Abstract
Brexpiprazole is an atypical antipsychotic that works as a partial agonist at serotonin 5-hydroxytryptamine1A and dopamine D2 receptors and an antagonist at serotonin 5-hydroxytryptamine2A. It has US Food and Drug Administration approval for monotherapy treatment of schizophrenia and adjunctive treatment to antidepressants for major depressive disorder. Two phase-3 clinical trials demonstrated efficacy and relatively fair tolerability with regard to adverse effects for each indication. Akathisia was frequently reported in the major depressive disorder trials but less so in the schizophrenia trials. Significant increases in body weight and triglycerides were seen across all studies. Brexpiprazole appears to be a viable option for treating an acute exacerbation of schizophrenia requiring hospitalization or adjunctive treatment of major depressive disorder in patients who showed an inadequate response to 1 to 3 antidepressants. Further clinical trials are warranted to determine the long-term efficacy of brexpiprazole, and comparison trials would be beneficial to establish its place in therapy.
Keywords: brexpiprazole, schizophrenia, major depressive disorder, atypical antipsychotic
Introduction
Brexpiprazole (Rexulti®, Otsuka Pharmaceutical Co, Ltd, Tokyo, Japan) is an atypical antipsychotic that was approved by the US Food and Drug Administration in July 2015 for treatment of schizophrenia and as an adjunctive therapy to antidepressant medications for the treatment of major depressive disorder (MDD).1 While the mechanism of action for brexpiprazole is unknown, it is believed that the combination of partial agonist activity at the serotonin 5-hydroxytryptamine (HT)1A and dopamine D2 receptors and antagonist activity at serotonin 5-HT2A mediates its efficacy. Brexpiprazole also has some affinity for the dopamine D3 (Ki 1.1 nM), noradrenergic α1A (Ki 3.8 nM), histamine H1 (Ki 19 nM), and muscarinic M1 (67% inhibition at 10 μM) receptors. Aripiprazole and brexpiprazole are thought to share a primary mechanism of efficacy through mediation of the same receptors (partial agonism at 5-HT1A and D2 and antagonism at 5-HT2A).1,2 In contrast, aripiprazole does not have strong affinity for the noradrenergic α1A (Ki 57 nM), histamine H1 (Ki 61 nM), or muscarinic M1 (IC50 >1000 nM) receptors.2 These medications may display different side-effect profiles due to variations in binding to these and other receptors.
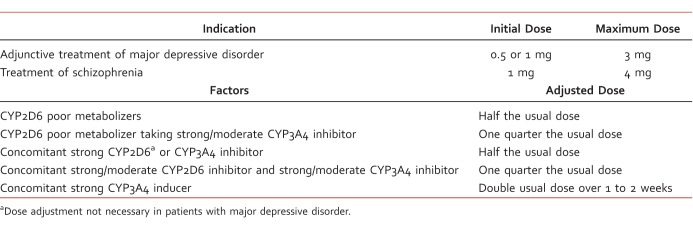
Dosing of brexpiprazole differs based on indication, cytochrome P450 (CYP) 2D6 poor metabolism status, and concomitant medications (Table 1).1 The maximum recommended dose for patients with moderate to severe hepatic impairment (Child-Pugh score ≥7) or moderate, severe, or end-stage renal impairment (creatinine clearance <60 mL/min) is 2 mg once daily for MDD and 3 mg once daily for schizophrenia. Peak plasma concentrations of brexpiprazole are reached within 4 hours after administration; the medication has 95% oral bioavailability and can be administered with or without food. The terminal elimination half-life of brexpiprazole is 91 hours, and steady state is reached within 10 to 12 days of daily administration. Brexpiprazole is highly protein bound in plasma to serum albumin and α1-acid glycoprotein, but binding is not affected by warfarin, diazepam, or digitoxin. Brexpiprazole is primarily metabolized by CYP3A4 and CYP2D6 to DM-3411, which is not thought to contribute to therapeutic effects. As noted by the dose adjustments in Table 1, brexpiprazole exposure is increased with concomitant strong CYP3A4 and CYP2D6 inhibitors and decreased with strong CYP3A4 inducers.1
Table 1.
