyops sp. and five species from Delphacinae mentioned above. Results from this work will facilitate the reconstruction of higher level phylogenetic relationships within Delphacidae and Fulgoroidea based on mitogenomic data in the future.
Materials and Methods
DNA Extraction, Amplification, and Sequencing
Adults of Ugyops sp. were collected in Sabah, Malaysia (5.443107°N, 116.451572°E). Samples were preserved in 100% ethanol and kept at −70°C until DNA extractions were conducted. The sequenced sample was deposited as voucher specimen in the Institute of Zoology, Chinese Academy of Sciences, Beijing, China.
Total genomic DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocols. The mitochondrial genome of the Ugyops sp. was amplified using 11 pairs of primers (Supp Table 1 [online only]), which were modified from universal insect mitochondrial primers (Simon et al. 1994, Simon et al. 2006). All PCRs were performed in 50 μl reaction volumes using TaKaRa LA Taq (Takara Biomedical, Dalian, China). The PCR thermal program was as follows: initial denaturation of 2 min at 94°C, followed by 35 cycles of 94°C for 1 min, 48–50°C for 1 min, 68°C for 10 min, and a final extension for 20 min at 68°C. The PCR products were electrophoresed in 1.2% agarose gel and sequencing was performed using BigDye v3.1 on an ABI 3730XL DNA Analyzer (Applied Biosystems, Carlsbad, CA). When purified PCR products were difficult to sequence directly, they were inserted into a pMD 19-T Vector (Takara Biomedical, Dalian, China). Multiple clones were independently sequenced.
Annotation and Genomic Analysis
The secondary structures of all tRNA genes were predicted using MITOS Web Server (Bernt et al. 2013). PCGs were identified using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) under the invertebrate mitochondrial genetic code. For some PCGs, start and stop codons were corrected according to alignment of homologous genes in mitogenomes of Auchenorrhyncha. The beginning and end of the rrnL gene were presumed to extend to the boundaries of the adjacent tRNA genes trnL1 (CUN) and trnV. The 5′ end of rrnS gene was determined by aligning rrnS sequences of auchenorrhynchan mitogenomes, and the 3′ end was assumed to be delimited by the beginning of trnV. Secondary structures of two rRNAs (rrnL and rrnS) were inferred using models predicted for Drosophila spp. (Cannone et al. 2002), Apis mellifera (Gillespie et al. 2006), Stenopirates sp. (Li et al. 2012), Cervaphis quercus (Wang et al. 2014), Panaorus albomaculatus (Li et al. 2016), and Taharana fasciana (Wang et al. 2017). Helix names followed the conventions of Gillespie et al. (2006).
Nucleotide composition was calculated in Bioedit (Hall 1999). To measure the base-compositional difference, AT skew and GC skew were calculated using the formulae AT skew = (A − T)/(A + T) and GC skew = (G − C)/(G + C) (Perna and Kocher 1995). Codon usage and the relative synonymous codon usage (RSCU) were calculated with MEGA 6.0 (Tamura et al. 2013). The software DnaSP 5.0 (Librado and Rozas 2009) was used to calculate the number of synonymous substitutions per synonymous site (Ks), the number of nonsynonymous substitutions per nonsynonymous site (Ka), and the ratio of Ka/Ks for each PCG. The repeat motifs in the control region were detected using Tandem Repeats Finder (Benson 1999). Comparation of nucleotide composition, evolutionary rate, and noncoding region used the following five complete mitogenomes of Delphacidae from GenBank: C. velitchkovskyi (MG049916), L. striatellus (JX880068), N. lugens (NC_021748), P. maidis (MG049917), and S. furcifera (NC_021417).
Sequence Alignment and Phylogenetic Analyses
In total, 15 species were used for phylogenetic analyses, including eight species of Delphacidae and seven outgroup taxa (Table 1). Nucleotide sequence of each PCG was aligned individually based on alignment of translated amino acid sequence using Muscle (Edgar 2004) implemented in MEGA 6 (Tamura et al. 2013). All alignments were checked manually and then assembled into the concatenated data set. For the maximum likelihood (ML) and Bayesian inference (BI) analyses, the optimal partitioning schemes and best-fitting models (Supp Table 2 [online only]) were selected using PartitonFinder 2.1.1 (Lanfear et al. 2017) with the greedy algorithm under the corrected Akaike Information Criterion (AICc).
Table 1.
List of species used for phylogenetic analyses in this study
| Superfamily | Family | Species | Accession number | |
|---|---|---|---|---|
| Ingroup | Fulgoroidea | Delphacidae | Changeondelphax velitchkovskyi | MG049916 |
| Laodelphax striatellus | JX880068 | |||
| Nilaparvata bakeri | NC_033388 | |||
| Nilaparvata lugens | NC_021748 | |||
| Nilaparvata muiri | NC_024627 | |||
| Peregrinus maidis | MG049917 | |||
| Sogatella furcifera | NC_021417 | |||
| Ugyops sp. | MH352481 | |||
| Outgroup | Fulgoroidea | Cixiidae | Pentastiridius sp. | KY039133 |
| Fulgoridae | Lycorma delicatula | NC_012835 | ||
| Issidae | Sivaloka damnosus | NC_014286 | ||
| Ricaniidae | Ricania speculum | NC_031369 | ||
| Cercopoidea | Aphrophoridae | Philaenus spumarius | NC_005944 | |
| Cercopidae | Abidama producta | NC_015799 | ||
| Callitettix braconoides | NC_025497 |
An ML tree was estimated using the IQ-TREE (Nguyen et al. 2015) Web Server in W-IQ-TREE (Trifinopoulos et al. 2016, http://iqtree.cibiv.univie.ac.at/) with 1,000 replicates of ultrafast likelihood bootstrap (Minh et al. 2013). Bayesian trees were inferred using MrBayes V3.2.6 (Ronquist et al. 2012). Two Markov chain Monte Carlo (MCMC) runs were employed for 4,000,000 generations and trees were sampled every 500 generations. The 50% majority consensus tree was computed after excluding the first 25% of samples as burn-in.
Results and Discussion
Genome Organization
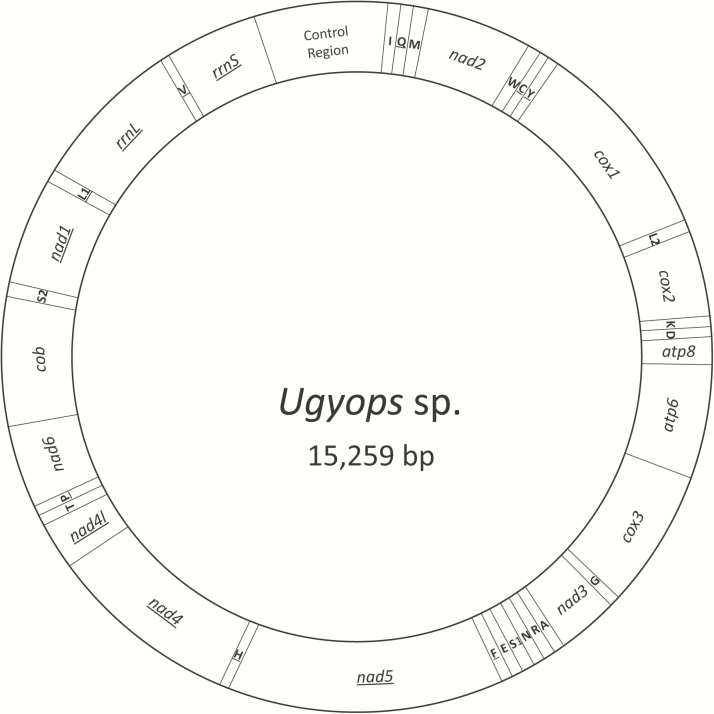
The mitochondrial genome of Ugyops sp. was 15,259 bp in length (GenBank MH352481), which is the smallest completely sequenced mitogenome in Fulgoroidea at present. The mitogenome contains 37 genes (13 PCGs, 22 tRNA genes, and two rRNA genes) and a control region, as found in most insects (Boore 1999) (Table 2).
