Abstract
Introduction:
Recently, controversy has surrounded a 2011 Food and Drug Administration warning against using citalopram at doses >40 mg/day due to QTc prolonging effects.
Methods:
Patients ≥18 years old at the VA North Texas Health Care System were included in this retrospective review if they had received at least 1 prescription for a 30-day supply of citalopram between January 1, 2007, and February 29, 2012, and had a baseline electrocardiogram (ECG) within 1 year before initiation or dose increase of citalopram and at least 1 repeat ECG within 3 months after citalopram initiation or dose increase. The primary endpoint was the prevalence of QTc prolongation (QTc interval ≥470 ms for men and ≥480 ms for women) after initiation or a dose increase of citalopram. For secondary objectives, Fisher exact tests were used determine if there was a dose-dependent difference in prevalence of QTc prolongation among the whole study sample and among the subgroup of patients ≥60 years old.
Results:
Among the entire study sample, QTc prolongation was identified in 12 patients (16.4%) after initiation or a dose increase of citalopram. In the subgroup of patients ≥60 years old, QTc prolongation was identified in 7 patients (21.9%). Prevalence of QTc prolongation increased with dose in the entire study population (P = .016) and in patients ≥60 years (not significant).
Discussion:
This retrospective study suggests that citalopram produces a dose-dependent increase in QTc interval.
Keywords: citalopram, antidepressant, SSRI, QTc prolongation, QT interval, elderly, ventricular arrhythmia
Introduction
In August 2011, the Food and Drug Administration (FDA) released a drug safety communication that significantly changed dosing recommendations for the selective serotonin reuptake inhibitor (SSRI) citalopram. This warning advised against prescribing citalopram at doses >40 mg/day due to “dose-dependent QT interval prolongation.” This safety warning also decreased the maximum dose to 20 mg in patients ≥60 years old, those with hepatic impairment, those who are CYP2C19 poor metabolizers, and/or those taking cimetidine or other CYP2C19 inhibitors. The FDA recommended frequent electrolyte and electrocardiogram (ECG) monitoring for QTc prolongation in patients that meet these criteria and are taking citalopram. This safety communication also recommended against the use of citalopram in certain patient populations, including those with symptomatic bradycardia, uncompensated heart failure, hypokalemia, or hypomagnesemia; those with recent acute myocardial infarction; or those taking other drugs that prolong the QT interval.1 The FDA updated the 2011 safety announcement in March 2012, changing congenital long QT syndrome from a contraindication to a precaution for citalopram.2
The FDA safety communication did not cite specific evidence supporting its recommendations but did discuss an “unpublished thorough QT/QTc study” of citalopram, which was a randomized, multicenter, double-blind, placebo-controlled, active drug-controlled crossover study.1,3 The primary outcome was the mean change in QTc interval in 119 healthy, nondepressed subjects randomized to receive 20 or 60 mg citalopram per day, 400 mg moxifloxacin per day, or placebo. The mean change in QTc interval was 8.5 ms (90% confidence interval [CI], 6.2-10.8) in the citalopram 20-mg group and 18.5 ms (90% CI, 16.0-21.0) in the citalopram 60-mg group. The investigators concluded that citalopram should not be used at doses >40 mg/day as a result of this statistically significant finding in the 60-mg group. Estimations of change in QTc with citalopram 40 mg were calculated using the mean values for the 20-mg and 60-mg groups, thus providing no evidence on the QTc-prolonging effects of the 40-mg dose. The guidelines of the Committee for Proprietary Medicinal Products suggest that the risk of developing torsades de pointes (TdP) increases significantly with an increase in QTc interval of 30 to 60 ms from baseline,4 which was not mentioned for any subjects in this study.
There are some existing case reports of QTc prolongation and TdP in patients taking citalopram,5 but large randomized controlled trials have provided only minimal evidence on the significance of this serious adverse event. Most citalopram studies have focused on efficacy and were not designed to evaluate QTc prolongation as the primary outcome. The Sequenced Treatment Alternatives to Relieve Depression trial did not find significant cardiac toxicity associated with citalopram; however, safety monitoring did not include ECGs.6-8 A systematic review by Rasmussen and colleagues9 evaluating trials of citalopram at doses up to 60 mg per day did not find a significant effect on QTc interval, but many of the studies included did not include patients with cardiovascular disease.