Brexpiprazole dosing recommendations and adjustments
Brexpiprazole is contraindicated in patients with a known hypersensitivity to the medication or any of its components.1 There is also a black box warning for increased mortality in elderly patients with dementia-related psychosis and suicidal thoughts and behaviors in children, adolescents, and young adults. Other warnings and precautions for brexpiprazole are similar to those of other atypical antipsychotics and include cerebrovascular adverse reactions, including stroke in elderly patients with dementia-related psychosis; neuroleptic malignant syndrome; tardive dyskinesia; metabolic changes; leukopenia, neutropenia, and agranulocytosis; orthostatic hypotension and syncope; seizures; body temperature dysregulation; dysphagia; and potential for cognitive and motor impairment.1
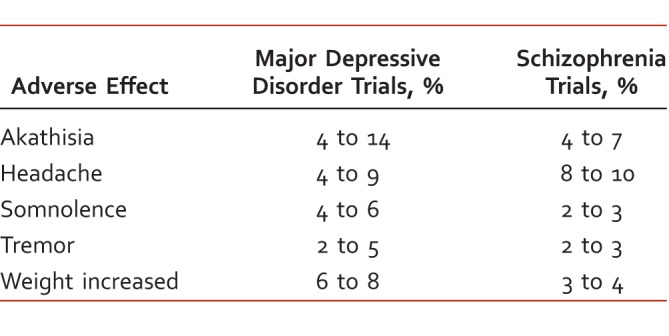
Brexpiprazole appears to be generally well tolerated.1 Commonly reported adverse effects are described in Table 2. In MDD trials, akathisia was reported in 9% of patients compared with 2% in those treated with placebo. In contrast, the rate of akathisia reported in the brexpiprazole group was very similar to that reported in the placebo group (6% versus 5%, respectively) in the schizophrenia trials. Changes in fasting total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were similar in brexpiprazole-treated and placebo-treated patients. The proportion of patients whose baseline triglyceride values shifted from clinically normal to high was 5% to 13% in the brexpiprazole groups versus 6% in the placebo groups. Increases in body weight ≥7% were seen in both the schizophrenia trials (10% to 11% brexpiprazole, 4% placebo) and MDD trials (2% to 5% brexpiprazole, 2% placebo).1
Table 2.
Adverse effects of brexpiprazole
Efficacy
Schizophrenia Trials
Two phase-3 clinical trials3,4 (the BEACON3 and VECTOR4 trials) were similarly designed and used to establish the efficacy, safety, and tolerability of brexpiprazole in the treatment of schizophrenia. Both studies3,4 enrolled subjects between 18 and 65 years of age with a diagnosis of schizophrenia defined by Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV-TR) who were experiencing an acute exacerbation and would benefit from hospitalization or continued hospitalization. Participants were screened for up to 14 days before entering a 6-week double-blind treatment period and a 30-day follow-up phase. The BEACON trial randomized participants in a 2:3:3:3 manner to 1 mg brexpiprazole, 2 mg brexpiprazole,4 mg brexpiprazole, or placebo, respectively, while the VECTOR trial randomized in a 1:2:2:2 manner to 0.25 mg brexpiprazole, 2 mg brexpiprazole, 4 mg brexpiprazole, or placebo, respectively. The primary endpoint for both trials was change in total score from baseline to week 6 on the Positive and Negative Syndrome Scale (PANSS). The key secondary endpoint in both trials was change from baseline to week 6 in Clinical Global Impressions-Severity score. The 2 trials included a safety population that consisted of all patients taking at least 1 dose of study medication and an efficacy population that included only patients who had efficacy evaluations completed at baseline and at least 1 time point after baseline.