Table 2.
Mitochondrial genome organization of Ugyops sp.
| Gene | Strand | Position | Size (bp) | Anticodon | Start codon | Stop codon | Intergenic nucleotides (bp) |
|---|---|---|---|---|---|---|---|
| trnI | J | 1–64 | 64 | GAT | – | – | – |
| trnQ | N | 65–131 | 67 | TTG | – | – | 0 |
| trnM | J | 130–193 | 64 | CAT | – | – | −2 |
| nad2 | J | 194–1153 | 960 | – | ATT | TAA | 0 |
| trnW | J | 1157–1219 | 63 | TCA | – | – | 3 |
| trnC | N | 1212–1272 | 61 | GCA | – | – | −8 |
| trnY | N | 1274–1334 | 61 | GTA | – | – | 1 |
| cox1 | J | 1333–2866 | 1,534 | – | CTG | T- | −2 |
| trnL2 (UUR) | J | 2867–2929 | 63 | TAA | – | – | −5 |
| cox2 | J | 2930–3601 | 672 | – | ATA | TAA | 0 |
| trnK | J | 3603–3674 | 72 | CTT | – | – | 1 |
| trnD | J | 3675–3736 | 62 | GTC | – | – | 0 |
| atp8 | J | 3737–3844 | 108 | – | ATA | TAA | 0 |
| atp6 | J | 3841–4492 | 652 | – | ATA | T- | −4 |
| cox3 | J | 4493–5273 | 781 | – | ATG | T- | 0 |
| trnG | J | 5274–5333 | 60 | TCC | – | – | 0 |
| nad3 | J | 5334–5684 | 351 | – | ATT | TAG | 0 |
| trnA | J | 5683–5743 | 61 | TGC | – | – | −2 |
| trnR | J | 5750–5809 | 60 | TCG | – | – | 6 |
| trnN | J | 5808–5871 | 64 | GTT | – | – | −2 |
| trnS1 (AGN) | J | 5871–5931 | 61 | GCT | – | – | −1 |
| trnE | J | 5931–5996 | 66 | TTC | – | – | −1 |
| trnF | N | 5995–6056 | 62 | GAA | – | – | −2 |
| nad5 | N | 6059–7739 | 1,681 | – | GTG | T- | 2 |
| trnH | N | 7740–7803 | 64 | GTG | – | – | 0 |
| nad4 | N | 7804–9121 | 1,318 | – | ATG | T- | 0 |
| nad4l | N | 9115–9387 | 273 | – | ATG | TAA | −7 |
| trnT | J | 9389–9451 | 63 | TGT | – | – | 1 |
| trnP | N | 9451–9514 | 64 | TGG | – | – | −1 |
| nad6 | J | 9516–10008 | 492 | – | ATT | T- | 1 |
| cytb | J | 10009–11130 | 1,122 | – | ATG | TAA | 0 |
| trnS2 (UCN) | J | 11130–11191 | 62 | TGA | – | – | −1 |
| nad1 | N | 11208–12123 | 916 | – | ATG | T- | 16 |
| trnL1 (CUN) | N | 12125–12186 | 62 | TAG | – | – | 1 |
| rrnL | N | 12187–13392 | 1,206 | – | – | – | 0 |
| trnV | N | 13393–13461 | 69 | TAC | – | – | 0 |
| rrnS | N | 13462–14228 | 767 | – | – | – | 0 |
| Control region | – | 14229–15259 | 1,031 | – | – | – | 0 |
The gene order of the Ugyops sp. mitogenome (Fig. 1) was identical to that of Drosophila yakuba, in which gene arrangement has been considered to be the ancestral gene order of insects (Clary and Wolstenholme 1985, Boore 1999). In Hemiptera, most species maintain the ancestral mitogenome arrangement of insects (Song et al. 2012, Cui et al. 2013, Wang et al. 2013, Liu et al. 2014, Li et al. 2016). Gene rearrangement, however, has been found in Aleyrodidae (Sternorrhyncha) (Thao et al. 2004), Cicadellidae (Auchenorrhyncha) (Du et al. 2017), Delphacidae (Auchenorrhyncha), and five families of true bugs (Heteroptera) (Hua et al. 2008, Li et al. 2012, Jiang et al. 2016, Song et al. 2016). Mitochondrial gene order changes, as one type of genomic changes, provide complementary markers with considerable potential for molecular systematics (Rokas and Holland 2000). In most insect orders, the synapomorphic rearrangements occur at many different taxonomic levels (Cameron 2014a). The rearrangement was observed in species of the derived subfamily Delphacinae, and the gene order remained unknown in other subfamilies such as Vizcayinae, Plesiodelphacinae, Kelisiinae, and Stenocraninae. Consequently, to explicate the origin and evolution of gene rearrangement, more delphacid mitogenomes are needed, particularly species from non-Delphacinae.
Fig. 1.
Structure of the mitochondrial genome of Ugyops sp.
Nucleotide Composition
Results of comparative nucleotide composition of six delphacid species are listed in Table 3. The A + T content of Ugyops sp. mitogenome was 77.65%, and the nucleotide composition of the whole mitogenome was strongly A-skewed (0.288) and highly C-skewed (−0.270). Comparatively, a slightly A-skewed pattern was observed in five species of Delphacinae (Table 3).
Table 3.
Nucleotide composition of mitochondrial genomes in six species of Delphacidae
| Species | A + T content (%) | AT skew | GC skew | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ugyops | S. f | P. m | N. l | L. s | C. v | Ugyops | S. f | P. m | N. l | L. s | C. v | Ugyops | S. f | P. m | N. l | L. s | C. v | |
| Whole genome | 77.65 | 76.19 | 77.75 | 76.95 | 77.17 | 75.72 | 0.288 | 0.093 | 0.108 | 0.091 | 0.119 | 0.130 | −0.270 | −0.141 | −0.244 | −0.183 | −0.184 | −0.272 |
| All PCGs | 76.41 | 74.44 | 75.74 | 76.01 | 75.74 | 74.48 | −0.102 | −0.170 | −0.151 | −0.156 | −0.144 | −0.151 | −0.064 | −0.068 | −0.081 | −0.073 | −0.092 | −0.101 |
| J-strand PCGs | 74.83 | 72.27 | 73.98 | 74.14 | 73.52 | 72.55 | 0.189 | −0.044 | −0.012 | −0.031 | 0.0002 | 0.006 | −0.271 | −0.170 | −0.256 | −0.231 | −0.244 | −0.299 |
| N-strand PCGs | 78.93 | 77.89 | 78.54 | 78.99 | 79.30 | 77.57 | −0.541 | −0.355 | −0.359 | −0.344 | −0.359 | −0.385 | 0.328 | 0.136 | 0.255 | 0.237 | 0.218 | 0.284 |
| First codon | 73.15 | 71.52 | 73.47 | 73.56 | 72.82 | 73.02 | −0.008 | −0.032 | −0.052 | −0.014 | −0.022 | −0.031 | 0.166 | 0.139 | 0.131 | 0.099 | 0.118 | 0.112 |
| Second codon | 68.36 | 68.94 | 69.59 | 69.87 | 69.50 | 69.56 | −0.401 | −0.407 | −0.405 | −0.399 | −0.386 | −0.398 | −0.174 | −0.150 | −0.156 | −0.121 | −0.151 | −0.140 |
| Third codon | 87.74 | 82.86 | 84.17 | 84.59 | 84.92 | 80.84 | 0.053 | −0.092 | −0.027 | −0.079 | −0.052 | −0.047 | −0.283 | −0.262 | −0.292 | −0.273 | −0.354 | −0.339 |
| rRNAs | 78.50 | 76.46 | 78.45 | 77.83 | 77.83 | 76.74 | −0.274 | −0.082 | −0.094 | −0.076 | −0.076 | −0.105 | 0.302 | 0.267 | 0.308 | 0.298 | 0.318 | 0.335 |
| Control region | 88.86 | 82.50 | 86.15 | 79.29 | 83.20 | 80.12 | 0.055 | 0.004 | −0.025 | −0.007 | 0.028 | −0.006 | −0.096 | 0.105 | −0.231 | 0.169 | 0.294 | −0.130 |
Ugyops sp. (Ugyops), Sogatella furcifera (S. f), Peregrinus maidis (P. m), Nilaparvata lugens (N. l), Laodelphax striatellus (L. s), and Changeondelphax velitchkovskyi (C. v).