Castro and colleagues10 performed a cross-sectional study of 38 397 adult patients with ECGs after receiving a prescription for an antidepressant or methadone. This study found dose-dependent QTc prolongation for citalopram (adjusted β 0.10 [SE 0.04], P < .01), escitalopram (adjusted β 0.10 [SE 0.04], P < .01), and amitriptyline (adjusted β 0.11 [SE 0.03], P < 0.001). However, the extent of QTc prolongation was small for each of these medications and the proportion of patients categorized with abnormal QTc intervals (451-500 ms for men and 471-500 ms for women) was similar for all antidepressants studied. This study also revealed significant within-subject QTc prolongation with increases in citalopram dose from 10 to 20 mg (7.8 ms, P < 0.05) and 20 to 40 mg (10.3 ms, P < 0.01).
Zivin and colleagues11 conducted a study using Veterans Health Administration data between 2004 and 2009. Patients with a diagnosis of depression who received at least 1 prescription for citalopram (N = 618 450) or sertraline (N = 365 898) were included. Daily doses of citalopram >40 mg were associated with lower risks of ventricular arrhythmia (adjusted hazard ratio = 0.68; 95% CI, 0.61-0.76), all-cause mortality (adjusted hazard ratio = 0.94; 95% CI, 0.90-0.99), and noncardiac mortality (adjusted hazard ratio = 0.90; 95% CI, 0.86-0.96) compared with daily doses of 1 to 20 mg. No dose groups were found to be at increased risk of cardiac mortality, and there was a lower risk of ventricular arrhythmia with citalopram daily doses of 21 to 40 mg compared with daily doses of 1 to 20 mg (adjusted hazard ratio = 0.80; 95% CI, 0.74-0.86). There is a possibility that results were skewed because patients who received higher doses had fewer medical comorbidities and were less likely to reach the outcome of death during the study period, although the investigators did adjust for confounders.
Van Haelst and colleagues12 performed a cross-sectional study of 794 elderly surgical patients either prescribed SSRIs or not prescribed SSRIs, matched 1 to 1 based on sex and year of outpatient surgery, excluding cardiac surgeries and cardioversion. After adjusting for confounding factors, the researchers did not find any difference in the primary outcome of occurrence of QTc interval prolongation (>450 ms for men and >470 ms for women) between SSRI users (6%) and non-SSRI users (5%). Of the 397 patients in the index group who received SSRIs, the most commonly prescribed SSRIs were citalopram (n = 114) and paroxetine (n = 172). A subgroup analysis revealed that the incidence of QTc prolongation in the citalopram group (10%) was not statistically greater than their matched non-SSRI user cohorts (7%).
The current study evaluates the risk of developing prolonged QTc interval based on citalopram dose and patient age in a Veterans Health Administration population, and includes patients with significant cardiac disease in attempts to replicate a real-world setting and enhance generalizability of the study results.
Methods
Study Design
This was a retrospective chart review of patients ≥18 years old at the Veterans Affairs North Texas Health Care System who received at least one 30-day prescription for citalopram from January 1, 2007, to February 29, 2012. In addition, patients had a baseline ECG within 1 year before citalopram initiation or dose increase and at least 1 repeat ECG within 3 months after citalopram initiation or dose increase. Patients were excluded if they had prolonged QTc at baseline. Patients were also excluded if they were receiving concurrent medications with a risk of prolonging QTc interval, which included all drugs on qtdrugs.org (Arizona Cert Center for Education and Research on Therapeutics) with risk of torsades or possible risk of torsades.13 Drugs with conditional risk of torsades were not excluded. After charts of 1423 patients receiving citalopram during the study period were reviewed, 73 patients were included. The most common reasons for exclusion were lack of ECG, inappropriate ECG timing, and concurrent drugs with risk of torsades or possible risk of torsades.
