Abstract
Introduction:
Multiple sclerosis (MS) is a chronic disease state that affects and disables many people each year. The most common clinical presentation is relapsing-remitting MS (RRMS). In the past 7 years, new medications have been approved for the treatment of RRMS, thereby providing more treatment options for patients and providers. The purpose of this article is to provide an update on medications for the treatment of MS that have been approved since January 2010.
Methods:
A review was performed utilizing CenterWatch to search for medications approved by the US Food and Drug Administration for the treatment of RRMS between January 2010 and April 2017. The package inserts of medications indicated for RRMS were analyzed, and key points were summarized. PubMed and EBSCOhost were utilized to identify articles relevant to RRMS background and treatment.
Results:
Seven medications with varying mechanisms of action have been approved to treat RRMS since 2010. Pharmacotherapy options include oral and injectable formulations. Efficacy across the agents is comparable, and each agent has safety data from clinical trials. The safety profile varies between oral and injectable agents, but potential adverse effects are important to consider before initiation. Therapeutic selection is based on patient preference, dosing (frequency and route), and safety considerations.
Discussion:
Multiple therapeutic options are available for the treatment of RRMS. Health care practitioners should be cognizant of the adverse effects, dosing route, and frequency in order to optimally tailor therapy to meet individual patient needs.
Keywords: multiple sclerosis, RRMS, relapsing-remitting, disease-modifying agents, treatment, pharmacotherapy
Introduction
Multiple sclerosis (MS) is a chronic, autoimmune, inflammatory disease of the central nervous system (CNS), that affects the brain and spinal cord.1 The disease results in injury to the myelin sheath, a protective covering composed of lipids that insulate nerves and transmit electrical impulses down the length of an axon.2 The process of myelin sheath destruction, termed demyelination, results in plaques or lesions, which lead to clinical symptoms.2 Symptoms vary widely among patients depending on the location of the lesions within the CNS and may include sensory disturbances in the extremities, optic nerve dysfunction, pyramidal tract dysfunction, bladder and bowel dysfunction, sexual dysfunction, depression, ataxia, and diplopia.3 As demyelination progresses, symptom severity also progresses, as nerves can no longer properly conduct electrical transmission.
Multiple sclerosis is the most common chronic demyelinating disease and the most common cause of nontraumatic disability in young adults. It impacts more than 400 000 individuals in the United States and approximately 2.5 million people worldwide.4 A person can acquire MS at any age, but it is more commonly diagnosed between the ages of 20 and 40 years.4 It affects females more than males (2:1) and is more frequently seen in whites, especially those of Northern European descent.
There are 4 types of MS based on the course of the disease: relapsing-remitting MS (RRMS), secondary-progressive MS, primary-progressive MS (PPMS), and progressive-relapsing MS. According to the National Multiple Sclerosis Society,5 a relapse is defined as “an exacerbation that results in new symptoms or worsening of old symptoms.” In order for an event to be considered a true relapse, the exacerbation must last a minimum of 24 hours and be separated from previous exacerbations by a minimum of 30 days.5 The most common type of MS is RRMS, accounting for approximately 85% of all diagnoses. It is characterized by spontaneous relapses or worsening of neurologic function followed by periods of remission or recovery where symptoms improve or resolve completely. No disease progression occurs between relapses.
Unfortunately, there is no cure for MS.5 As a result, treatment typically focuses on slowing the progression of MS, shortening the duration and frequency of relapses, and managing symptoms.6 Prior to 1993, MS treatment options were limited: corticosteroid therapy was the primary treatment option for acute exacerbations.7 Interferon beta-1b (Betaseron, Bayer HealthCare Pharmaceuticals Inc, Whippany, NJ) was the first disease-modifying agent that received US Food and Drug Administration (FDA) approval for the treatment of RRMS.8 Interferon beta-1b (Extavia) was approved in 2009 and differs only in the size of the needle, package size, and cost. In 1995, interferon beta-1a (Avonex), received FDA approval for the treatment of relapsing forms of MS and is administered intramuscularly once weekly.9 In 2002, a subcutaneous injectable form emerged, interferon beta-1a (Rebif).9 It is administered 3 times weekly and was more effective in reducing relapse frequency and number of lesions found on magnetic resonance imaging in a head-to-head comparison with Avonex.10 Interferon beta-1a offers multiple routes of administration, fewer injections compared with its predecessor, and a safety profile similar to that of interferon beta-1b.
