Abstract
A strong association exists between epilepsy and psychiatric comorbidities, especially depression, anxiety, attention deficit disorders, and psychosis. The impact of psychotropic medications in lowering seizure threshold both directly and indirectly, hypersensitivity reactions to antiepileptic and other psychotropic medications, and how antiepileptic drugs affect psychiatric disorders are explored through three patient cases. Ultimately, in selecting an appropriate psychotropic medication for an individual with epilepsy and psychiatric comorbidities, it is important to consider the clinical and quality-of-life impacts that a particular medication will have on that individual.
Keywords: antiepileptic drugs (AEDs), psychiatric comorbidities, proconvulsant, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, psychiatric disorders, suicidal tendencies
Take Home Points:
Individuals with seizure disorders and comorbid psychiatric conditions are frequently undertreated with psychotropic medications due to a misconception that all psychotropic medications are proconvulsant. Only a subset of psychotropic medications, such as chlorpromazine and bupropion, have the highest likelihood to provoke seizures.
Aromatic antiepileptic drugs (AEDs) and tricyclic antidepressants are associated with idiosyncratic cutaneous reactions that can cross-react whereas other aromatic antidepressants rarely cross-react and can be used safely.
Seizure disorders are associated with behavioral disturbances while taking AEDs, especially in children and individuals with developmental delay. The causes of these behavioral manifestations are multifactorial and include, for example, suppression of seizure activity, seizure complexity, AED pharmacology, polypharmacy, drug interactions, genetics, and environmental influences.
Introduction
Epilepsy is a common chronic but complex medical disease that affects approximately 5.1 million adults and children in the United States and 50 million worldwide.1-3 It is characterized by more than 25 syndromes and multiple seizure types, which can vary in both severity and response to treatment.4 Due to the diverse symptomatology of epilepsy, persons with this condition may be challenged with psychiatric symptoms, such as cognitive and behavior changes, that can complicate epilepsy management by mimicking psychiatric disorders.5,6 Likewise, individuals with a psychiatric disorder, such as psychosis, anxiety, mood, and attention deficit disorder, have a higher likelihood of developing seizures and other neurological disorders, such as migraines and stroke, than the general population.6-9 Postmortem hippocampi were compared in individuals with mesial temporal lobe epilepsy, a common intractable seizure type, in the presence or absence of major depression versus interictal psychosis (a schizophrenia mimic).10,11 A closely related pattern of neuroinflammatory chemical abnormalities was seen in the presence of mesial temporal lobe epilepsy and either psychiatric disorder. This neuroinflammatory chemical finding may suggest greater insight into the relationship between the pathophysiology of epilepsy and comorbid psychiatric disorders.
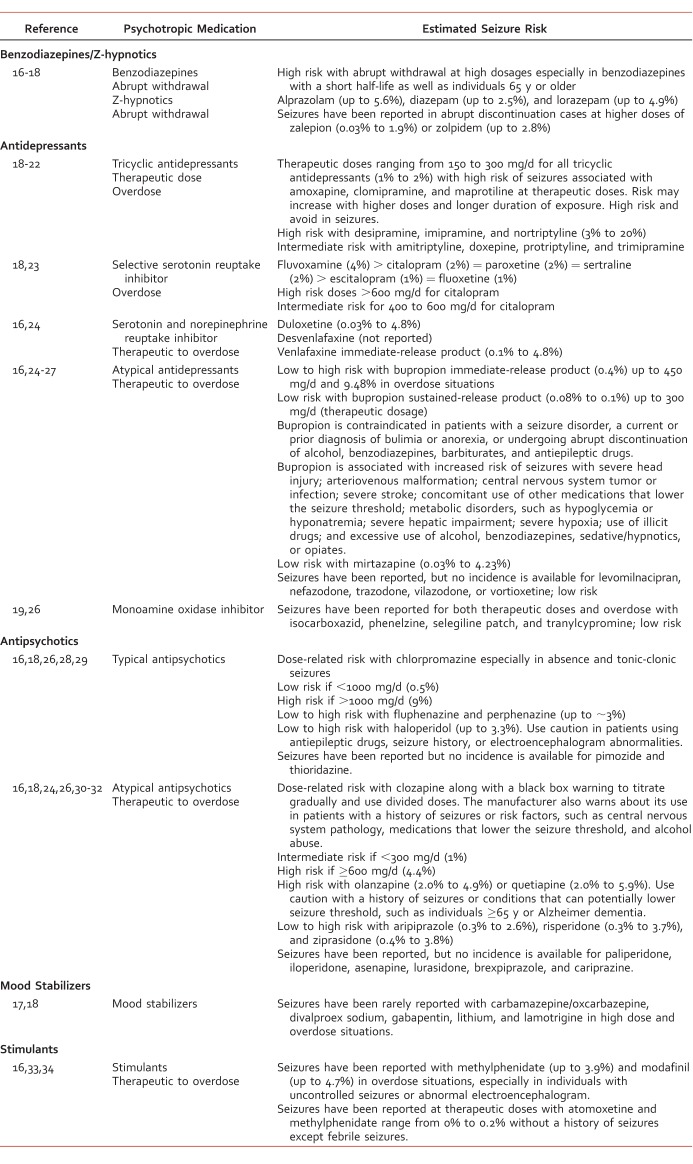
Common psychiatric comorbidities seen with epilepsy (Table 1) are depression, anxiety, attention deficit disorder, and psychosis at a prevalence rate of 20% to 30%.5,12,13 The most common psychiatric comorbidity is depression with a prevalence of 20% to 55%; however, in select populations, the prevalence can reach as high as 80% (Table 1).5,14 The prevalence of anxiety disorders is 19%, but these coexist with depression in up to 66% of patients with epilepsy.5,14,15 The prevalence of attention deficit disorder and psychosis with epilepsy is lower—up to 40% and 10%, respectively.5
TABLE 1.