The BEACON trial3 screened 1005 patients, of which 674 were randomized to double-blind treatment and included in the safety sample, while 657 were included in the efficacy sample. Randomization led to 4 treatment groups with similar baseline characteristics: 184 patients on placebo, 120 on 1 mg brexpiprazole, 186 on 2 mg brexpiprazole, and 184 on 4 mg brexpiprazole. The average effect of 2 mg and 4 mg brexpiprazole was statistically significant compared with placebo (least squares [LS] mean difference –4.78, P = .0093) when looking at the change in PANSS total score from baseline to week 6; however, individual comparisons of the 2 doses with placebo only demonstrated statistical significance with 4 mg brexpiprazole (mean difference –6.47, P = .0022). Secondary endpoints were not statistically tested because only the 4-mg brexpiprazole group had a statistically significant reduction in PANSS total score compared with placebo. While 58.6% of patients reported at least 1 treatment-emergent adverse event, none of these events met criteria for common adverse events (≥5% in the brexpiprazole group and ≥2 times the placebo rate), and the overwhelming majority of patients reported their treatment-emergent adverse events as mild or moderate. Akathisia was reported more frequently in the placebo group (7.1%) than in any of the brexpiprazole groups (4.2% in the 1-mg group, 4.8% in the 2-mg group, and 6.5% in the 4-mg group). Moderate weight gain was observed in the brexpiprazole groups, and clinically relevant increases in body weight (≥7%) occurred more often in brexpiprazole-treated patients (10% in the 1-mg group, 12.2% in the 2-mg group, 11.4% in the 4-mg group, and 3.9% in the placebo group). Study authors concluded that the optimal effective dose of brexpiprazole was 4 mg.
The VECTOR trial4 screened 949 patients and randomized 636 of these patients to the safety population, while 623 were included in the efficacy population. Randomization led to 4 groups with similar baseline characteristics: 184 patients on placebo, 90 on 0.25 mg brexpiprazole, 182 on 2 mg brexpiprazole, and 180 on 4 mg brexpiprazole. The average effect of the 2-mg and 4-mg brexpiprazole groups on the primary endpoint of change from baseline to week 6 based on the PANSS total score was statistically significant compared with placebo. When analyzed individually, statistically significant greater mean improvements were found with both the 2-mg and 4-mg doses of brexpiprazole (2 mg = –8.72, P < .0001; 4 mg = –7.64, P = .0006). Significant differences from placebo were noted starting at week 1 for the 2-mg group and week 2 for the 4-mg group, and these differences were maintained throughout the 6-week treatment period. Overall, there were fewer reported treatment-emergent adverse events in the brexpiprazole groups (48.9% to 56.7%) than in the placebo group. Akathisia occurred most often during the first 3 weeks of treatment; all cases were mild or moderate and were more frequently reported with 2 mg and 4 mg brexpiprazole than with placebo (4.4% and 7.2% versus 2.2%, respectively). Clinically significant increases in body weight (≥7%) from baseline were seen in 8.8% and 9.0% of the 2-mg and 4-mg brexpiprazole groups, respectively, compared with 4.4% in the placebo group. Superiority of 2 mg and 4 mg brexpiprazole over placebo was also noted to be clinically significant as a significantly greater change in Clinical Global Impressions-Severity score was seen at week 6.4
While the 2-mg dose of brexpiprazole was not found to be statistically significantly different compared with placebo in PANSS total score at week 6 in the BEACON trial,3 a meta-analysis of the 2 phase-3 studies demonstrated statistical significance when the results for the 2-mg dose were pooled.5 The LS mean change for the 2-mg and 4-mg doses of brexpiprazole for PANSS total score at week 6 were –5.46 (95% confidence interval [CI] –8.46, –2.47; P = .0004) and –6.69 (95% CI –9.67, –3.70; P < .0001), respectively compared with placebo.