Another disease-modifying agent with a unique mechanism of action, glatiramer acetate (Copaxone, TEVA Pharmaceuticals USA, Inc, Kansas City, MO), was approved for reducing the frequency of relapses in patients with RRMS in 1997 and received approval in 2014 as an extended dosage form.9 The first monoclonal antibody, natalizumab (Tysabri) was FDA approved in 2004 for relapsing forms of MS in patients who had inadequate responses to, or who were unable to tolerate, previous MS medications.9
The prevalence of MS has increased since 1955; current statistics indicate a prevalence of 3.6 per 100 000 person-years for women versus 2.0 per 100 000 person-years for men.11 Therefore, new medications have been developed in an effort to provide patients with various options for this debilitating disease. The purpose of this review is to compare the agents approved for RRMS since January 2010 focusing on advantages, disadvantages, and place in therapy.
Methods
The search engines PubMed and EBSCOhost were utilized to identify articles relevant to RRMS background and treatment using the search terms multiple sclerosis, RRMS, relapsing-remitting, disease-modifying agents, treatment, and pharmacotherapy. A review was performed utilizing CenterWatch in order to search for medications approved by the FDA for the treatment of RRMS between January 2010 and April 2017. The package inserts of individual agents were evaluated.
Results
Since January 2010, the FDA has approved 7 novel agents for the treatment of RRMS. In addition, a new dosage formulation of an agent originally approved in 1996 has come to market.
Fingolimod
The first of the newer medications, fingolimod (Gilenya, Novartis Pharmaceuticals Corporation, East Hanover, NJ), was also the first FDA-approved oral medication for treating RRMS. It was approved in September 2010 and is categorized as a sphingosine 1-phosphate receptor modulator, which blocks lymphocytes from emerging from lymph nodes.12 This mechanism decreases lymphocytes available in the CNS, resulting in reduced central inflammation. Efficacy has been established for patients with RRMS to reduce the frequency of clinical exacerbations and to delay the accumulation of physical disability. The recommended daily dose of fingolimod is 0.5 mg once daily by mouth taken with or without food. Capsules are only available in 0.5-mg strength as higher doses are associated with no additional benefit but increased adverse events. Patients being initiated on fingolimod will need to undergo continuous monitoring and observation for a minimum of 6 hours after the first dose of medication due to possible bradycardia and QTc prolongation.12 Hourly pulse and blood pressure measurements should be obtained as well as electrocardiogram results prior to and at the end of the observation period. First-dose bradycardia can occur within 2 hours of fingolimod administration but will resolve within 24 hours. Furthermore, if patients discontinue fingolimod for more than 14 days after their first month of treatment, they will need to repeat 6 hours of observation upon reinitiating the medication. No dosage adjustment is offered in the package insert for renal impairment; however, caution is advised in patients with severe renal impairment. A small pharmacokinetic study found that patients with stable severe impairment (CrCl <30 mL/min) not on dialysis had greater exposure to fingolimod and its metabolites, but this was not found to be clinically meaningful, and adjusting the dose may not be necessary. Similarly, no dose adjustment is necessary for mild to moderate hepatic impairment (Child-Pugh Class A and B). No recommendation for dose adjustments in severe hepatic impairment is offered in the package insert. Patients with hepatic impairment should be closely monitored as exposure to fingolimod can increase, resulting in a higher risk of adverse reactions.12
Fingolimod is primarily metabolized by CYP4F2 to its active form fingolimod-phosphate and other inactive metabolites; CYP2D6, 2E1, 3A4, and 4F12 also contribute to its metabolism. After administration, peak plasma concentrations of fingolimod are reached in 12 to 16 hours. The most common adverse reactions associated with fingolimod therapy (incidence ≥10%) are headache, liver transaminase elevation, diarrhea, cough, influenza, sinusitis, and back pain. Fingolimod is advantageous in that it offers once-a-day oral administration and a high percentage of reduction in relapse rates; it has also been proven to be more efficacious in reducing relapse rates and the number of lesions when compared head to head with interferon beta-1a. Specifically, fingolimod 1.25 mg and 0.5 mg had an annualized relapse rate of 0.20 (95% confidence interval [CI], 0.16-0.26) and 0.16 (95% CI, 0.12-0.21), respectively, compared with 0.33 for interferon (95% CI, 0.26-0.42; P < .001 for both doses).13 Disadvantages include the first-dose monitoring requirement, contraindications in heart disease, risk of macular edema, and potential elevation of liver enzymes.