Prevalence rate comparing epilepsy and psychiatric comorbidities5
In this article, three cases explore the impact of psychotropic medications in lowering seizure threshold both directly and indirectly, antiepileptic and other psychotropic hypersensitivity reaction considerations, and how antiepileptic drugs (AEDs) affect psychiatric disorders and suicidality.
Psychotropic Proconvulsants
A 68-year-old patient was brought into the emergency room by family members for increased anxiousness, decreased appetite, fatigue, and insomnia over the previous 3 weeks. The family also stated that the patient had seizure-like activity that lasted about 2 minutes. The patient has a past psychiatric history of major depressive disorder with psychotic features with a first hospitalization 4 years earlier. Medical history was significant for focal seizures diagnosed 1 year earlier. The patient graduated from college with a teaching degree and taught high school science until retiring at age 58. The patient lived independently until having a focal seizure and now lives with family members. Current medications include olanzapine 15 mg by mouth daily, bupropion hydrochloride extended-release 150 mg by mouth daily (started 3 weeks earlier), and levetiracetam 750 mg by mouth twice daily. Previous antidepressant history includes escitalopram, which caused diarrhea and was discontinued 3 weeks previously. A mental status exam noted that the patient appeared older than stated age with poor grooming, poor eye contact, depressed mood, and auditory hallucinations calling the patient worthless. Vital signs and laboratory results were normal. Height: 5′6″; weight: 60 kg; and body mass index: 26 kg/m2.
Several psychotropic medications are associated with a proconvulsant effect or withdrawal syndromes that may be responsible for drug-related seizures (Table 2).16-34 The seizure incidence associated with psychotropic medications varies. At therapeutic doses, the incidence ranges from approximately 0.1% to 1.7%, and with either intentional or unintentional overdoses, the incidence ranges from 4% to 58% (Table 2).16-34 More recently, however, reports suggest that there are limitations in estimating the likelihood for each psychotropic medication to provoke seizures because many of these epidemiology studies are retrospective, of small sample size, and lack controls.16 In fact, a small observational study evaluated electroencephalogram (EEG) changes of olanzapine in relationship to total daily dose and plasma concentration of patients diagnosed with paranoid schizophrenia.35 This study found EEG changes are common, especially when doses of olanzapine 20 mg/d are used although the predictability of future seizures is limited. When applied to this case, the potential for olanzapine to lower the seizure threshold is low because a total daily dose of 15 mg was used. In general, the risk of EEG changes varies between typical and atypical antipsychotics, and the greatest risk is seen with clozapine and olanzapine followed by risperidone and typical antipsychotics.36 Despite these methodological drawbacks, the proconvulsant concept has prompted the addition of generic warning labeling for most psychotropic medications that recommends using caution or avoiding their use altogether in patients with seizures. Studies show that the ability to provoke seizures has the highest likelihood in a subset of psychotropic medications, such as chlorpromazine and bupropion, yet when warnings are applied equally to all patients with epilepsy and a psychiatric comorbidity, suboptimal or lack of treatment of the psychiatric disorder is frequently the unfortunate outcome.24,39 Other factors that can promote seizures include the addition of psychotropic medications in vulnerable patient populations (eg, preexisting seizures, traumatic brain injury, eating disorders), polypharmacy (especially with AEDs, which are associated with pharmacokinetic, pharmacodynamic, or a combination of interactions), rapid downward titrations, and very high dosages or overdoses.26,40,41
TABLE 2.