Two 52-week, open-label extension studies were completed with flexibly dosed brexpiprazole from 1 mg to 6 mg in the phase-2 trial (STEP 210), which included 28 patients, and from 1 mg to 4 mg in the phase-3 trial (ZENITH), which included 1031 patients at the time of cut-off.6 Data were pooled from these 2 trials and showed 34.0% completion at 52 weeks, a mean duration (SD) of treatment of 200.0 (136.2) days, and a mean dose (SD) of 3.1 (1.0) mg. At least 1 treatment-emergent adverse event was reported by 58.6% of patients with the most frequent being insomnia and schizophrenia. Akathisia was reported by 4.6% of patients and led to a discontinuation rate of only 0.4%. Notably, 18.2% of the patients had a weight increase ≥7%, and for those exposed to brexpiprazole for 52 weeks or more, 5.6% gained ≥15 kg. Another long-term 52-week study demonstrated a significant difference in time to exacerbation of psychotic symptoms/impending relapse with brexpiprazole compared with placebo.7
Brexpiprazole was also studied in an open-label fashion to explore changes in efficacy, cognitive functioning, and safety of 6-week treatment with flexibly dosed brexpiprazole or aripiprazole, which was included for assay sensitivity.8 In that study, 64 patients received a mean daily dose of 3.58 mg brexpiprazole and 33 patients received a mean daily dose of 18.20 mg aripiprazole. Improvements were seen in PANSS total score from baseline to week 6 with both the brexpiprazole and aripiprazole groups (LS mean = –22.9, P < .0001; and LS mean = –19.4, P < .0001, respectively); these improvements were seen as early as week 1 for both groups. While this study was not powered to determine differences between the 2 treatment groups, it should be noted that there was a lower incidence of extrapyramidal symptom-related adverse effects with brexpiprazole (14.1% versus 30.3%) and a higher incidence of weight gain ≥7% with brexpiprazole (35% versus 19%).8
Depression Trials
Food and Drug Administration approval of brexpiprazole as adjunctive treatment in MDD was based on the result of 2 phase-3 trials.1 The first study9 evaluated adjunctive brexpiprazole 2 mg daily (the Pyxis trial), while the second study10 evaluated adjunctive brexpiprazole 1 mg or 3 mg daily (the Polaris trial). Both were 6-week, randomized, double-blind, fixed-dose, placebo-controlled trials studying adjunctive brexpiprazole in adult patients with MDD who had inadequate response to 1 to 3 previous antidepressant trials. Each study also included an 8-week single-blind prospective treatment phase, where subjects were started and titrated to a maximum tolerated dose of a standard antidepressant. Brexpiprazole was added to this standard antidepressant at the beginning of the treatment phase. Inclusion criteria were the same across both studies: outpatients aged 18 to 65 years who were diagnosed with a nonpsychotic episode of MDD per DSM-IV-TR lasting at least 8 weeks and who had a total score ≥18 on the 17-item Hamilton Depression Rating Scale (HDRS-17) and an inadequate response to a maximally tolerated dose of a standard antidepressant (defined as HDRS-17 score ≥14, <50% reduction in HDRS-17 score from baseline, <50% reduction in score on the Montgomery-Asberg Depression Rate Scale [MADRS], and a score ≥3 on the Clinical Global Impressions-Improvement scale at each prospective visit). Key exclusion criteria included treatment of current depressive episode with an adjunctive antipsychotic for >3 weeks, hospitalization, current electroconvulsive therapy or prior inadequate response to electroconvulsive therapy, current diagnosis of other psychiatric or medical condition, serious risk of suicide, and abnormal electrocardiogram or laboratory measures. Though concurrent monoamine oxidase inhibitors and neuroleptics were not permitted, subjects remained on a standard antidepressant and were allowed to use some medications concurrently to manage symptoms or adverse effects, including benzodiazepines and nonbenzodiazepine sleep aids (short-term use only), anticholinergics, and propranolol.
The primary outcome measure for both the Pyxis and Polaris trials9,10 was change from baseline to week 6 in MADRS total score. Safety and tolerability were evaluated by recording adverse events, administering extrapyramidal symptom scales, conducting electrocardiograms, measuring vital signs, and monitoring suicidality. Efficacy data from the Pyxis trial9 included 175 subjects in the 2-mg brexpiprazole group and 178 subjects in the placebo group. Patients taking 2 mg brexpiprazole showed a significant reduction in MADRS score from baseline to week 6, compared with those in the placebo group (LS mean difference = –3.21, P = .0002). The brexpiprazole group also had a higher proportion of responders compared with placebo (23.4% versus 15.7%, P = .0429), defined as subjects showing ≥50% reduction in MADRS total score from baseline, though there was no