Electronic medical records were reviewed to obtain the following data: age at time of citalopram initiation, sex, race, psychiatric diagnoses, laboratory data (AST/ALT, serum creatinine, and creatinine clearance), ECG data, citalopram dose, and concurrent drugs with conditional risk of torsades. The data were used to evaluate the demographics of the study population and the study endpoints. Data collection also included adverse events, including sudden cardiac death, TdP, and other cardiac events.
Endpoints
The primary endpoint was the percent of ECGs with QTc prolongation (≥470 ms for men and ≥480 ms for women) after initiation or dose increase of citalopram. Secondary endpoints included prevalence of QTc prolongation by dose, prevalence of QTc interval ≥500 ms or an increase of ≥60 ms from baseline, mean change in QTc interval by dose, and prevalence of cardiac arrest and cardiac events. Citalopram doses were stratified into the following groups to evaluate the secondary outcome of prevalence of QTc prolongation by dose: 10-20 mg, 30-40 mg, and 60-80 mg. Subgroup analyses of all primary and secondary endpoints were performed in patients ≥60 years old. For each patient, all QTc data that met inclusion criteria were collected, so patients who received different doses of citalopram during the study period are represented in multiple-dosage groups. However, only 1 patient experienced QTc prolongation with 2 different citalopram doses. This patient was included in the total percentage of patients who developed QTc prolongation but was omitted when comparing an effect among the different citalopram doses in order to maintain independence among the dose groups.
Statistical Analysis
Nominal data, including prevalence of QTc prolongation at various citalopram doses, were analyzed using a Fisher exact test. A Student t test was used to analyze continuous data, which included mean change in QTc interval from baseline in each dose group.
Results
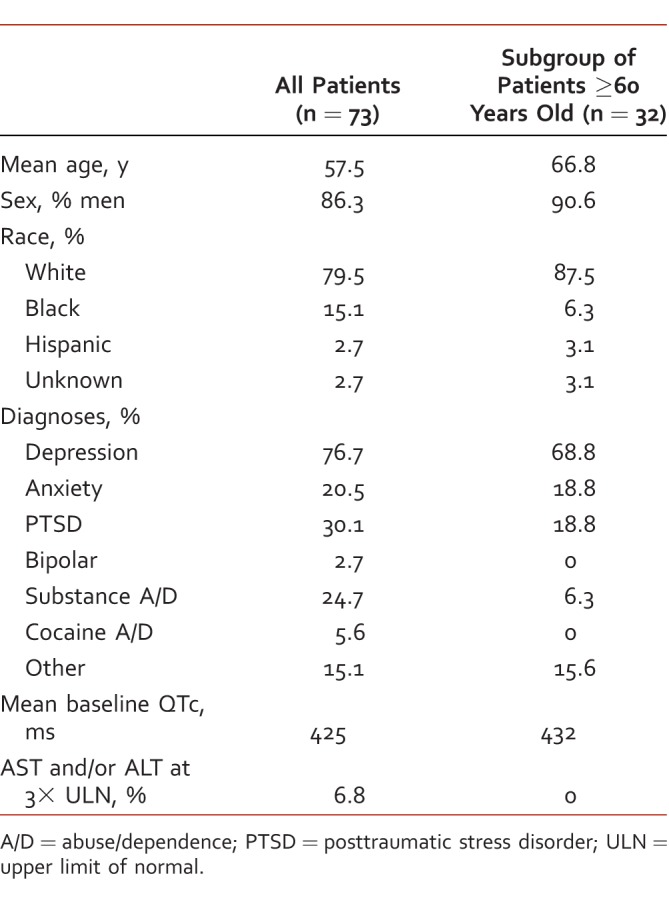
Seventy-three patients were included in this retrospective review, and 32 patients were ≥60 years old. Baseline characteristics are listed in Table 1. The mean age of the patients included in this study was 57.5 years, and the majority were men (86.3%) and white (79.5%). The most common psychiatric diagnosis was depression, and 18 patients (25%) had at least 1 documented form of current or past substance abuse.
TABLE 1: .