Mitochondrial genomes usually show specific-strand bias in nucleotide composition, due to asymmetrical mutation pressure (Hassanin et al. 2005). In all compared species, the gene set on the J-strand was C-skewed and that on the N-strand was G-skewed. The comparison between AT bias on both strands indicated that the minority gene set was strongly T-skewed in each species, but the AT skew of majority gene set was different among the six compared species. In Ugyops sp., the gene set on the J-strand was moderately A-skewed (0.188). The AT skew was approximately zero in L. striatellus and C. velitchkovskyi, lacking significant A or T bias (Table 3), while the set of PCGs on the J-strand was subtly T-skewed in the remaining species.
For each codon of all PCGs, the second codon had lower AT content than the first and third codons in the six examined species. The first and second codons were T-skewed (Table 3). The value of AT skew at third codon position was positive in Ugyops sp. (0.053), whereas those were negative in other five delphacids.
Protein-Coding Genes
The mitogenome of Ugyops sp. contained 13 PCGs typical to animal mitochondrial genomes. The canonical start codons ATN (Met/Ile) were assigned to 11 of all PCGs. Three genes (atp8, atp6, and cox2) started with ATA, three genes (nad2, nad3, and nad6) with ATT, and five genes (cox3, cytb, nad1, nad4, and nad4l) with ATG. The exceptions were cox1 and nad5, which used the noncanonical start codon CTG and GTG, respectively. In Hemiptera, employing GTG as start codon of nad5 was also found in the white-backed planthopper S. furcifera (Zhang et al. 2014) and the kissing bug Triatoma dimidiata (Dotson and Beard 2001). Furthermore, GTG was used as start codon of nad5 across a range of insect taxa, such as in some species of Diptera (Zhang et al. 2016b), Mecoptera (Beckenbach 2011), and Plecoptera (Stewart and Beckenbach 2006). Seven genes (atp6, cox1, cox3, nad1, nad4, nad5, and nad6) ended with incomplete stop codons T, which are presumably completed by polyadenylation after transcription (Ojala et al. 1981). The remaining genes had the complete termination codons TAA (atp8, cox2, cytb, nad2, nad4l, and nad6), except for nad3, in which TAG was used.
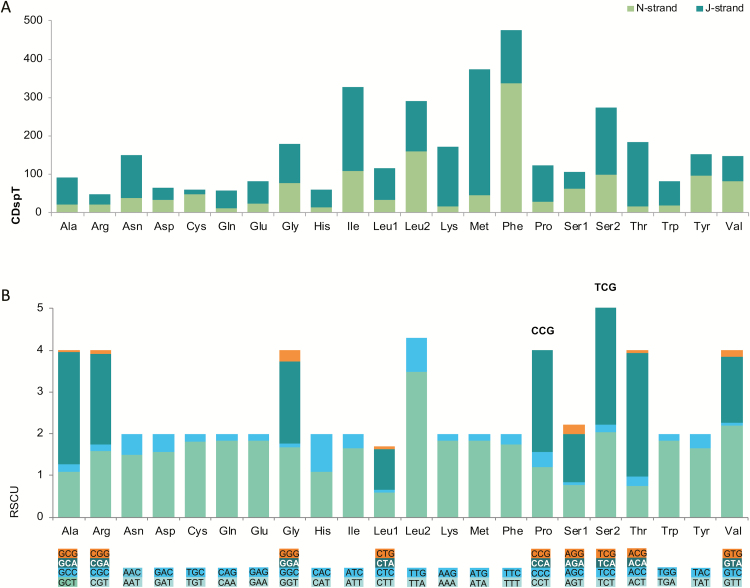
The total number of codons was 3,612, excluding stop codons. Approximately equivalent codon numbers were detected in S. furcifera (3,606), C. velitchkovskyi (3,607), P. maidis (3,607), N. lugens (3,608), and L. striatellus (3,613). The three most abundant codon families were Phe, Met, and Ile (Fig. 2A), all of which were twofold degenerate in codon usage and rich in A and T. When codons were calculated on the majority and minority strands separately, the most frequently used codon families were Met and Phe, respectively. The RSCU also reflected nucleotide compositional bias. Generally, codons with A or T in the third codon position were greatly preferred within each synonymous codon family, compared to codons with G or C in the third position. Both CCG (Pro) and UCG (Ser2 (UCN)) were lost in Ugyops sp. (Fig. 2B).
Fig. 2.
Codon distribution (A) and RSCU in the Ugyops sp. mitogenome (B). Codon Families are provided on the x-axis. CDspT, codons per thousand codons. Absent codons are marked at the top of bars.
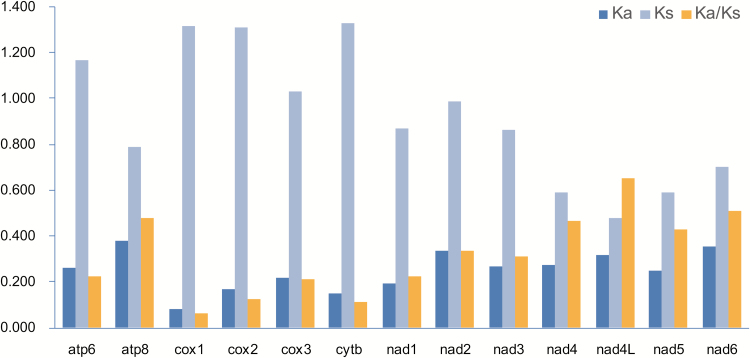
The average ratio of Ka/Ks was calculated to evaluate the evolutionary rate of each PCG in the six delphacid mitogenomes. Among the 13 PCGs, nad4l had the highest rate (Fig. 3), followed by nad6 which located in the rearranged gene cluster trnT-trnP-nad6. Three lowest genes were cox1, cytb, and cox2, respectively (Ka/Ks < 0.2). For each PCG, the ratio of Ka/Ks was less than 1, indicating the probable purifying selection in evolution of these genes. Furthermore, a negative correlation was detected between the Ka/Ks ratio and the G + C content of each PCG (R2 = 0.867, P < 0.01).