difference seen in proportion of remitters (14.9% versus 9%, P = .0671). Efficacy data from the Polaris trial10 included 211 subjects in the 1-mg brexpiprazole group, 213 subjects in the 3-mg brexpiprazole group, and 203 subjects in the placebo group. Patients in the 3-mg brexpiprazole group showed a significant reduction in MADRS score from baseline to week 6, compared with those in the placebo group (LS mean difference = -1.95, P = .0079). Patients in the 1-mg brexpiprazole group did not show a statistically significant difference compared with those in the placebo group. Patients in the 1-mg and 3-mg brexpiprazole groups had higher proportions of responders compared with those in the placebo group (23.2%, 23.0%, and 14.3%, respectively). Evidence also shows improvements in irritability and work/school functioning with open-label brexpiprazole plus standard antidepressant treatment compared with placebo.11,12
In the Pyxis trial,9 weight gain (8%) and akathisia (7.4%) were the most commonly reported adverse events (compared with 3.1% and 1%, respectively, for placebo). A mean body weight change of 1.64 kg for 2 mg brexpiprazole and 0.36 kg for placebo was seen at week 6. In the Polaris trial,10 akathisia (13.5%), headache (6.1%), weight gain (5.7%), somnolence (5.7%), and tremor (5.2%) were the most commonly reported adverse events in the 3-mg brexpiprazole group (compared with 2.3%, 7.7%, 0.9%, 0.5%, and 3.2%, respectively, for placebo). Mean increase in body weight was 1.4 kg in the 1-mg brexpiprazole group, 0.4 kg in the 3-mg brexpiprazole group, and 0.5 kg in the placebo group. In the Pyxis trial,9 a change from normal to high triglyceride concentrations occurred in 13% of patients taking brexpiprazole versus 4.2% of patients taking placebo. Small increases in prolactin were seen in both trials, along with small changes in lipid and other metabolic measures.9,10
Other Trials
Brexpiprazole is also being studied in the treatment of agitation associated with dementia of the Alzheimer type; 2 trials13,14 are actively recruiting for participants and a third study15 is planned for follow-up on safety. A clinical trial16 was also planned to investigate the use of brexpiprazole as adjunctive treatment to paroxetine or sertraline in adults with posttraumatic stress disorder; however, this study was terminated due to challenges with patient eligibility.
Conclusion
Brexpiprazole is an atypical antipsychotic indicated for treatment of schizophrenia and adjunctive treatment of MDD. Though brexpiprazole's mechanism of action in these disease states is unknown, its efficacy may be due to partial agonist activity at 5-HT1A and D2 receptors and antagonist activity at 5-HT2A receptors. Dosing of brexpiprazole differs depending on indication and factors that will affect metabolism, such as CYP2D6 poor metabolizers and inducers/inhibitors of CYP2D6 or CYP3A4.
The most common adverse effects reported during all phase-3 clinical trials were akathisia, increased weight, and changes in triglyceride concentrations. Significant changes were not seen in other metabolic parameters during phase-3 trials; however, open-label extension trials of 52 weeks for schizophrenia continued to find an increased percentage of weight gain. Other common adverse effects reported during phase-3 trials for MDD included headache and somnolence. Because clinical trials used to establish the efficacy of brexpiprazole lasted only 6 weeks, it is unknown how the medication will affect patients over time. Information about long-term adverse effects of brexpiprazole through open-label extension trials and clinical use will be necessary to determine the long-term risks and benefits of the medication.
Based on currently available data, brexpiprazole appears to be a suitable choice for treatment of an acute exacerbation of schizophrenia requiring hospitalization or adjunctive treatment of MDD in patients who have had an inadequate response to 1 to 3 antidepressants. Due to the lack of head-to-head trials or direct comparisons with other current treatments, the exact place in therapy for brexpiprazole is difficult to determine. Ideally, future trials should be designed to compare brexpiprazole to currently approved antipsychotic medications, including aripiprazole, in order to better compare the efficacy and adverse effect profile of brexpiprazole to medications that have been utilized extensively in clinical practice. November 2016 pricing shows that the average wholesale price of brexpiprazole is slightly more expensive than generic aripiprazole and paliperidone and slightly less expensive than brand lurasidone and cariprazine. Based on the established efficacy and relatively mild adverse effect profile, brexpiprazole is a suitable option for the treatment of an acute exacerbation of schizophrenia or adjunctive treatment of MDD with inadequate response to 1 to 3 antidepressants, if cost is not an issue.