Teriflunomide
In September 2012, the FDA approved teriflunomide (Aubagio, Genzyme Corporation, Cambridge, MA), a pyrimidine synthesis inhibitor indicated for the treatment of patients with relapsing forms of MS. It works as an immunomodulation agent that results in antiproliferative and anti-inflammatory properties which may decrease the number of activated lymphocytes in the CNS.14 The recommended daily dosing of teriflunomide is 7 mg or 14 mg once daily by mouth taken with or without food. Tablets are only available in 7-mg or 14-mg strengths and are individually packaged in blister packs with a 28-day supply. No dose adjustments are necessary in patients with mild, moderate, or severe renal impairment.
Teriflunomide is primarily metabolized by hydrolysis to its minor metabolites. Secondary metabolism includes oxidation, conjugation, and N-acetylation. After administration, peak plasma concentrations of teriflunomide are reached in 1 to 4 hours. However, teriflunomide has a relatively long half-life of approximately 19 days. Because of this, it is recommended to administer cholestyramine or activated charcoal to accelerate elimination of teriflunomide if drug-induced liver injury is suspected. With regards to hepatic impairment, no dose adjustment is necessary for mild to moderate impairment. However, teriflunomide use is contraindicated in patients with severe hepatic impairment. Teriflunomide has a black box warning as its use is contraindicated in patients with preexisting acute or chronic liver disease.14
Teriflunomide has a second black box warning stating that its use is contraindicated in women of childbearing potential. Teriflunomide is classified as pregnancy X because of the major birth defects observed in animal studies. Women must not be started on teriflunomide until confirmation of reliable contraception has been obtained.
The most common adverse reactions associated with teriflunomide therapy (incidence ≥10%) are headache, diarrhea, nausea, alopecia, and increased alanine transaminase levels (ALT). Advantages of this medication are that it offers once daily oral administration, limited adverse reactions, and less frequent monitoring. Disadvantages associated with the use of teriflunomide are its hepatotoxicity and teratogenicity effects.14
Pegylated Interferon Beta-1a
In August 2014, the FDA approved pegylated interferon beta-1a (Plegridy, Biogen Idec, Inc, Cambridge, MA), an interferon beta indicated for the treatment of relapsing forms of MS. Interferon beta has been a staple treatment medication utilized in MS for over 20 years, but it requires cumbersome administration regimens: up to 3 injections weekly.15 Pegylated interferon beta-1a features a polyethylene glycol side chain attached to the parent molecule, which results in an extended half-life and increased systemic exposure, ultimately leading to fewer required injections. This medication emerged in an effort to reduce the number of injections and may promote greater treatment adherence.
The mechanism of action in the treatment of MS is unknown but interferon treatment is thought to reduce T-cell proliferation and antigen presentation, alter cytokine and matrix metalloproteinase expression, and restore suppressor function.16 The recommended dosing of pegylated interferon beta-1a is 125 μg administered subcutaneously every 14 days. In efforts to limit flu-like symptoms, initial dosing should be titrated up to 125 μg by administering 63 μg on day 1, followed by 94 μg administered on day 15, then subsequent doses will remain constant at 125 μg given on day 29 and every 14 days after.16 Pegylated interferon beta-1a is available as a starter pack that includes a 63-μg and 94-μg dose as a solution in a pen injector. It also is available in 125-μg doses via pen injectors.
No dosage adjustments for renal impairment are offered in the package insert. While the manufacturer advises caution in severe renal impairment, there is no specific guidance for dosage adjustments. Similarly, no dose adjustment is provided for hepatic impairment. Pegylated interferon beta-1a is not extensively metabolized by the liver and is eliminated renally. After administration, peak plasma concentrations of pegylated interferon beta-1a are reached in 1 to 1.5 days. The elimination half-life is approximately 78 hours. The most common adverse reactions associated with pegylated interferon beta-1a therapy (incidence ≥10%) include injection-site erythema, influenza-like illness, pyrexia, headache, myalgia, chills, injection-site pain, injection-site pruritus, arthralgia, and asthenia. The greatest advantage of this medication is that it requires fewer injections compared with its predecessors, which makes it superior to older beta-1a agents.16
Dimethyl Fumarate
The third and latest oral medication, dimethyl fumarate (Tecfidera, Biogen Idec, Inc), was approved by the FDA in March 2013. It is indicated for treatment of patients with relapsing forms of MS and is categorized as a fumaric acid derivative and a systemic immunomodulator. Its mechanism is not fully known, but dimethyl fumarate is thought to activate the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway,17 which is involved in the cellular response to oxidative stress. Therefore, dimethyl fumarate reduces peripheral and central inflammatory response and protects the CNS from oxidative stress.