Psychotropic medications known to lower seizure threshold
This patient case illustrates the potential consequence of using bupropion to treat depression in an individual with a preexisting seizure disorder. Bupropion and its primary active metabolite (hydroxybupropion) have been associated with an increased risk of seizures, in part, due to their sympathomimetic amine structures (similar to amphetamine) and proposed mechanism of increased noradrenergic and dopaminergic activity.27,42 It is contraindicated in epilepsy or certain comorbid conditions, including a history of eating disorders or abrupt discontinuation of AEDs, barbiturates, benzodiazepines, and ethanol.38 This phenomenon is dose-related and associated with the immediate-release product where a 10-fold increase in seizure risk is seen when the dose of bupropion is increased from 450 mg/d to 600 mg/d or greater.43 Typically, seizures present within 6 hours of ingestion of the immediate-release product.44,45 In contrast, the time to seizure onset with bupropion overdose is delayed with other formulations occurring at 10 hours (sustained-release) or 12 hours (extended-release), respectively, but can occur up to 24 hours.44,46-49 For sustained-release or extended-release products, the labeling is the same, but the seizure rate is lower based on a reduced peak plasma concentration associated with the longer-acting products.46,49 One could argue, however, that the risk of using either long-acting product within the therapeutic dose is comparable to the general population.46,49 Nevertheless, the contraindication labeling should dissuade a clinician from recommending any bupropion formulation from both a medical-legal perspective and clinical experience.24
AED Hypersensitivity Reactions and Cross-Reactivity
A 32-year-old Asian American female of Chinese descent with a history of depression and no seizure disorder intentionally overdosed on approximately 60 bupropion sustained-release 150 mg tablets and was found down after about 3 hours in her apartment by her roommate. She was transported by paramedics to the emergency room and during transport suffered what appeared to be a generalized tonic-clonic seizure lasting 30 seconds, which self-terminated. She was alert and oriented to place and person as well as able to follow commands. Her vital signs, laboratory, urine toxicology, and physical exam were normal except the electrocardiogram showed a sinus tachycardia of 110 beats per minute. A prolactin level was drawn 15 minutes after the ictal event and was abnormal with a measured level of 45 mcg/L, indicating a seizure took place. In the emergency room, she complained of nausea and had another generalized tonic-clonic seizure lasting 30 minutes despite treatment with 2 doses of lorazepam 4 mg intravenous (IV) via slow push. She was then loaded with phenytoin 1000 mg IV and started on phenytoin 300 mg by mouth daily, escitalopram 10 mg by mouth daily for depression, and trazodone 100 mg by mouth at bedtime for sleep.
By day 6 of the admission, her mental status exam was unremarkable. She was noticeably less depressed, participating in groups, goal oriented, eating 100% of her meals, and was sleeping about 8 hours per night. During medication group, she complained of a rash on her back, legs, and arms, which she said started early that morning. Upon physical exam, her pulse was 87 beats per minute, blood pressure 135/77 mmHg, respirations 17 breaths per minute, and temperature 39°C (102.2°F). She appeared moderately uncomfortable. Her skin revealed a scattered, erythematous, maculopapular rash along with painful cervical and axillary lymphadenopathy. Laboratory results revealed a white blood count of 16 000 mm3 with 37% neutrophils cells, 35% lymphocytes, 19% monocytes, and 8% eosinophils as well as elevated liver enzymes (aspartate aminotransferase 165 units/L and alanine aminotransferase 210 units/L). The rest of the physical exam and laboratory results were normal.
The patient was diagnosed with presumptive drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome based on clinical and laboratory findings. Phenytoin was immediately discontinued, and her seizures were managed with an alternative AED while escitalopram was continued. She was treated symptomatically with both systemic and topical corticosteroids, hydroxyzine, and IV fluids. By hospital day 10, all symptoms and laboratory findings were normalized, and she was discharged with a plan to assess the appropriateness of continuing the AED at her first outpatient appointment.
Aromatic AEDs (including carbamazepine, eslicarbazepine, ethosuximide, lamotrigine, lacosamide, oxcarbazepine, phenobarbital, phenytoin, primidone, and zonisamide) are associated with idiosyncratic cutaneous eruptions, which are diverse and range from common, nonsevere drug reactions known as AED hypersensitivity syndromes to rare life-threatening reactions, such as DRESS, Stevens Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN).50-53 Both SJS and TEN occur acutely and progress quickly. Painful skin lesions and mucosal membrane blisters develop and erode, leading to skin detachment and large areas of body surface area (BSA) necrosis. Frequently, the liver or other organs are involved. The amount of BSA detachment, however, is the distinguishing feature between SJS (BSA: 10% or less) and TEN (BSA: above 30%).