Baseline characteristics
Primary and secondary endpoints are displayed in Table 2 for the entire study sample and in Table 3 for patients ≥60 years old. QTc prolongation was identified in 12 patients (16.4%) among the entire study sample and in 7 patients (21.7%) in the subgroup of patients ≥60 years old. The prevalence of QTc prolongation increased with citalopram dose in the entire study population (P = .016). This trend was also observed in patients ≥60 years old but did not meet statistical significance.
TABLE 2: .
Secondary endpoints in all patients
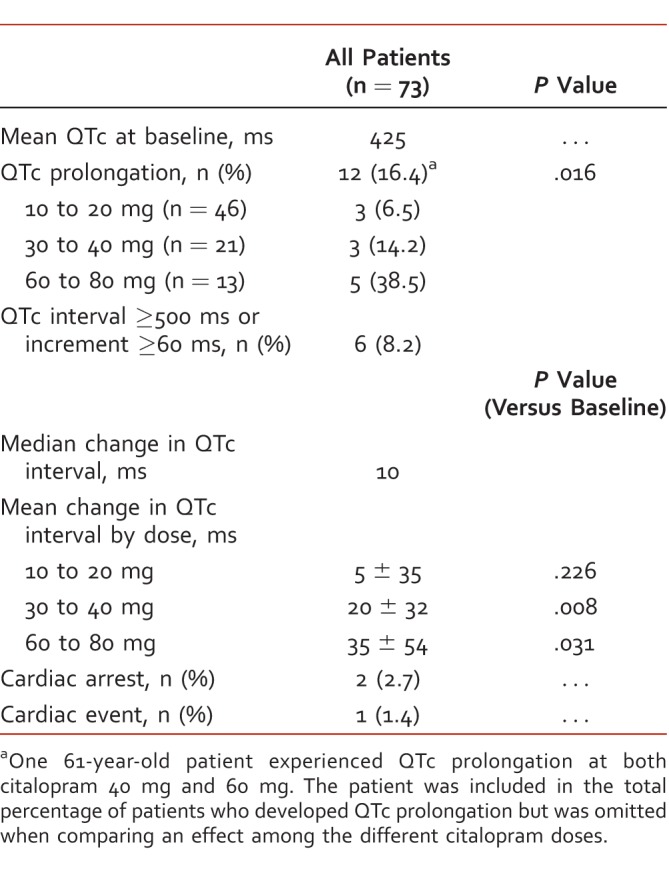
TABLE 3: .
Secondary endpoints in patients ≥60 years old
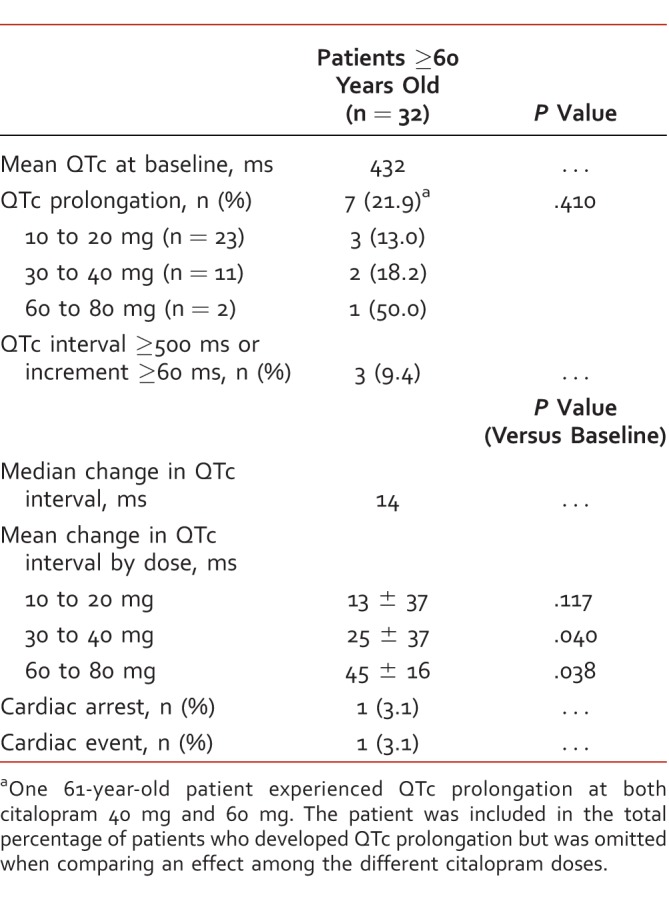
The mean change in QTc interval compared with baseline values after the initiation or a dose increase of citalopram was significant in both the 30- to 40-mg group and the 60- to 80-mg group, among the entire sample, and in the subgroup of patients ≥60 years old, as reflected in Tables 2 and 3. Six patients (8.2%) among the entire study population and 3 patients (9.4%) among patients ≥60 years old had QTc intervals ≥500 ms or an increase ≥60 ms from baseline. At the time of this ECG finding, 4 patients were receiving citalopram 60 mg, 1 patient was receiving citalopram 40 mg, and 1 patient was receiving citalopram 20 mg.