The recommended daily dose of dimethyl fumarate is 120 mg by mouth twice daily for the first 7 days, then 240 mg by mouth twice daily as a maintenance dose taken with or without food. It is available in 120-mg and 240-mg delayed-released capsules, which are not to be split but taken whole.17 No dose adjustment is necessary for renal or hepatic impairment as dimethyl fumarate undergoes rapid hydrolysis by esterases before it reaches systemic circulation. Its active metabolite, monomethyl fumarate, is metabolized through the citric acid cycle and does not involve the CYP450 system. Elimination occurs primarily through exhalation of CO2, accounting for 60% of dimethyl fumarate removal. Therefore, no drug interactions are associated with dimethyl fumarate.
Flushing is the most common adverse effect associated with dimethyl fumarate, but it can be lessened with aspirin 325 mg at least 30 minutes before each dose. Other adverse effects with dimethyl fumarate include diarrhea, nausea, vomiting, and abdominal pain. Flushing and gastrointestinal effects are most likely to occur in the first month of therapy.17 Dimethyl fumarate offers a multitude of advantages, including its safety in patients with renal/hepatic impairments, noninvasive administration, limited adverse effects that subside over time, and no risk of drug interactions. Disadvantages include twice daily dosing, which may lead to adherence concerns and the risk of lymphopenia resulting in annual complete blood count monitoring.
Glatiramer Acetate
Glatiramer acetate (Copaxone) was originally approved in 1996 and has been available in an extended dosage form since January 2014.9 It is indicated for reducing the frequency of relapses in patients with RRMS. The exact mechanism of action is unknown, but it is thought that glatiramer acetate induces and activates T-lymphocyte suppressor cells.18 It has demonstrated reductions in relapse rates and magnetic resonance imaging lesions as well as progression on the expanded disability status scale.19,20 Administration is via the subcutaneous route, and the medication is given as 20 mg every day or 40 mg 3 times a week at least 48 hours apart.18
Glatiramer acetate has no drug interactions or dosage adjustments for patients with renal or hepatic impairment. The pharmacokinetics of glatiramer acetate in patients with impaired renal function have not been determined.
Common adverse reactions with glatiramer acetate therapy (incidence ≥10%) are injection-site reactions, chest pain, rash, dyspnea, and vasodilation. Glatiramer acetate is the safest medication utilized in pregnancy to date and is the only medication for RRMS that is listed as category B. Also, it may be the least expensive therapy option available because a generic form was approved for the 20-mg dose in April 2015.9 Disadvantages of glatiramer acetate include the potential to modify immune response as well as to cause an immediate postinjection reaction (flushing, chest pain, palpitations, anxiety, dyspnea, throat constriction, and/or urticaria) that can occur several months after initiation, though it is generally transient and self-limiting.18 Lipoatrophy and skin necrosis may occur, and it is important to instruct patients about proper injection technique and to rotate injection sites.
Alemtuzumab
Alemtuzumab (Lemtrada, Genzyme Corporation) was FDA approved in November 2014 for relapsing forms of MS. It is an anti-CD52 directed monoclonal antibody that binds to CD52 antigens present on the surface of B lymphocytes, T lymphocytes, monocytes, macrophages, natural killer cells, and some granulocytes, halting their ability to enter the brain and destroy myelin.21 Once bound, antibody-dependent cellular cytolysis and complement-mediated lysis occurs, which results in a decrease in circulating immune cells.21 The recommended dosing of alemtuzumab is 12 mg daily via intravenous infusion (infused over 4 hours) for 5 consecutive days, followed 1 year later by 12 mg daily via intravenous (IV) infusion for 3 consecutive days for a total duration of therapy of 24 months. Premedication with high-dose corticosteroids (methylprednisolone 1000 mg or equivalent) is recommended and should be utilized immediately prior to and for the first 3 days of infusions. Antiviral prophylaxis for herpes infection should start on the first day of each course and continue for 2 months or until CD4+ lymphocyte count is ≥200 cells/μL (whichever occurs later). Continuous observation for infusion reactions should occur during and 2 hours after infusion. Alemtuzumab is only available as a single-use vial containing 1.2 mL (10 mg/mL) solution for mixing with 100 mL of 0.9% sodium chloride or 5% dextrose in water.