DRESS syndrome is life-threatening with a mortality risk primarily from liver failure in up to 10% to 20% of patients.54 It is characterized by cutaneous skin eruptions (87%), fever >38°C (90% to 100%), lymphadenopathy (70% to 75%), hematologic abnormalities (especially eosinophilia; 52%), and mild-to-severe involvement of internal organs (eg, liver: 50% to 80%, kidneys: 40%, lung: 33%, heart: 15%, or pancreas: 5%).50,55-57 The symptoms of DRESS commonly manifest after approximately 2 to 6 weeks of treatment but can appear up to 3 months after exposure to the offending agent with an estimated incidence of 1 to 10 per 10 000 drug exposures.50,54,58
The pathogenesis of DRESS syndrome is not fully understood. Three mechanisms have been proposed. Aromatic AEDs are preferentially metabolized via cytochrome P450 to an arene oxide intermediate.59 This intermediate can be detoxified enzymatically to a nontoxic metabolite by epoxide hydrolase or glutathione transferase. It is postulated that individuals with a defect in 1 of these enzymes will accumulate the arene oxide intermediate, which either triggers an immune response or covalently binds to macromolecules leading to cell death. Another proposed mechanism is the reactivation of several human herpesviruses including human herpesvirus 6 and 7, Epstein-Barr virus, and cytomegalovirus, which may trigger the DRESS syndrome.54,57 However, it remains unclear whether the reactivation of human herpesviruses is a trigger or is a complication of the syndrome.60,61 Last, there is a genetic relationship between the human leukocyte antigen (HLA) haplotypes that predispose an individual to a drug hypersensitivity reaction, such as with SJS/TEN.62-64 For example, individuals of Asian descent, particularly Han Chinese, Malaysian, South Chinese, and Thai individuals, are more likely to have the HLA-B*1502 allele, which leads to a tenfold increased risk of developing carbamazepine-induced SJS/TEN.65 In contrast, there is a strong association between the HLA-A*3101 allele and carbamazepine-induced SJS/TENS, and this allele is found more frequency in individuals from Korea, Japan, or Northern Europe compared to other genetic backgrounds.65 In at-risk populations, genetic testing should be conducted. When patients test positive for either HLA allele, carbamazepine should be avoided along with other aromatic AEDs known to cross-react with carbamazepine, including oxcarbazepine or phenytoin, unless the benefit clearly outweighs the risk.66
This case demonstrates the importance of recognizing the symptoms of DRESS early. It also emphasizes the need to immediately discontinue any medication(s) that may have caused DRESS as well as to avoid any medications that may cause it in the future. It is difficult in the early stages to predict whether the rash will remain benign or progress to a severe skin reaction. Moreover, the high rate of cross-reactivity (70%) among the aromatic AEDs is also a concern when selecting an alternative AED as there are limited genetic tools to assist in this decision.67,68 As seen with this case, however, the patient's seizures were provoked by a bupropion overdose. These seizures often can be managed by acute benzodiazepine treatment alone.69 In selecting alternative AEDs for unresolved or recurrent seizures as seen with this case, evaluate all potential medications that both cross-react and interact with phenytoin as well as AEDs that are effective in treating generalized tonic-clonic seizures. In reviewing the literature, psychotropic medications reported to cause DRESS include not only the aromatic AEDs mentioned above but amitriptyline, citalopram, clomipramine, ethosuximide, felbamate, fluoxetine, olanzapine, paroxetine, and topiramate.51-53,70,71 According to the International League Against Epilepsy, AEDs that are effective against generalized tonic-clonic seizures and avoid the potential of cross-reactivity include valproate (third-line agent), gabapentin (fourth-line intervention), levetiracetam (fourth-line intervention), vigabatrin (fourth-line intervention), benzodiazepines (insufficient evidence), pregabalin (insufficient evidence), tiagabine (insufficient evidence), and brivaracetam (insufficient evidence).72 For this patient, it would be prudent to avoid using valproate as it reduces the elimination of phenytoin via the CYP2C pathway and thus prolongs the recovery of her hypersensitivity reaction. Valproate is also associated with a high risk of teratogenicity in women of childbearing age where pregnancy planning as well as contraception advice should be discussed.73 For this patient, an AED from the later intervention category with minimal drug-drug interactions, such as levetiracetam or gabapentin, would be a viable alternative. If this patient instead had complex partial seizures, AEDs that are effective for this seizure type and avoid the potential of cross-reactivity include levetiracetam (first-line agent), valproate (second-line agent), gabapentin (third-line agent), topiramate (third-line agent), vigabatrin (third-line agent), clonazepam (fourth-line intervention), and other benzodiazepine agents (insufficient evidence).72
There is less information known about the risk of cross-reactivity between antidepressants and other drugs, and these reactions do not occur frequently with antidepressants.74 It would be prudent to avoid tricyclic antidepressants in individuals with known AED hypersensitivity reactions as there is evidence that they do cross-react especially with carbamazepine and its derivatives.75 In contrast, bupropion, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and atypical antidepressants have not been reported to cross-react with AEDs, and they rarely have been reported to cause DRESS despite having an aromatic chemical structure.71 As seen in this case and supported by the literature, escitalopram was safely continued without further worsening of symptoms after the offending AED was discontinued.76 In rare situations, however, when an antidepressant aggravates DRESS, the offending medication should be discontinued, and an alternative antidepressant from a different class should be considered.71
Agitation, Irritability, Hyperactivity, and Suicide Related to AED Use
A patient was brought in by the police with suicidal thoughts and irritability after being found standing on a 200-foot bridge. The patient had no precipitant or past suicide attempts or hospitalizations. Past medical history includes status-posttraumatic brain injury secondary to a car accident (including an intracranial hemorrhage, burr hole, and 3-month coma), and a history of 2 recent complex partial seizures within the last year. The patient lives at home with family. Current medications include escitalopram 20 mg by mouth daily, levetiracetam 1000 mg by mouth twice daily (started 2 weeks earlier), lithium carbonate 300 mg by mouth twice daily, ferrous fumerate 1 tablet by mouth daily, and norethindrone acetate and ethinyl estradiol 1 tablet by mouth daily. Previous AEDs include phenytoin, which caused hirsutism, and topiramate, which caused cognitive impairment and reduced effectiveness of oral contraceptive. The mental status exam was unremarkable except the patient appeared poorly groomed with minimal eye contact and depressed, irritable mood. Vital signs and laboratory results were normal. Lithium level was 0.7 mmol/L.