Of the 73 patients included in the study, there were 2 documented instances of cardiac arrest involving a code response team. One patient was prescribed citalopram 30 mg daily and the other patient was prescribed citalopram 40 mg daily. The patient on citalopram 40 mg daily did not have prolonged QTc on any ECG, but did have a history of cocaine use, which significantly increases the risk of cardiac events. QTc prolongation was documented on a routine ECG before the cardiac arrest for the patient prescribed citalopram 30 mg daily. A finding of TdP was not captured on ECG for either patient, but one of the patients was found to be in asystole in the field for an unknown duration of time. Both patients were resuscitated from cardiac arrest, but 1 patient later died in the intensive care unit after resuscitation. There was 1 cardiac event involving a noncoding patient with supraventricular tachycardia who was admitted to the intensive care unit.
Thirty-nine patients (53%) received at least 1 concomitant agent with a conditional risk of TdP. The most common of these agents was trazodone, which was prescribed for 29 patients (40%). Other agents with conditional TdP risk (trimethoprim/sulfamethoxazole, nortriptyline, amitriptyline, ciprofloxacin, diphenhydramine, and fluconazole) were each prescribed in fewer than 5 patients. Of the 12 patients who experienced prolonged QTc interval, 5 received at least 1 drug concurrently with citalopram with a conditional risk of TdP. Of these patients, 3 received trazodone alone, 1 received ciprofloxacin, and 1 received trazodone and diphenhydramine.
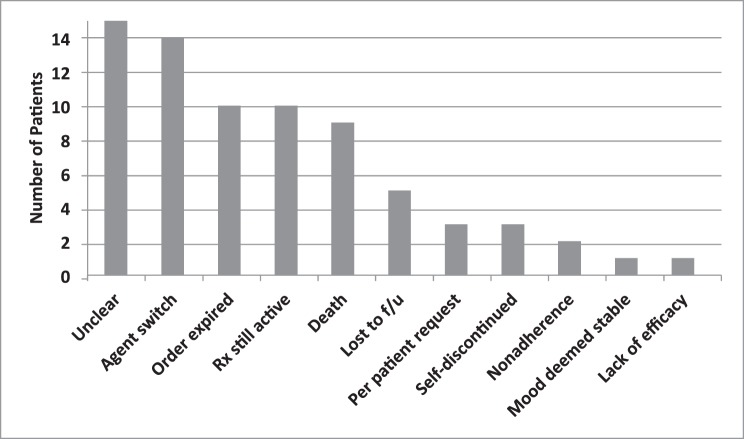
The Figure depicts citalopram discontinuations for various reasons. In the majority of cases, the reason for citalopram discontinuation was unclear or there was a switch to an alternative antidepressant agent. Ten patients still had an active prescription for citalopram at the time charts were reviewed.