Fig. 3.
Evolutionary rates of 13 protein-coding genes in the mitogenomes of six delphacid species. The rate of nonsynonymous substitutions (Ka), the rate of synonymous substitutions (Ks), and the ratio of the rate of nonsynonymous substitutions to the rate of synonymous substitutions (Ka/Ks) are calculated for each PCG.
tRNAs and rRNAs
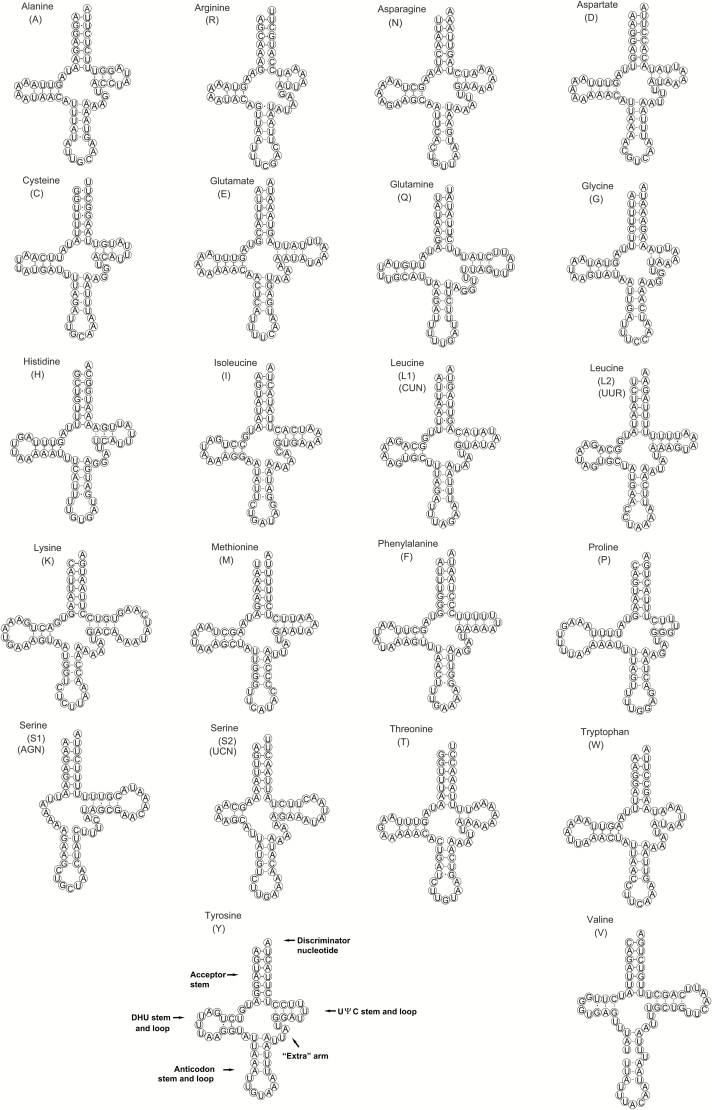
The length of all 22 tRNA genes ranged from 60 to 72 bp. The predicted secondary structures were typical cloverleaf except for trnS1 (AGN) (Fig. 4), in which the dihydrouridine (DHU) stem was replaced by a 6-bp simple loop. Similarly, trnS1 lacks the DHU arm in most other metazoans (Cameron 2014a). In Ugyops sp., the anticodon stem of trnV was longer than conservative length (5 bp), forming a 6-bp stem with an unpaired nucleotide. This type of oversized anticodon stem was also observed in trnS1 (AGN) of other hemipteran insects, including the aphid Cavariella salicicola (Wang et al. 2013) and some species of true bugs (Li et al. 2012, 2013, 2016; Yuan et al. 2015).
Fig. 4.
Predicted secondary structures for the 22 tRNAs of the Ugyops sp. mitogenome. Watson–Crick pairs are indicated by lines, and wobble GU pairs are indicated by dots.
In total, 28 G–U wobble pairs were present in 10 acceptor stems, seven DHU stems, nine anticodon stems, and two TΨC stems of the tRNA secondary structures (Fig. 4). In addition, four mismatched pairs (5 A–A, 3 A–C, 2 A–G, and 10 U–U) were detected in the acceptor stem, the DHU stem, and the anticodon stem. Wobble and mismatched pairs, which are common in insect tRNAs, are usually corrected via editing processes (Lavrov et al. 2000).
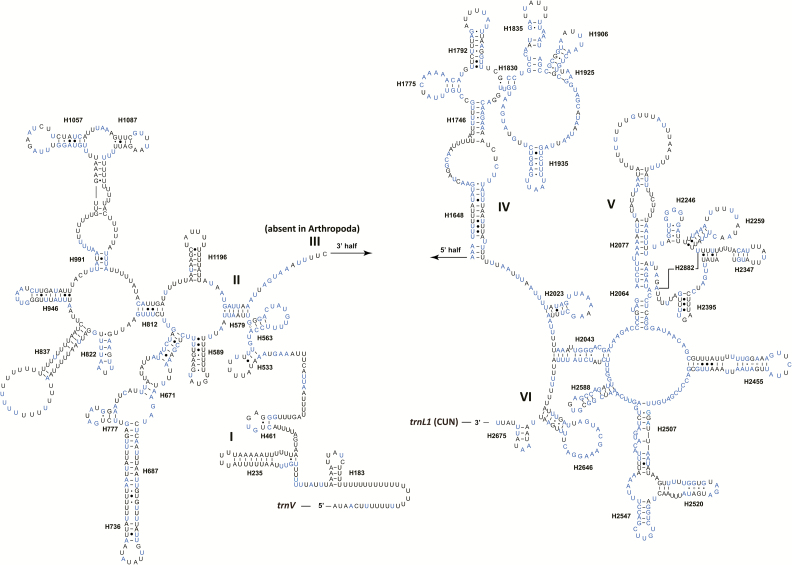
The rrnL gene was 1,206 bp in size with an A + T content of 80.76%, while the rrnS gene is 767 bp long, with a little lower A + T content (74.93%). The secondary structure of rrnL of Ugyops sp. contained six structural domains (domain III is absent in arthropods) and 44 helices (Fig. 5). Helix H2735 at the 3′ end was not present, which was also absent in the leafhopper T. fasciana (Wang et al. 2017). Domains IV and V were more conserved than others according to sequence alignment of the six compared delphacids. Four helices (H1775, H1830, H1935, and H2574) were most conserved with no more than one nucleotide substitution among the compared delphacid species. Some helices (H183, H235, H837, H991, and H2077) were highly variable in sequence and secondary structure.
Fig. 5.
Predicted secondary structure for the rrnL in the mitogenome of Ugyops sp. Base pairing is illustrated as follows: Watson–Crick pairs by lines; wobble GU pairs by dots; AG pairs by circles; other noncanonical pairs by solid circles. The 100% identical nucleotides in the six compared species of Delphacidae are marked in blue.