Antiepileptic drugs can be associated with both positive and negative psychiatric effects in individuals with seizures. These types of behaviors associated with seizures, including agitation, hyperactivity, irritability, psychosis, and restlessness, are more common in children and individuals with developmental delay (Table 3).77-82 In a large, single-center chart review, 16% of adults with epilepsy experienced psychiatric/behavioral side effects (PSE); more than 60% of these side-effects were attributed to the addition of new AEDs.84 The study also found that fewer PSEs were associated with gabapentin (0.6%, P < .001) and lamotrigine (4.8%, P < .001) when compared to other, newer AEDs. Levetiracetam, however, was associated with the highest incidence of PSEs at 15.7% (P < .001). In fact, individuals treated with AEDs who have a previous psychiatric history are prone to PSEs. In some individuals with epilepsy, suppression of seizure activity can result in psychotic episodes or behavioral/mood disturbances. This phenomenon is known as forced normalization and is most common in patients with temporal lobe and generalized epilepsies.82,85 Other factors that have been shown to predispose individuals to PSEs while taking AEDs include pharmacology, polypharmacy, drug interactions, genetics, and environmental influences.
TABLE 3.
The impact of antiepileptic drugs on psychiatric disorder (incidence provided where available)77-83
Proposed mechanisms of action for AEDs that influence psychiatric pharmacotherapy include reduction of glutamate excitation or enhanced gamma amino butyric acid inhibition.80 For example, benzodiazepines, felbamate, lamotrigine, phenobarbital, primidone, tiagabine, topiramate, valproic acid, vigabatrin, and zonisamide are associated with increased inhibitory gamma amino butyric acid neurotransmission. This form of neurotransmission can cause weight gain, sedation, fatigue, and cognitive impairment along with antianxiety and mood stabilization properties.81 Excitatory glutamate neurotransmission is inhibited by lamotrigine, perampanel, phenobarbital, and topiramate. Symptoms associated with excitatory neurotransmission appears to be contrary to that seen with inhibitory neurotransmission, such as weight loss, anxiety, depression, psychosis, and aggression.86,87
In 2008, the US Food and Drug Administration (FDA)88 issued a warning related to the risk of suicidal tendencies in individuals treated with 11 AEDs based on a meta-analysis of double-blind studies. The meta-analysis showed a near doubling of the risk of suicidal behavior in individuals treated with AEDs (4.3 per 1000) versus placebo (2.2 per 1000). As a result, the FDA issued a blanket warning stating individuals taking AEDs are at increased risk of suicidal tendencies. Upon closer review, the methodologies and results were fraught with several inconsistencies. The data was based on manufacturer spontaneous, self-reporting practices; usage of nonsystematic and retrospective data collection methodologies; and unilateral application of the FDA warning to all 11 AEDs analyzed despite statistical significance being reached for only 2 AEDs (lamotrigine and topiramate).89 Nevertheless, increased awareness of suicidal tendencies is still warranted as psychiatric comorbidities are common in individuals with epilepsy. In fact, risk factors for suicidal behaviors are complex, and individuals should be screened for the presence of preexisting mental illness. Other associated risk factors include seizure onset before 18 years of age, polytherapy, poor seizure control, and female gender.5,89
As seen in this case, levetiracetam is associated with suicidal tendencies in individuals with epilepsy.90 At doses above 1000 mg/d, aggression and irritability can also be seen.91 Other reported PSEs include depression, decreased memory/cognition, abnormal thinking, psychosis, anxiety, nervousness, emotional ability, unmanageable anger, rage, aggression, agitation, hostility, paranoia, somnolence, and insomnia.90-92 In determining the cause of PSEs, it is prudent to conduct a thorough medication review and determine which medications, if any, may be contributing to the observed PSEs along with other risk factors of suicidal tendencies. By removing the offending agent, the symptoms typically resolve back to baseline. For this patient, once levetiracetam was discontinued, the symptoms of suicidal ideation and depression improved without de-escalation of current medications.