FIGURE:
Reasons for citalopram discontinuation; Rx = prescription; f/u = follow-up
Discussion
To our knowledge, this is the first study that investigates the prevalence of QTc prolongation after initiation or dose increase of citalopram in a cohort of patients ≥60 years old. Broad exclusion due to lack of ECG monitoring and ECG timing suggests that ECGs are not being routinely monitored in patients prescribed citalopram, which increases the risk of undetected prolonged QTc that could lead to TdP or other arrhythmias. This study also suggests that there is an association between QTc prolongation and citalopram dose, as the prevalence of QTc prolongation increased significantly as dose increased in the entire study group. Although this same trend occurred in the subgroup of patients ≥60 years old, it did not meet statistical significance. This subgroup of patients was likely too small to show a statistical difference in prevalence of QTc prolongation with increasing citalopram dose. Upon comparison of QTc intervals to mean baseline values, there appears to be a citalopram dose-dependent increase in QTc interval in patients taking citalopram 30 mg or greater.
About 22% (n = 7) of patients ≥60 years old experienced QTc prolongation with citalopram compared with about 16% (n = 12) of all patients. The prevalence of QTc prolongation at each dose was higher in patients ≥60 years old, suggesting that elderly patients are at greater risk of QTc prolongation when exposed to high doses of citalopram. Of note, 2 of the 3 patients ≥60 years old prescribed citalopram 60 to 80 mg experienced QTc prolongation. Although the sample of patients who received 60 to 80 mg citalopram is very small, it is clear that the percentage of patients with QTc prolongation increased with dose. Our data support the FDA recommendation not to prescribe citalopram in doses greater than 20 mg daily in patients ≥60 years old. Given the findings of this retrospective study, we recommend ECG monitoring in adults receiving citalopram doses 30 mg and higher, especially in patients ≥60 years old and/or in patients with preexisting cardiovascular risk. These patients should have a baseline ECG and a follow-up ECG within 3 months of citalopram initiation. If there is evidence of QTc prolongation and citalopram is suspected to be the cause based on clinical judgment, it is recommended to discontinue citalopram in favor of another antidepressant agent without this known effect.11
It is important to note that greater than 8% of the entire study population had QTc intervals ≥500 ms or an increase ≥60 ms from baseline, which is thought to be associated with an increased risk of developing TdP.4 It must also be mentioned that 4 of the 6 patients with this ECG finding were receiving citalopram 60 mg daily. Of 73 patients, 2 (2.7%) experienced cardiac arrest, possibly as a result of ventricular arrhythmias. Although citalopram cannot be identified as the cause of cardiac arrest, it certainly suggests that it is vital to monitor ECGs in patients receiving high doses of citalopram and in patients with other risk factors for prolonged QTc interval. In this study, trazodone was the most frequently prescribed concurrent medication with a conditional risk of torsades according to qtdrugs.org, but there was not a clear signal that trazodone increased QTc prolongation risk. Almost half of the patients included in the study received trazodone, and almost half of the patients with QTc prolongation received trazodone. We are also not able to track the frequency of the as needed use of trazodone in this patient population, thus making it even more difficult to establish a link with QTc prolongation in patients receiving citalopram.
Some important limitations should be taken into consideration. First, this study is retrospective in nature, which makes it impossible to measure adherence to citalopram. Another concern is that the prevalence of QTc prolongation was overestimated because ECGs were not randomly obtained. In other words, not all patients who were screened for inclusion received ECGs, and those who did receive ECGs and were subsequently included in the study may have had other risk factors present for prolonged QTc interval. The study design excluded patients taking concomitant medications with significant risk of prolonging QTc but did not exclude patients with risk factors such as preexisting cardiac disease, which could increase the risk of QTc prolongation. On the other hand, including patients with cardiac disease is advantageous because this more accurately reflects a real-world setting and makes the results of this study more generalizable.
A second limitation relates to the prevalence of QTc prolongation among the various dose groups. The number of patients in each dose group is not equivalent, so it may not be reasonable to compare prevalence of QTc prolongation between groups. Another important consideration is the fact that our threshold for QTc prolongation (≥470 ms for men and ≥480 ms for women) was higher than in some previous studies.10,12 Using a more conservative cutoff for QTc prolongation would have resulted in a higher incidence of the primary outcome in our study. Also, we did not study the prevalence of QTc prolongation with other SSRI antidepressants, and therefore, can provide little guidance about whether other SSRIs provide a safer alternative to citalopram.