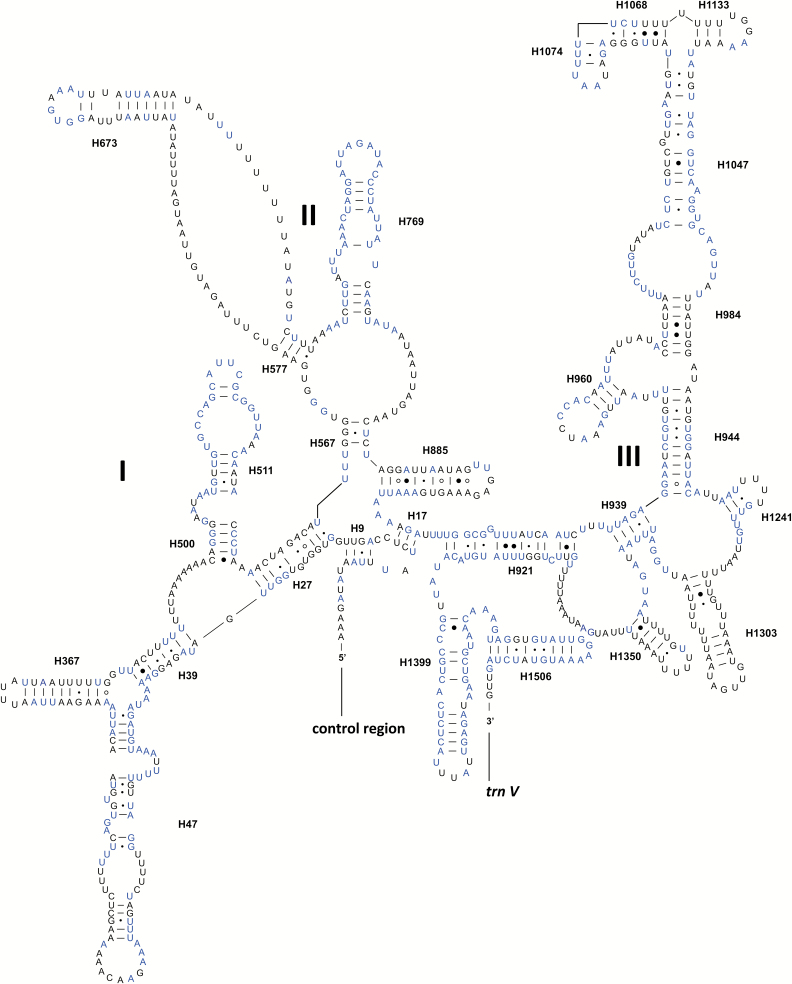
The secondary structure of rrnS consisted of three domains and 27 helices (Fig. 6). Domain I and II were less conserved than domain III. Two helices H511 and H769 were most conserved among the compared species of Delphacidae. In domain III, different possible secondary structures could be predicted from the region including H1047, H1068, H1074, and H1113, because of several noncanonical base pairs (Gillespie et al. 2006, Cameron and Whiting 2008). The helix H1068 has been absent in some hemipteran species (e.g., Wang et al. 2013, 2017; Yuan et al. 2015), while this helix was identified in Ugyops sp.
Fig. 6.
Predicted secondary structure for the rrnS in the mitogenome of Ugyops sp. Base pairing is illustrated as follows: Watson–Crick pairs by lines; wobble GU pairs by dots; AG pairs by circles; other noncanonical pairs by solid circles. The 100% identical nucleotides in the six compared species of Delphacidae are marked in blue.
Overlapping Sequences and Noncoding Regions
There were 12 overlaps (33 bp) found in the Ugyops sp. mitogenome (Table 2), and the longest one (8 bp) occurred between trnW and trnC, which oriented on different strands. In many insects, nad4l-nad4 and atp8-atp6 always overlap by 7 bp (ATGNTAA) in different reading frames (Stewart and Beckenbach 2005). The nad4l-nad4 overlap was almost identical in the six delphacid species, but the atp8-atp6 overlap was different in size (Fig. 7). In Ugyops sp., P. maidis and N. lugens, a 4-bp overlap (ATAA) was observed between atp8 and atp6, whereas the atp8-atp6 overlap (ATRTTAA) was 7 bp in other three species.
Fig. 7.
Sequence alignments of atp8/atp6 and nad4/nad4l in six species of Delphacidae.
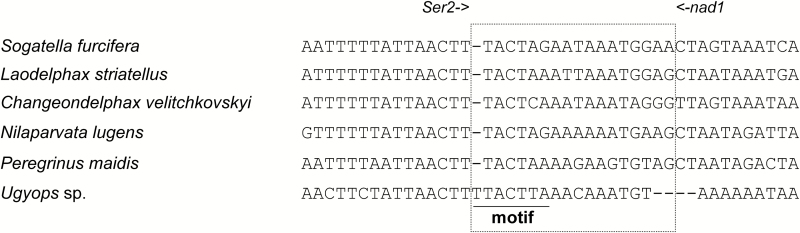
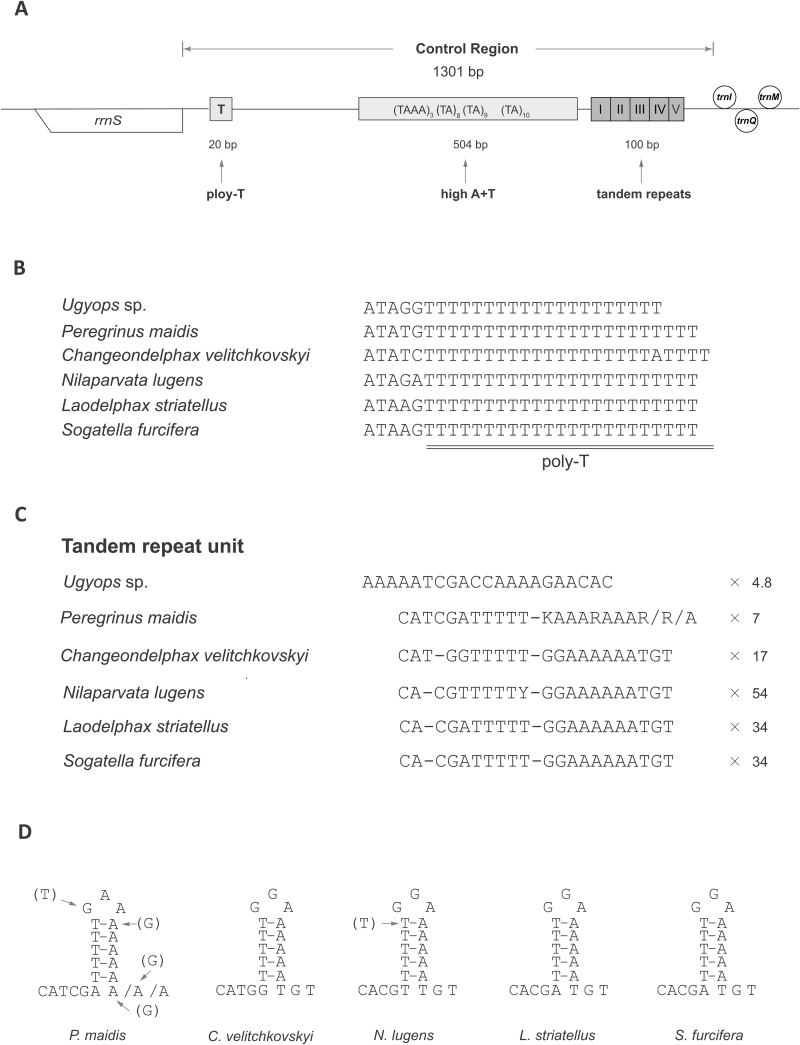
In total, 10 noncoding regions were spread throughout the mitogenome of Ugyops sp., including nine intergenic spacers (1–16 bp), and the control region (Table 2). The intergenic spacer between trnS2 (UCN) and nad1 is common to many insects (Cameron and Whiting 2008), and it corresponds to the binding site of a transcription termination peptide (Roberti et al. 2003) and has a highly conserved 7-bp motif that is conserved across insects (Cameron 2014b). In Ugyops sp., this spacer was 16 bp in length, while it was 17 bp long in other five species. The corresponding motif was TTACTTA in Ugyops sp., and TACTMR in other examined species of the subfamily Delphacinae (Fig. 8). The control region was the largest noncoding region in the mitogenome of Ugyops sp. and spanned 1,031 bp, located between rrnS and trnI. The A + T content (88.85%) of this region was higher compared with that of the whole mitogenome (77.65%). Three parts were recognized in the control region of Ugyops sp. as given in Fig. 9A: a 20-bp poly-thymidine (poly-T) stretch downstream of rrnS, a subregion of higher A + T content, and a tandem repeat sequence. The higher A + T content subregion (504 bp) was heavily biased toward A + T (94.05%) and included four microsatellite-like elements (TAAA)3, (TA)8, (TA)9, and (TA)10.