Conclusion
Epilepsy is associated with several psychiatric comorbidities, especially depression, but these are often undiagnosed. If recognized, psychiatric comorbidities may be inadequately treated as a result of efforts to minimize the risk of precipitating a seizure. As was discussed, only a few psychotropic medications, namely bupropion and chlorpromazine, will substantially lower the seizure threshold, especially in vulnerable individuals, but only bupropion carries a contraindication. Besides specific medications, inherent drug properties, medication combinations, rapid titrations, very high dosages or overdoses, and/or an individual's response to these factors all play a role in whether an individual will be at risk for a breakthrough seizure or not. Antiepileptic drugs can both positively and negatively influence psychiatric behaviors and suicidality in a select patient population. Antiepileptic drugs can also cause predictable side effects or idiosyncratic cutaneous reactions that can cross-react with other structurally similar psychotropic agents or AEDs, which can lead to life-threatening complications. Ultimately, in choosing an appropriate psychotropic medication for an individual with epilepsy and psychiatric comorbidities, it is important to consider the clinical impact and quality of life aspects that a particular medication will have on that individual.
Footnotes
Disclosures: I have nothing personal to disclose. Psychopharmacology Pearls are review articles intended to highlight both the evidence base available and/or controversial areas of clinical care for psychiatric and neurologic conditions as well as strategies of clinical decision making used by expert clinicians. As pearls, articles reflect the views and practice of each author as substantiated with evidence-based facts as well as opinion and experience. Articles are edited by members of the Psychopharmacology Pearls Editorial Board as well as peer reviewed by MHC reviewers. This article was developed as part of the 2017 Psychopharmacology Pearls product for BCPP recertification credit. The course information and testing center is at cpnp.org/322903.
References
- 1. Centers for Disease Control and Prevention. Epilepsy in adults and access to care — United States, 2012. MMWR. 2012; 61 45: 909- 13. PubMed PMID: 23151949. [PubMed] [Google Scholar]
- 2. Russ SA, Larson K, Halfon N. . A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012; 129 2: 256- 64. DOI: 10.1542/peds.2010-1371. PubMed PMID: 22271699. [DOI] [PubMed] [Google Scholar]
- 3. Brodie MJ, French JA. . Management of epilepsy in adolescents and adults. Lancet. 2000; 356 9226: 323- 9. DOI: 10.1016/S0140-6736(00)02515-0. PubMed PMID: 11071202. [DOI] [PubMed] [Google Scholar]
- 4. Institute of Medicine (US). Committee on the Public Health Dimensions of the Epilepsies; England MJ, Liverman CT, Schultz AM, Strawbridge LM, . Epilepsy across the spectrum: Promoting health and understanding. Washington: National Academies Press; 2012. [PubMed] [Google Scholar]
- 5. Hamed SA. . Psychiatric symptomatologies and disorders related to epilepsy and antiepileptic medications. Expert Opin Drug Saf. 2011; 10 6: 913- 34. DOI: 10.1517/14740338.2011.588597. PubMed PMID: 21619486. [DOI] [PubMed] [Google Scholar]
- 6. Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. . Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012; 72 2: 184- 91. DOI: 10.1002/ana.23601. PubMed PMID: 22887468. [DOI] [PubMed] [Google Scholar]
- 7. Chang Y-T, Chen P-C, Tsai I-J, Sung F-C, Chin Z-N, Kuo H-T, et al. Bidirectional relation between schizophrenia and epilepsy: a population-based retrospective cohort study. Epilepsia. 2011; 52 11: 2036- 42. DOI: 10.1111/j.1528-1167.2011.03268.x. PubMed PMID: 21929680. [DOI] [PubMed] [Google Scholar]
- 8. Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KMA. . Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology. 2003; 60 8: 1308- 12. PubMed PMID: 12707434. [DOI] [PubMed] [Google Scholar]
- 9. Larson SL, Owens PL, Ford D, Eaton W. . Depressive disorder, dysthymia, and risk of stroke: thirteen-year follow-up from the Baltimore epidemiologic catchment area study. Stroke. 2001; 32 9: 1979- 83. DOI: 10.1161/hs0901.094623. PubMed PMID: 11546884. [DOI] [PubMed] [Google Scholar]