Many black box warnings are associated with alemtuzumab and include risks of serious and sometimes fatal autoimmune conditions, infusion reactions, and various malignancies. It is contraindicated in human immunodeficiency virus due to CD4+ lymphocyte count reductions. The most common adverse reactions associated with alemtuzumab therapy (incidence ≥10%) are rash, headache, vomiting, nausea, diarrhea, flushing, abdominal pain, pyrexia, nasopharyngitis, urinary tract infection, fatigue, insomnia, upper respiratory tract infection, herpes viral infection, urticaria, pruritus, thyroid gland disorders, fungal infections, arthralgia, extremity pain, back pain, sinusitis, oropharyngeal pain, paresthesia, and dizziness.21 Moreover, it is only obtainable through a risk evaluation mitigation strategy (REMS) program due to concern about serious adverse reactions. Certified physicians through the alemtuzumab REMS program can prescribe the medication to patients who are enrolled and willing to receive ongoing monitoring.22 No renal or hepatic dosage adjustment is provided as alemtuzumab has not been studied in these populations. Information on drug interactions with alemtuzumab is limited. Serologic tests for varicella zoster virus should be assessed before initiating alemtuzumab. All necessary immunizations, including varicella zoster vaccine, should be completed at a minimum of 6 weeks prior to administration of alemtuzumab. Antiviral prophylaxis may also be indicated with alemtuzumab, along with ongoing monitoring due to the risk of herpes infections. Nevertheless, in clinical trials alemtuzumab was significantly more effective in reducing the number of relapses and risk of disability compared with interferon therapy (Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis [CARE MS] I trial 0.45, 95% CI, 0.32-0.63; CARE MS II trial 0.51, 95% CI, 0.39-0.65).23,24 Alemtuzumab is given for 5 consecutive days (60 mg as total dose); 12 months later, another 3-day treatment (36 mg as total dose) is given for a complete treatment (2-year duration). This administration regimen may be an advantage, especially in patients with poor adherence. Unfortunately, the numerous adverse effects, black box warnings, unknown long-term effects, invasive administration, cost, and extensive monitoring limit alemtuzumab to a last-line therapy reserved for those with inadequate responses to 2 or more MS medications.
Daclizumab
Daclizumab (Zinbryta, Biogen Idec, Inc) received FDA approval in May 2016 for the treatment of relapsing forms of MS. It is an anti-interleukin-2 directed monoclonal antibody that binds to CD25 subunits on high-affinity interleukin-2 (IL-2) receptors to prevent signaling. Because IL-2 plays a role in activating and regulating the immune system, antagonism at IL-2 receptors may produce benefits in MS.25 The recommended dosing of daclizumab is 150 mg given subcutaneously once monthly. Patients can receive daclizumab up to 2 weeks following a missed dose. If the missed dose duration is longer than 2 weeks, the next dose should be administered on the original schedule.25 Prior to administration, patients should be screened for hepatitis B and C, as use in these populations is contraindicated. Presence of tuberculosis or other active infections should be ruled out prior to initiation, along with completion of any live vaccines. Daclizumab is only available as a 1 mL (150 mg/mL) solution in a single-dose prefilled pen for subcutaneous administration.
Daclizumab can be obtained through a REMS program due to risk of hepatic injury, autoimmune hepatitis, and other immune-mediated conditions.26 As a protein, daclizumab is metabolized via catabolism to peptides and amino acids. Thus, no dosage adjustments for renal impairment are provided in the package insert; conversely, use in preexisting hepatic disease or impairment (ALT or aspartate transaminase ≥2 times the upper limit) or history of autoimmune hepatitis is contraindicated as daclizumab was not studied in these populations since patients may be at an increased risk of hepatotoxicity. Information on drug interactions with daclizumab is limited; however, hepatotoxic drugs should be evaluated as concomitant use is associated with increased risks of hepatotoxicity. Two black box warnings accompany daclizumab, including risk of severe liver injury and immune-mediated disorders, such as skin reactions, lymphadenopathy, and noninfectious colitis. The most common adverse reactions associated with daclizumab therapy (incidence ≥5%) compared with interferon beta-1a therapy were influenza, bronchitis, eczema, lymphadenopathy, nasopharyngitis, upper respiratory tract infection, rash, and dermatitis. Rash (7% to 11%), depression (7% to 10%), upper respiratory tract infection (9% to 17%), pharyngitis (25%), and elevated ALT levels (5% to 6%) were observed compared with placebo.25
Once-monthly injections, along with clinical trials that demonstrated statistically significant superiority (P < .0001) over interferon beta-1a therapy in reducing annualized relapse rates and number of new lesions, render daclizumab advantageous. The study revealed that daclizumab reduced annualized relapse rates on average 45% more than interferon-1a, and there was a 54% relative reduction in new or newly enlarging lesions.25 Nonetheless, daclizumab use is limited by its increased risks of serious side effects compared with its predecessors as well as the black box warnings, contraindications, invasive administration, and inability to lower the risk of disability progression in clinical trials. Due to these reasons, daclizumab is currently a last-line therapy option and is reserved for patients who have had inadequate responses to 2 or more MS medications.