Fig. 8.
Alignments of the intergenic spacer between nad1 and trnS2 (UCR) in six species of Delphacidae.
Fig. 9.
Control region of Ugyops sp. mitogenome, and the comparison of two elements in control regions of six delphacid species. (A) Map of the control region in Ugyops sp. (B) The poly-T stretch in six species of Delphacidae. (C) Sequences of tandem repeat unit in the six examined species of Delphacidae. (D) Predicted secondary structures of tandem repeat unit.
We compared the poly-T stretches and repeat sequences among the six delphacids. In the five species of Delphacinae, the poly-T stretch was 23 bp in length, longer than that found in Ugyops sp. (Fig. 9B). Despite length variations, the poly-T stretch seemed to be conserved in Delphacidae.
Tandem repetition has been frequently found in the control regions of insect mitogenomes (Zhang and Hewitt 1997). It has been proposed that the occurrence and persistence of tandem repeat units results from slipped-strand mispairing during mitochondrial DNA replication (Moritz et al. 1987, Macey et al. 1998). Tandem repeat sequences were detected in mitogenomes from all suborders of Hemiptera (Li and Liang 2018). In the six examined species of Delphacidae, repeat units occurred multiple times (Fig. 9C). A 21-bp consensus motif (AAAAATCGACCAAAAGAACAC) repeated 4.8 times in the control region of Ugyops sp., four complete units and a partial copy (16 bp) near trnI. The size of repeat unit varied in P. maidis, ranging from 20 to 22 bp (Fig. 9C). The repeat units of the remaining species were similar in both sequence and second structure (Fig. 9D). Particularly, the repeat unit of S. furcifera was identical to that of L. striatellus (Zhang et al. 2014). It was presumed that the subfamily Delphacinae has undergone a substantial radiation associated with host plant divergence (Urban et al. 2010, Huang et al. 2017), to which the similarity of repeat units might be related in the five species of Delphacinae. The sequence homology between Ugyops sp. and five Delphacinae species seemed limited (Fig. 9B), which might imply that evolution of control region in Delphacidae is very complicated. Further investigations of additional delphacid species from different groups would likely to provide useful information for understanding the way repeat units evolve in control region.
Phylogenetic Analyses
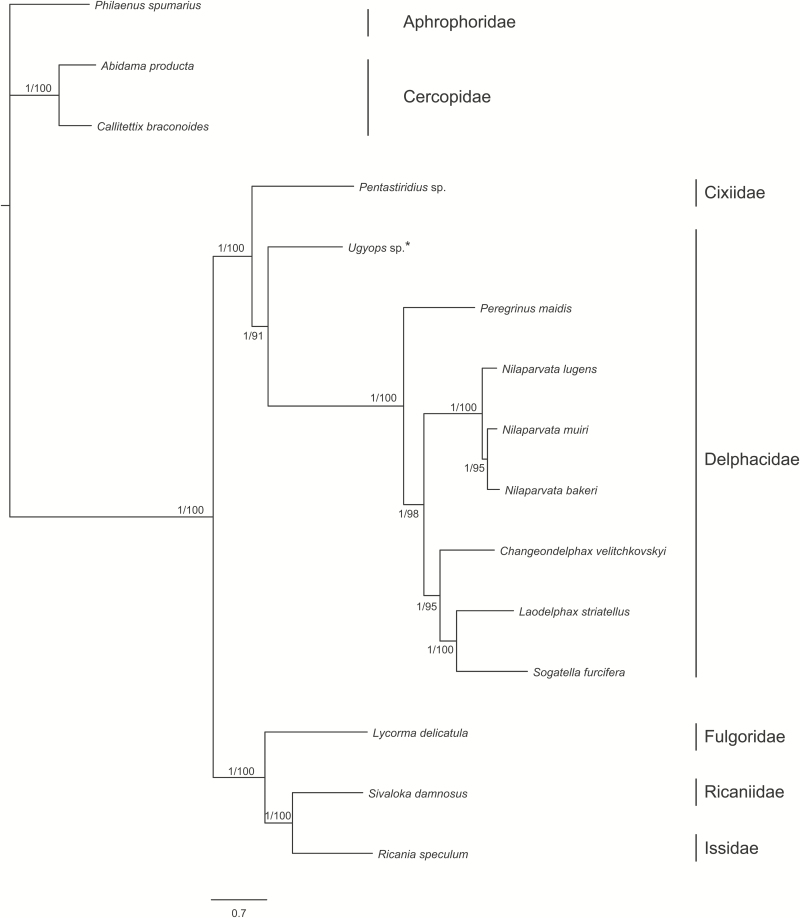
The topology of ML tree was consistent with that of BI tree. In both analyses (Fig. 10), Delphacidae was monophyletic (bootstrap = 100, posterior probability = 1.00) and sister group to Cixiidae (represented by Pentastiridius sp.). In Delphacidae, two clades were strongly supported (bootstrap = 91, posterior probability = 1.00), the Ugyops sp. clade and the Delphacinae clade (Fig. 10). In the Delphacinae clade, C. velitchkovskyi, L. striatellus, S. furcifera, and N. lugens clustered together, indicating their relatively close relationships, which was likely supported by their similar tandem repeat unit in control regions.
Fig. 10.
Phylogenetic tree inferred from ML and BI using the data set of 13 PCGs. Nodal supports are indicated above or below the branches.
Although the findings of the current study improved our understanding of the mitogenomics of the basal group Asiracinae, the other subfamilies aside from Delphacinae remain poorly known. Additional taxonomic sampling will be needed to explore the diversity of their mitochondrial genomes and provide more complete insights into the evolution of Delphacidae.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
We wish to thank Zhi Shun Song for collecting specimens and Qi Qi Wang, Institute of Zoology, Chinese Academy of Sciences, Beijing, China, for giving advice on prediction of rRNA secondary structures. We are grateful to Michael C. Orr, Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese Academy of Sciences, Beijing, China, for offering suggestions on writing. The work on which this paper is based was supported by the Cooperative Research Projects between the Chinese mainland and Taiwan in Biodiversity jointly supported by the National Natural Science Foundation of China and the K.T. Li Foundation for the Development of Science and Technology (grant 31561163003) and the National Natural Science Foundation of China (grant 31572298).