- 10. Kandratavicius L, Peixoto-Santos J, Monteiro M, Scandiuzzi R, Carlotti C, Assirati J, et al. Mesial temporal lobe epilepsy with psychiatric comorbidities: a place for differential neuroinflammatory interplay. J Neuroinflammation. 2015; 12: 38 DOI: 10.1186/s12974-015-0266-z. PubMed PMID: 25889039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tadokoro Y, Oshima T, Kanemoto K. . Interictal psychoses in comparison with schizophrenia--a prospective study. Epilepsia. 2007; 48 12: 2345- 51. DOI: 10.1111/j.1528-1167.2007.01230.x. PubMed PMID: 17666070. [DOI] [PubMed] [Google Scholar]
- 12. Gaitatzis A, Carroll K, Majeed A, Sander JW. . The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004; 45 12: 1613- 22. DOI: 10.1111/j.0013-9580.2004.17504.x. PubMed PMID: 15571520. [DOI] [PubMed] [Google Scholar]
- 13. Hesdorffer D, Krishnamoorthy E. . Neuropsychiatric disorders in epilepsy: epidemiology and classification. : Trimble M, Schmitz B, . Neuropsychiatric disorders in epilepsy: epidemiology and classification. Cambridge: Cambridge University Press; 2011. p 3- 13. [Google Scholar]
- 14. Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. . Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007; 48 12: 2336- 44. DOI: 10.1111/j.1528-1167.2007.01222.x. PubMed PMID: 17662062. [DOI] [PubMed] [Google Scholar]
- 15. Beyenburg S, Mitchell AJ, Schmidt D, Elger CE, Reuber M. . Anxiety in patients with epilepsy: systematic review and suggestions for clinical management. Epilepsy Behav. 2005; 7 2: 161- 71. DOI: 10.1016/j.yebeh.2005.05.014. PubMed PMID: 16054870. [DOI] [PubMed] [Google Scholar]
- 16. Kumlien E, Lundberg PO. . Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010; 19 2: 69- 73. DOI: 10.1016/j.seizure.2009.11.005. PubMed PMID: 20036167. [DOI] [PubMed] [Google Scholar]
- 17. Lee KC, Finley PR, Alldredge BK. . Risk of seizures associated with psychotropic medications: emphasis on new drugs and new findings. Expert Opin Drug Saf. 2003; 2 3: 233- 47. DOI: 10.1517/14740338.2.3.233. PubMed PMID: 12904103. [DOI] [PubMed] [Google Scholar]
- 18. Ruffmann C, Bogliun G, Beghi E. . Epileptogenic drugs: a systematic review. Expert Rev Neurother. 2006; 6 4: 575- 89. DOI: 10.1586/14737175.6.4.575. PubMed PMID: 16623656. [DOI] [PubMed] [Google Scholar]
- 19. Judge BS, Rentmeester LL. . Antidepressant overdose-induced seizures. Psychiatr Clin North Am. 2013; 36 2: 245- 60. DOI: 10.1016/j.psc.2013.02.004. PubMed PMID: 23688690. [DOI] [PubMed] [Google Scholar]
- 20. Activas Pharma, Inc. Amoxapine tablet [rev. 2015 Feb; cited 2017 Aug 24]. In DailyMed [Internet]. 2015 [about 18 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a16297df-3158-48db-85e5-5cd506885556 [Google Scholar]
- 21. Mallinckrodt, Inc. Clomipramine Hydrochloride capsule [rev. 2017 June; cited 2017 Aug 24]. In DailyMed [Internet]. 2017 [about 28 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d7cee3fa-d05c-4702-8f75-f446627bdb49 [Google Scholar]
- 22. Mylan Pharmaceuticals, Inc. Maprotiline Hydrochloride tablet, film coated [rev. 2014 Dec; cited 2017 Aug 24]. In DailyMed [Internet]. 2014 [about 17 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c3ca69e6-1ea0-4c2c-abcb-7264b2e79a87 [Google Scholar]
- 23. Isbister GK, Bowe SJ, Dawson A, Whyte IM. . Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004; 42 3: 277- 85. PubMed PMID: 15362595. [DOI] [PubMed] [Google Scholar]
- 24. Alper K, Schwartz KA, Kolts RL, Khan A. . Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007; 62 4: 345- 54. DOI: 10.1016/j.biopsych.2006.09.023. PubMed PMID: 17223086. [DOI] [PubMed] [Google Scholar]
- 25. Johnston JA, Lineberry CG, Ascher JA, Davidson J, Khayrallah MA, Feighner JP, et al. A 102-center prospective study of seizure in association with bupropion. J Clin Psychiatry. 1991; 52 11: 450- 6. PubMed PMID: 1744061. [PubMed] [Google Scholar]
- 26. Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R. . Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002; 25 2: 91- 110. DOI: 10.2165/00002018-200225020-00004. PubMed PMID: 11888352. [DOI] [PubMed] [Google Scholar]