Ocrelizumab
Ocrelizumab (Ocrevus, Genentech, Inc, San Francisco, CA) was FDA approved March 2017 and is the latest medication approved for the treatment of patients with relapsing forms of MS. Ocrelizumab is also the first treatment for PPMS. Ocrelizumab is a recombinant humanized monoclonal antibody that specifically targets and depletes CD-20-positive B lymphocytes.27 Similar to alemtuzumab, antibody-dependent cellular cytolysis and complement-mediated lysis occur, which results in a decrease of circulating immune cells.27 Recommended initial dosing for RRMS and PPMS is ocrelizumab 300 mg IV infusion followed by a second dose 2 weeks later. The infusion rate should begin at 30 mL/h and may be increased by 30 mL/h every 30 minutes if tolerated, to a maximum rate of 180 mL/h infused at a minimum of 2.5 hours. Subsequent doses should be started 6 months following the initial dose at a target dose of 600 mg IV every 6 months. The infusion rate should begin at 40 mL/h and may be increased by 40 mL/h every 30 minutes if tolerated, to a maximum rate of 200 mL/h infused at a minimum of 3.5 hours.27 Ocrelizumab is available as a single-use vial containing 10 mL (30 mg/mL) solution for diluting only with 0.9% sodium chloride. Prior to receiving ocrelizumab, patients must be screened for hepatitis B, and all necessary immunizations should be completed at a minimum of 6 weeks prior to therapy. Additionally, patients should be premedicated with an antihistamine and methylprednisolone 100 mg IV (or equivalent corticosteroid) 30 minutes prior to each infusion. An antipyretic may also be considered.
The manufacturer provides no black box warnings and no renal or hepatic dosage adjustments for ocrelizumab; however, its use is contraindicated in patients with an active hepatitis B infection and any history of life-threatening infusion reactions occurring from ocrelizumab.27 The most common adverse reactions associated with ocrelizumab therapy are upper respiratory tract infections (40% to 49%), lower respiratory tract infections (8% to 10%), infusion reactions (34% to 40%), and infection of the skin and/or subcutaneous tissue (14%).27 Approval of ocrelizumab was based on 2 clinical trials evaluating annualized relapse rates as the primary outcome. Both trials included the active comparator interferon beta-1a (Rebif), and in both trials ocrelizumab was associated with lower annualized relapse rates (46% and 47%, respectively) compared with interferon beta-1a.28 Hence, ocrelizumab should be considered a first-line agent in patients with PPMS as it is the only medication currently approved for this disease state. For relapsing forms of MS treatment, ocrelizumab offers patients the lowest number of administrations compared with all other MS medications; however, infusion reactions and infection rates may limit its use.