In conclusion, this study suggests that there is a citalopram dose-dependent increase in QTc interval with significant increases compared with baseline at doses ranging from 30 to 80 mg. The incidence of QTc prolongation with citalopram may be higher in patients ≥60 years old, and the incidence of cardiac arrest caused by ventricular arrhythmias may be greater than what has previously been reported. Caution is recommended when prescribing citalopram in patients with cardiovascular comorbidities, and timely ECG monitoring should be performed. The limitations of this small retrospective cohort study must be taken into consideration, and these results should be replicated in larger trials.
Footnotes
Disclosures: The authors have no disclosures of interest.
References
- 1. FDA [Internet]. Silver Spring (MD): FDA. FDA Drug Safety Communication: abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide) . August 24, 2011. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm269086.htm.
- 2. FDA [Internet]. Silver Spring (MD): FDA. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses; March 28, 2012. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. [Google Scholar]
- 3. Howland RH. . A critical evaluation of the cardiac toxicity of citalopram: part 1. J Psychosoc Nurs Ment Health Ser. 2011; 49 11: 13- 6. DOI: 10.3928/02793695-20111011-01. PubMed PMID: 22007855. [DOI] [PubMed] [Google Scholar]
- 4. Committee for proprietary medicinal products (CPMP) [Internet]. Silver Spring (MD): FDA. Points to consider. The assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. Available from: http://www.fda.gov/ohrms/dockets/ac/03/briefing/pubs/cpmp.pdf.
- 5. Kanjanauthai S, Kanluen T, Chareonthaitawee P. . Citalopram induced torsade de pointes, a rare life threatening side effect. Int J Cardiol. 2008; 131 1: e33- 4. DOI: 10.1016/j.ijcard.2007.08.006. PubMed PMID: 17919753. [DOI] [PubMed] [Google Scholar]
- 6. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006; 163 1: 28- 40. DOI: 10.1176/appi.ajp.163.1.28. PubMed PMID: 16390886. [DOI] [PubMed] [Google Scholar]
- 7. Howland RH. . Sequenced Treatment Alternatives to Relieve Depression (STAR*D). Part 1: study design. J Psychosoc Nurs Ment Health Serv. 2008; 46 9: 21- 4. PubMed PMID: 18822996. [DOI] [PubMed] [Google Scholar]
- 8. Howland RH. . Sequenced Treatment Alternatives to Relieve Depression (STAR*D). Part 2: Study outcomes. J Psychosoc Nurs Ment Health Serv. 2008; 46 10: 21- 4. PubMed PMID: 18935932. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen SL, Overø KF, Tanghøj P. . Cardiac safety of citalopram: prospective trials and retrospective analyses. J Clin Psychopharmacol. 1999; 19 5: 407- 15. PubMed PMID: 10505582. [DOI] [PubMed] [Google Scholar]
- 10. Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, et al. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ. 2013; 346:f288. DOI: 10.1136/bmj.f288. PubMed PMID: 23360890; PubMed Central PMCID: PMC3558546. [DOI] [PMC free article] [PubMed]
- 11. Zivin K, Pfeiffer PN, Bohnert ASB, Ganoczy D, Blow FC, Nallamothu BK, et al. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatry. 2013; 170 6: 642- 50. DOI: 10.1176/appi.ajp.2013.12030408. PubMed PMID: 23640689. [DOI] [PubMed] [Google Scholar]
- 12. van Haelst IMM, van Klei WA, Doodeman HJ, Warnier MJ, De Bruin ML, Kalkman CJ, et al. QT interval prolongation in users of selective serotonin reuptake inhibitors in an elderly surgical population: a cross-sectional study. J Clin Psychiatry. 2014; 75 1: 15- 21. DOI: 10.4088/JCP.13m08397. PubMed PMID: 24345304. [DOI] [PubMed] [Google Scholar]
- 13. Credible Meds [Internet]. Oro Valley (AZ): Credible Meds. Drug lists by risk groups. Available from: www.azcert.org/medical-pros/drug-lists. Accessed September 17, 2012.