References Cited
- Asche M. 1985. Zur Phylogenie der Delphacidae Leach, 1815 (Homoptera: Cicadina: Fulgoromorpha). Marburger Entomol. Publ. 2: 1–910. [Google Scholar]
- Asche M. 1990. Vizcayinae, a new subfamily of Delphacidae with revision of Vizcaya Muir (Homoptera: Fulgoroidea) - a significant phylogenetic link. Bishop Mus. Occas. Pap. 30: 154–187. [Google Scholar]
- Beckenbach A. T. 2011. Mitochondrial genome sequences of representatives of three families of scorpionflies (Order Mecoptera) and evolution in a major duplication of coding sequence. Genome. 54: 368–376. [DOI] [PubMed] [Google Scholar]
- Benson G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt M., A. Donath F. Jühling F. Externbrink C. Florentz G. Fritzsch J. Pütz M. Middendorf, and Stadler P. F.. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 69: 313–319. [DOI] [PubMed] [Google Scholar]
- Boore J. L. 1999. Animal mitochondrial genomes. Nucleic Acids Res. 27: 1767–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgoin T. 2018. FLOW (Fulgoromorpha Lists on The Web): a world knowledge base dedicated to Fulgoromorpha. Version 8, updated (2018-05-02). http://hemiptera-databases.org/flow/ (accessed on 8 February 2018).
- Cameron S. L. 2014a. Insect mitochondrial genomics: implications for evolution and phylogeny. Annu. Rev. Entomol. 59: 95–117. [DOI] [PubMed] [Google Scholar]
- Cameron S. L. 2014b. How to sequence and annotate insect mitochondrial genomes for systematic and comparative genomics research. Syst. Entomol. 39: 400–411. [Google Scholar]
- Cameron S. L. and Whiting M. F.. 2008. The complete mitochondrial genome of the tobacco hornworm, Manduca sexta, (Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene. 408: 112–123. [DOI] [PubMed] [Google Scholar]
- Cannone J. J., S., Subramanian M. N., Schnare J. R., Collett L. M., D’Souza Y., Du B., Feng N., Lin L. V., Madabusi K. M., Müller et al. 2002. The comparative RNA web (CRW) site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics. 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D. O. and Wolstenholme D. R.. 1985. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J. Mol. Evol. 22: 252–271. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. 1982. Replication of animal mitochondrial DNA. Cell. 28: 693–705. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. 1992. Transcription and replication of animal mitochondrial DNAs. Int. Rev. Cytol. 141: 217–232. [DOI] [PubMed] [Google Scholar]
- Cui Y., Xie Q., Hua J. M., Dang K., Zhou J. F., Liu X. G., Wang G., Yu X., and Bu W. J.. 2013. Phylogenomics of Hemiptera (Insecta: Paraneoptera) based on mitochondrial genomes. Syst. Entomol. 38: 233–245. [Google Scholar]
- Dotson E. M. and Beard C. B.. 2001. Sequence and organization of the mitochondrial genome of the Chagas disease vector, Triatoma dimidiata. Insect Mol. Biol. 10: 205–215. [DOI] [PubMed] [Google Scholar]
- Du Y., C. Zhang C. H. Dietrich Y. Zhang, and Dai W.. 2017. Characterization of the complete mitochondrial genomes of Maiestas dorsalis and Japananus hyalinus (Hemiptera: Cicadellidae) and comparison with other Membracoidea. Sci. Rep. 7: 14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeljanov A. F. 1996. On the question of the classification and phylogeny of the Delphacidae (Homoptera, Cicadina), with reference to larval characters. Entomol. Rev. 75: 134–150. [Google Scholar]
- Fennah R. G. 1979. Tribal classification of Asiracine Delphacidae (Homoptera: Fulgoroidea). Entomol. Rec. I/V/79: 116. [Google Scholar]
- Gillespie J. J., J. S. Johnston J. J. Cannone, and Gutell R. R.. 2006. Characteristics of the nuclear (18S, 5.8S, 28S and 5S) and mitochondrial (12S and 16S) rRNA genes of Apis mellifera (Insecta: Hymenoptera): structure, organization, and retrotransposable elements. Insect Mol. Biol. 15: 657–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41: 95. [Google Scholar]
- Hassanin A., N. Léger, and Deutsch J.. 2005. Evidence for multiple reversals of asymmetric mutational constraints during the evolution of the mitochondrial genome of metazoa, and consequences for phylogenetic inferences. Syst. Biol. 54: 277–298. [DOI] [PubMed] [Google Scholar]
- Hogenhout S. A., E. D. Ammar A. E. Whitfield, and Redinbaugh M. G.. 2008. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46: 327–359. [DOI] [PubMed] [Google Scholar]
- Hua J., M. Li P. Dong Y. Cui Q. Xie, and Bu W.. 2008. Comparative and phylogenomic studies on the mitochondrial genomes of Pentatomomorpha (Insecta: Hemiptera: Heteroptera). BMC Genomics. 9: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J., M. Li P. Dong Y. Cui Q. Xie, and Bu W.. 2009. Phylogenetic analysis of the true water bugs (Insecta: Hemiptera: Heteroptera: Nepomorpha): evidence from mitochondrial genomes. BMC Evol. Biol. 9: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. X., and Qin D. Z.. 2018a. Sequencing and analysis of the complete mitochondrial genome of Changeondelphax velitchkovskyi (Hemiptera: Fulgoroidea). Mitochondrial DNA B Resour. 3: 90–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. X., and Qin D. Z.. 2018b. The complete mitochondrial genome sequence of the corn planthopper, Peregrinus maidis (Hemiptera: Fulgoroidea). Mitochondrial DNA B Resour. 2: 783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. X., L. F. Zheng C. R. Bartlett, and Qin D. Z.. 2017. Resolving phylogenetic relationships of Delphacini and Tropidocephalini (Hemiptera: Delphacidae: Delphacinae) as inferred from four genetic loci. Sci. Rep. 7: 3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Li H., Song F., Cai Y., Wang J. Y., Liu J. P., and Cai W. Z.. 2016. Duplication and remolding of tRNA genes in the mitochondrial genome of Reduvius tenebrosus (Hemiptera: Reduviidae). Int. J. Mol. Sci. 17: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R., P. B. Frandsen A. M. Wright T. Senfeld, and Calcott B.. 2017. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34: 772–773. [DOI] [PubMed] [Google Scholar]
- Lavrov D. V., W. M. Brown, and Boore J. L.. 2000. A novel type of RNA editing occurs in the mitochondrial tRNAs of the centipede Lithobius forficatus. Proc. Natl. Acad. Sci. USA. 97: 13738–13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., and Liang A. P.. 2018. Hemiptera mitochondrial control region: new sights into the structural organization, phylogenetic utility and roles of tandem repetitions of the noncoding segment. Int. J. Mol. Sci. 19: 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., H. Liu A. Shi P. Stys X. Zhou, and Cai W.. 2012. The complete mitochondrial genome and novel gene arrangement of the unique-headed bug Stenopirates sp. (Hemiptera: Enicocephalidae). PLoS One. 7: e29419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., C. Gao Y. Cui Q. Xie, and Bu W.. 2013. The complete mitochondrial genome of the stalk-eyed bug Chauliops fallax Scott, and the monophyly of Malcidae (Hemiptera: Heteroptera). PLoS One. 8: e55381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., J. Yang Y. Li Y. Cui Q. Xie W. Bu, and Hillis D. M.. 2016. A mitochondrial genome of Rhyparochromidae (Hemiptera: Heteroptera) and a comparative analysis of related mitochondrial genomes. Sci. Rep. 6: 35175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P. and Rozas J.. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- Liu J., C. Bu B. Wipfler, and Liang A.. 2014. Comparative analysis of the mitochondrial genomes of Callitettixini Spittlebugs (Hemiptera: Cercopidae) confirms the overall high evolutionary speed of the AT-rich region but reveals the presence of short conservative elements at the tribal level. PLoS One. 9: e109140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey J. R., J. A. Schulte A. 2nd Larson, and Papenfuss T. J.. 1998. Tandem duplication via light-strand synthesis may provide a precursor for mitochondrial genomic rearrangement. Mol. Biol. Evol. 15: 71–75. [DOI] [PubMed] [Google Scholar]