Discussion
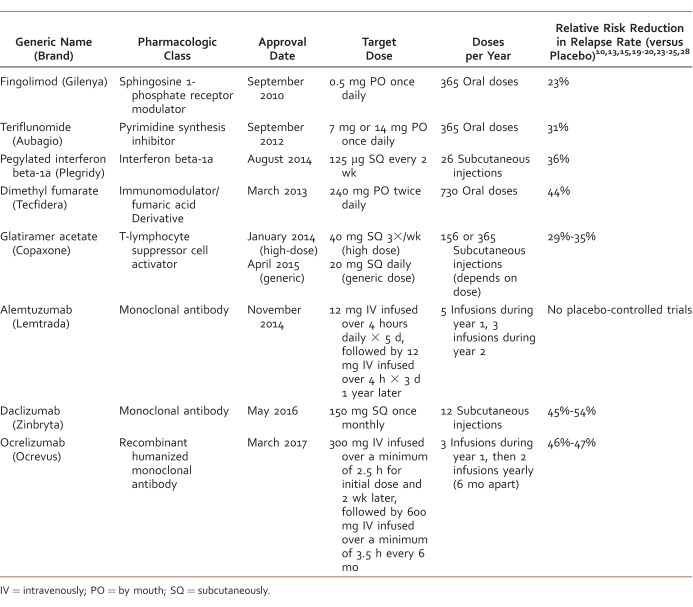
The Table provides a summary of new agents for RRMS approved since 2010 with pharmacologic class, approval date, dosing, and relative risk reduction in relapse rates. Unfortunately, there are no clinical practice guidelines or widely accepted treatment algorithms to date for RRMS; however, the Multiple Sclerosis Coalition has published an evidence-based consensus paper that summarizes the current evidence about disease modification in MS.29 The coalition recommends early treatment to prevent disability and improve quality of life in individuals with MS and recognizes that adherence is a key factor for efficacy with pharmacologic treatment. Individuals with MS may become nonadherent due to many factors, such as route of administration, tolerability and side effects, length of time needed for treatment, and costs. Patients and providers need to be educated about the various pharmacologic treatment options for MS, especially nonadherent patients.
TABLE:
Medications approved for relapsing-remitting multiple sclerosis since 2010
Previous therapies of RRMS were limited to a few drugs, which included only invasive forms of administration. This type of administration could affect adherence and, thus, the progression of MS. Multiple drugs have been approved since 2010 that offer different mechanisms of action, administration routes, safety profiles, and reductions in relapse rates and provide patients with more choices. Depending on patient preference for managing RRMS symptoms, most subcutaneous injectable therapies offer the greatest safety profile, the oral therapies offer the greatest convenience, and IV infusion therapies demonstrate the greatest reductions in relapse rates. Multiple factors, such as preferred route of administration, adverse effects, adherence, safety, drug interactions, and cost need to be considered before determining which agent is optimal for the patient.
Footnotes
Disclosures: Authors of this manuscript do not have any financial or personal relationships with commercial entities that may have direct or indirect interest in the subject matter of this manuscript.
References
- 1. Miller RM, Happe LE, Meyer KL, Spear RJ. . Approaches to the management of agents used for the treatment of multiple sclerosis: consensus statements from a panel of U.S. managed care pharmacists and physicians. J Manag Care Pharm. 2012; 18 1: 54- 62. DOI: 10.18553/jmcp.2012.18.1.54. PubMed PMID: 22235955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sturm D, Gurevitz SL, Turner A. . Multiple sclerosis: a review of the disease and treatment options. Consult Pharm. 2014; 29 7: 469- 79. DOI: 10.4140/TCP.n.2014.469. PubMed PMID: 25203107. [DOI] [PubMed] [Google Scholar]
- 3. Goodin DS, Frohman EM, Garmany GP, Halper J, Likosky WH, Lublin FD, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002; 58 2: 169- 78. PubMed PMID: 11805241. [DOI] [PubMed] [Google Scholar]
- 4. Pietrangelo A, Higuera V. . Multiple sclerosis by the numbers: Facts, statistics, and you. [Medically reviewed by Steven Kim, MD, 2015. Mar 24.] Healthline [Internet]. [cited 2016 Nov 10.] Available from: http://www.healthline.com/health/multiple-sclerosis/facts-statistics-infographic
- 5. Managing Relapses. National Multiple Sclerosis Society [Internet]. [cited 2016. Nov 10.] Available from: http://www.nationalmssociety.org/Treating-MS/Managing-Relapses
- 6. Moore CD. . Managing multiple sclerosis: The pharmacist's expertise. 2016 Feb 16. Pharmacy Times [Internet]. [cited 2016. Nov 10.] Available: http://www.pharmacytimes.com/publications/issue/2016/february2016/managing-multiple-sclerosis-the-pharmacists-expertise
- 7. English C, Aloi JJ. . New FDA-approved disease-modifying therapies for multiple sclerosis. Clin Ther. 2015; 37 4: 691- 715. DOI: 10.1016/j.clinthera.2015.03.001. PubMed PMID: 25846320. [DOI] [PubMed] [Google Scholar]
- 8. Timeline of progress in MS research. National Multiple Sclerosis Society [Internet]. [cited 2016. Nov 10.] Available from: http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Timeline-of-Research.pdf
- 9. FDA approved drugs. Centerwatch [Internet]. [cited 2017. Apr 8.] Available from: http://www.centerwatch.com/drug-information/fda-approved-drugs/.