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1. Rexulti [package insert]. Tokyo: Otsuka Pharmaceutical Co, Ltd; 2015. [Google Scholar]
- 2. Abilify [package insert]. Tokyo: Otsuka Pharmaceutical Co, Ltd; 2014. [Google Scholar]
- 3. Kane JM, Skuban A, Ouyang J, Hobart M, Pfister S, McQuade RD, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015; 164 1-3: 127- 35. DOI: 10.1016/j.schres.2015.01.038. PubMed PMID: 25682550. [DOI] [PubMed] [Google Scholar]
- 4. Correll CU, Skuban A, Ouyang J, Hobart M, Pfister S, McQuade RD, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015; 172 9: 870- 80. DOI: 10.1176/appi.ajp.2015.14101275. PubMed PMID: 25882325. [DOI] [PubMed] [Google Scholar]
- 5. Correll CU, Skuban A, Hobart M, Ouyang J, Weiller E, Weiss C, et al. Efficacy of brexpiprazole in patients with acute schizophrenia: review of three randomized, double-blind, placebo-controlled studies. Schizophr Res. 2016; 174 1-3: 82- 92. DOI: 10.1016/j.schres.2016.04.012. PubMed PMID: 27157799. [DOI] [PubMed] [Google Scholar]
- 6. Kane JM, Skuban A, Hobart M, Ouyang J, Weiller E, Weiss C, et al. Overview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr Res. 2016; 174 1-3: 93- 8. DOI: 10.1016/j.schres.2016.04.013. PubMed PMID: 27188270. [DOI] [PubMed] [Google Scholar]
- 7. Hobart M, Ouyang J, Forbes A, Pfister S, McQuade RD, Carson WH, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study. Poster presented at the American Society of Clinical Psychopharmacology Annual Meeting; 2015. June 24; Miami. [DOI] [PMC free article] [PubMed]
- 8. Citrome L, Ota A, Nagamizu K, Perry P, Weiller E, Baker RA. . The effect of brexpiprazole (OPC-34712) and aripiprazole in adult patients with acute schizophrenia. Int Clin Psychopharmacol. 2016; 31 4: 192- 201. DOI: 10.1097/YIC.0000000000000123. PubMed PMID: 26963842. [DOI] [PubMed] [Google Scholar]
- 9. Thase ME, Youakim JM, Skuban A, Hobart M, Augustine C, Zhang P, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015; 76 9: 1224- 31. DOI: 10.4088/JCP.14m09688. PubMed PMID: 26301701. [DOI] [PubMed] [Google Scholar]
- 10. Thase ME, Youakim JM, Skuban A, Hobart M, Zhang P, McQuade RD, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015; 76 9: 1232- 40. DOI: 10.4088/JCP.14m09689. PubMed PMID: 26301771. [DOI] [PubMed] [Google Scholar]
- 11. Fava M, Ménard F, Davidsen CK, Baker RA. . Adjunctive brexpiprazole in patients with major depressive disorder and irritability: an exploratory study. J Clin Psychiatry. 2016; 77 12: 1695- 1701. DOI: 10.4088/JCP.15m10470. PubMed PMID: 27379823. [DOI] [PubMed] [Google Scholar]
- 12. Weisler RH, Ota A, Tsuneyoshi K, Perry P, Weiller E, Baker RA, et al. Brexpiprazole as an adjunctive treatment in young adults with major depressive disorder who are in a school or work environment. J Affect Disord. 2016; 204: 40- 7. DOI: 10.1016/j.jad.2016.06.001. PubMed PMID: 27322768. [DOI] [PubMed] [Google Scholar]
- 13. Otsuka Pharmaceutical Development & Commercialization, Inc. A study of two fixed-doses of brexpiprazole in the treatment of subjects with agitation associated with dementia of the Alzheimer's type [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. [cited 2016 Aug 23]. Available from: http://clinicaltrials.gov/show/NCT01862640
- 14. Otsuka Pharmaceutical Development & Commercialization, Inc. Safety and tolerability study of flexible dosing of brexpiprazole in the treatment of subjects with agitation associated with dementia of the Alzheimer's type [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. [cited 2016 Aug 23]. Available from: http://clinicaltrials.gov/show/NCT01922258
- 15. Otsuka Pharmaceutical Development & Commercialization, Inc. Safety follow-up study for subjects with agitation associated with dementia of the Alzheimer's type who previously participated in a double-blind trial of brexpiprazole or placebo [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. [cited 2016 Aug 23]. Available from: http://clinicaltrials.gov/show/NCT0219554
- 16. H. Lundbeck A/S. Brexpiprazole as an additional treatment to paroxetine or sertraline in adult patients suffering from post-traumatic stress disorder (PTSD) [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. [cited 2016 Aug 23]. Available from: http://clinicaltrials.gov/show/NCT01987960