- Minh B. Q., M. A. Nguyen, and von Haeseler A.. 2013. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 30: 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz C., Dowling T. E., and Brown W. M.. 1987. Evolution of animal mitochondrial DNA: relevance for population biology and systematics. Annu. Rev. Ecol. Syst. 18: 269–292. [Google Scholar]
- Nguyen L. T., H. A. Schmidt A. von Haeseler, and Minh B. Q.. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala D., J. Montoya, and Attardi G.. 1981. tRNA punctuation model of RNA processing in human mitochondria. Nature. 290: 470–474. [DOI] [PubMed] [Google Scholar]
- Perna N. T. and Kocher T. D.. 1995. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 41: 353–358. [DOI] [PubMed] [Google Scholar]
- Roberti M., P. L. Polosa F. Bruni C. Musicco M. N. Gadaleta, and Cantatore P.. 2003. DmTTF, a novel mitochondrial transcription termination factor that recognises two sequences of Drosophila melanogaster mitochondrial DNA. Nucleic Acids Res. 31: 1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokas A. and Holland P. W.. 2000. Rare genomic changes as a tool for phylogenetics. Trends Ecol. Evol. 15: 454–459. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., Mark P. V. D., Ayres D. L., Darling A. S., Höhna S., Larget B., Liu L., Suchard M. A., and Huelsenbeck J. P.. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C., Frati F., Beckenbach A., Crespi B., Liu H., and Flook P.. 1994. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87: 651–701. [Google Scholar]
- Simon C., Buckley T. R., Frati F., Stewart J. B., and Beckenbach A. T.. 2006. Incorporating molecular evolution into phylogenetic analysis and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecolog. Evol. Syst. 37: 545–579. [Google Scholar]
- Song N. and Liang A.. 2009. The complete mitochondrial genome sequence of Geisha distinctissima (Hemiptera: Flatidae) and comparison with other hemipteran insects. Acta Biochim. Biophys. Sin. (Shanghai). 41: 206–216. [DOI] [PubMed] [Google Scholar]
- Song N., A. P. Liang, and Ma C.. 2010. The complete mitochondrial genome sequence of the planthopper, Sivaloka damnosus. J. Insect Sci. 10: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N., A. P. Liang, and Bu C. P.. 2012. A molecular phylogeny of Hemiptera inferred from mitochondrial genome sequences. PLoS One. 7: e48778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F., H. Li R. Shao A. Shi X. Bai X. Zheng E. Heiss, and Cai W.. 2016. Rearrangement of mitochondrial tRNA genes in flat bugs (Hemiptera: Aradidae). Sci. Rep. 6: 25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. B. and Beckenbach A. T.. 2005. Insect mitochondrial genomics: the complete mitochondrial genome sequence of the meadow spittlebug Philaenus spumarius (Hemiptera: Auchenorrhyncha: Cercopoidae). Genome. 48: 46–54. [DOI] [PubMed] [Google Scholar]
- Stewart J. B. and Beckenbach A. T.. 2006. Insect mitochondrial genomics 2: the complete mitochondrial genome sequence of a giant stonefly, Pteronarcys princeps, asymmetric directional mutation bias, and conserved plecopteran A+T-region elements. Genome. 49: 815–824. [DOI] [PubMed] [Google Scholar]
- Taanman J. W. 1999. The mitochondrial genome: structure, transcription, translation and replication. Biochim. Biophys. Acta. 1410: 103–123. [DOI] [PubMed] [Google Scholar]
- Tamura K., G. Stecher D. Peterson A. Filipski, and Kumar S.. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao M. L., L. Baumann, and Baumann P.. 2004. Organization of the mitochondrial genomes of whiteflies, aphids, and psyllids (Hemiptera, Sternorrhyncha). BMC Evol. Biol. 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifinopoulos J., L. T. Nguyen A. von Haeseler, and Minh B. Q.. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44: W232–W235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. M., Bartlett C. R., and Cryan J. R.. 2010. Evolution of Delphacidae (Hemiptera: Fulgoroidea): combined-evidence phylogenetics reveals importance of grass host shifts. Syst. Entomol. 35: 678–691. [Google Scholar]
- Wang Y., X. L. Huang, and Qiao G. X.. 2013. Comparative analysis of mitochondrial genomes of five aphid species (Hemiptera: Aphididae) and phylogenetic implications. PLoS One. 8: e77511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., X. L. Huang, and Qiao G. X.. 2014. The complete mitochondrial genome of Cervaphis quercus (Insecta: Hemiptera: Aphididae: Greenideinae). Insect Sci. 21: 278–290. [DOI] [PubMed] [Google Scholar]
- Wang J., H. Li, and Dai R.. 2017. Complete mitochondrial genome of Taharana fasciana (Insecta, Hemiptera: Cicadellidae) and comparison with other Cicadellidae insects. Genetica. 145: 593–602. [DOI] [PubMed] [Google Scholar]
- Wilson S. W. 2005. Keys to the families of Fulgoromorpha with emphasis on planthoppers of potential economic importance in the southeastern United States (Hemiptera: Auchenorrhyncha). Fla. Entomol. 88: 464–481. [Google Scholar]
- Yuan M. L., Zhang Q. L., Guo Z. L., Wang J., and Shen Y. Y.. 2015. Comparative mitogenomic analysis of the superfamily Pentatomoidea (Insecta: Hemiptera: Heteroptera) and phylogenetic implications. BMC Genomics. 16: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. X., and Hewitt G. M.. 1997. Insect mitochondrial control region: a review of its structure, evolution and usefulness in evolutionary studies. Biochem. Syst. Ecol. 25: 99–120. [Google Scholar]
- Zhang K. J., W. C. Zhu X. Rong Y. K. Zhang X. L. Ding J. Liu D. S. Chen Y. Du, and Hong X. Y.. 2013. The complete mitochondrial genomes of two rice planthoppers, Nilaparvata lugens and Laodelphax striatellus: conserved genome rearrangement in Delphacidae and discovery of new characteristics of atp8 and tRNA genes. BMC Genomics. 14: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. J., W. C. Zhu X. Rong J. Liu X. L. Ding, and Hong X. Y.. 2014. The complete mitochondrial genome sequence of Sogatella furcifera (Horváth) and a comparative mitogenomic analysis of three predominant rice planthoppers. Gene. 533: 100–109. [DOI] [PubMed] [Google Scholar]
- Zhang Q. X., Guan D. L., Niu Y., and Xu S. Q.. 2016a. Characterization of the complete mitochondrial genome of the Asian planthopper Ricania speculum (Hemiptera: Fulgoroidea: Ricannidae). Conserv. Genet. Resour. 8: 463–466. [Google Scholar]
- Zhang X., Z. Kang M. Mao X. Li S. L. Cameron H. d. Jong M. Wang, and Yang D.. 2016b. Comparative mt genomics of the Tipuloidea (Diptera: Nematocera: Tipulomorpha) and its implications for the phylogeny of the Tipulomorpha. PLoS One. 11: e0158167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.