- 10. Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O'Connor P, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE Trial. Neurology. 2002; 59 10: 1496- 506. PubMed PMID: 12451188. [DOI] [PubMed] [Google Scholar]
- 11. Alonso A, Hernan MA. . Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology. 2008; 71 2: 129- 35. DOI: 10.1212/01.wnl.0000316802.35974.34. PubMed PMID: 18606967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. GilenyaTM [Package Insert]. Novartis Pharmaceuticals Corporation, East Hanover (NJ); February 2016. [cited 2016 Nov 10.] Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/gilenya.pdf
- 13. Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010; 362 5: 402- 15. DOI: 10.1056/NEJMoa0907839. PubMed PMID: 20089954. [DOI] [PubMed] [Google Scholar]
- 14. AubagioTM [Package Insert]. Genzyme Corporation, Cambridge (MA); June 2016. [cited 2016 Nov 10.] Available from: http://products.sanofi.us/aubagio/aubagio.pdf
- 15. Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996; 39 3: 285- 94. DOI: 10.1002/ana.410390304. PubMed PMID: 8602746. [DOI] [PubMed] [Google Scholar]
- 16. PlegridyTM [Package Insert]. Biogen Idec, Inc, Cambridge (MA); October 2015. [cited 2016 Nov 10.] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125499s000lbl.pdf
- 17. TecfideraTM [Package Insert]. Biogen Idec, Inc, Cambridge (MA); March 2013. [cited 2016 Nov 10.] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204063lbl.pdf
- 18. CopaxoneTM [Package Insert]. TEVA Pharmaceuticals USA, Inc, Kansas City (MO); February 2009. [cited 2016 Nov 10.] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020622s057lbl.pdf
- 19. Comi G, Filippi M, Wolinsky JS. . European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Gla. Ann Neurol. 2001; 49 3: 290- 7. PubMed PMID: 11261502. [PubMed] [Google Scholar]
- 20. Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995; 45 7: 1268- 76. PubMed PMID: 7617181. [DOI] [PubMed] [Google Scholar]
- 21. LemtradaTM [Package Insert]. Genzyme Corporation, Cambridge (MA); November 2014. [cited 2016 Nov 10.] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/103948s5139lbl.pdf
- 22. LEMTRADA REMS (Risk Evaluation and Mitigation Strategy) Program. Genzyme Corporation, a Sanofi company; 2017. [cited 2017 Jun 29.] Available from: https://www.lemtradarems.com/.
- 23. Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung H-P, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012; 380 9856: 1819- 28. DOI: 10.1016/S0140-6736(12)61769-3. PubMed PMID: 23122652. [DOI] [PubMed] [Google Scholar]
- 24. Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012; 380 9856: 1829- 39. DOI: 10.1016/S0140-6736(12)61768-1. PubMed PMID: 23122650. [DOI] [PubMed] [Google Scholar]
- 25. ZinbrytaTM [Package Insert]. Biogen Idec, Inc, Cambridge (MA); May 2016. [cited 2016 Nov 10.] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761029s000lbl.pdf
- 26. ZINBRYTA (daclizumab) Risk Evaluation and Mitigation Strategy (REMS) Program. Biogen and AbbVie Inc ; 2016. [cited 29 Jun 2017.] Available from: https://www.zinbrytarems.com/.
- 27. OcrevusTM [Package Insert]. Genentech, Inc, San Francisco (CA); March 2017. [cited 2017 Apr 17.] Available from: https://www.gene.com/download/pdf/ocrevus_prescribing.pdf
- 28. Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017; 376 3: 221- 34. DOI: 10.1056/NEJMoa1601277. PubMed PMID: 28002679. [DOI] [PubMed] [Google Scholar]
- 29. Multiple sclerosis coalition consensus paper. The use of disease-modifying therapies in multiple sclerosis: Principles and current evidence. March 2017: 1- 73. [cited 2017. Jun 21.] Available from: http://www.nationalmssociety.org/getmedia/5ca284d3-fc7c-4ba5-b005-ab537d495c3c/DMT_Consensus_MS_Coalition_color [Google Scholar]