- 27. Heritage Pharmaceuticals Inc. Bupropion Hydrochloride tablet [rev. 2017 May; cited 2017 Aug 24]. In DailyMed [Internet]. 2017 [about 22 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9196568e-aec8-4d56-993e-4cbb78d4a3a6 [Google Scholar]
- 28. Sandoz Inc. Chlorpromazine Hydrochloride tablet, sugar coated [rev. 2017 Aug; cited 2017 Aug 24]. In DailyMed [Internet]. 2017 [about 22 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b028fb3d-2939-4197-b31c-ac327f9593ec [Google Scholar]
- 29. Major Pharmaceuticals. Haloperidol tablet [rev. 2015 Mar; cited 2017 Aug 24]. In DailyMed [Internet]. 2015 [about 12 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6cf978f3-4945-4624-a103-b800837101a2 [Google Scholar]
- 30. Mylan Pharmaceuticals Inc. Clozapine tablet [rev. 2017 Feb; cited 2017 Aug 24]. In DailyMed [Internet]. 2017 [about 39 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=883b5d43-0339-7dc1-f775-93791fb9b978 [Google Scholar]
- 31. Teva Pharmaceuticals USA, Inc. Olanzapine tablet, film coated [rev. 2017 Mar; cited 2017 Aug 24]. In DailyMed [Internet]. 2017 [about 51 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676ace4e-7706-742d-cab0-430dd1f83408 [Google Scholar]
- 32. Jubilant Cadista Pharmaceuticals Inc. Quetiapine Fumerate tablet [rev. 2017 Mar; cited 2017 Aug 24]. In DailyMed [Internet]. 2017 [about 42 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=904f80af-44b6-d964-1680-ac9e6b61327b [Google Scholar]
- 33. Wernicke JF, Holdridge KC, Jin L, Edison T, Zhang S, Bangs ME, et al. Seizure risk in patients with attention-deficit-hyperactivity disorder treated with atomoxetine. Dev Med Child Neurol. 2007; 49 7: 498- 502. DOI: 10.1111/j.1469-8749.2007.00498.x. PubMed PMID: 17593120. [DOI] [PubMed] [Google Scholar]
- 34. UCB Pharma Inc. Methylphenidate Hydrochloride tablet [rev. 2006 Sept; cited 2017 Aug 24]. In DailyMed [Internet]. 2006 [about 12 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6281ca7c-2aec-4f14-b7e4-82401a701c96 [Google Scholar]
- 35. Degner D, Nitsche MA, Bias F, Rüther E, Reulbach U. . EEG alterations during treatment with olanzapine. Eur Arch Psychiatry Clin Neurosci. 2011; 261 7: 483- 8. DOI: 10.1007/s00406-011-0208-4. PubMed PMID: 21431467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centorrino F, Price BH, Tuttle M, Bahk W-M, Hennen J, Albert MJ, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002; 159 1: 109- 15. DOI: 10.1176/appi.ajp.159.1.109. PubMed PMID: 11772698. [DOI] [PubMed] [Google Scholar]
- 37. Messing RO, Closson RG, Simon RP. . Drug-induced seizures: A 10-year experience. Neurology. 1984; 34 12: 1582 DOI: 10.1212/WNL.34.12.1582. [DOI] [PubMed] [Google Scholar]
- 38. Thundiyil JG, Kearney TE, Olson KR. . Evolving epidemiology of drug-induced seizures reported to a Poison Control Center System. J Med Toxicol. 2007; 3 1: 15- 9. PubMed PMID: 18072153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alldredge BK. . Seizure risk associated with psychotropic drugs: clinical and pharmacokinetic considerations. Neurology. 1999; 53 5 Suppl 2: S68- 75. PubMed PMID: 10496236. [PubMed] [Google Scholar]
- 40. Horne RL, Ferguson JM, Pope HG, Hudson JI, Lineberry CG, Ascher J, et al. Treatment of bulimia with bupropion: a multicenter controlled trial. J Clin Psychiatry. 1988; 49 7: 262- 6. PubMed PMID: 3134343. [PubMed] [Google Scholar]
- 41. Annegers JF, Hauser WA, Coan SP, Rocca WA. . A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998; 338 1: 20- 4. DOI: 10.1056/NEJM199801013380104. PubMed PMID: 9414327. [DOI] [PubMed] [Google Scholar]
- 42. Gobbi G, Slater S, Boucher N, Debonnel G, Blier P. . Neurochemical and psychotropic effects of bupropion in healthy male subjects. J Clin Psychopharmacol. 2003; 23 3: 233- 9. DOI: 10.1097/01.jcp.0000084023.22282.03. PubMed PMID: 12826985. [DOI] [PubMed] [Google Scholar]
- 43. J Davidson . . Seizures and bupropion: a review. J Clin Psychiatry. 1989; 50 7: 256- 61. PubMed PMID: 2500425. [PubMed] [Google Scholar]
- 44. Starr P, Klein-Schwartz W, Spiller H, Kern P, Ekleberry SE, Kunkel S. . Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am J Emerg Med. 2009; 27 8: 911- 5. DOI: 10.1016/j.ajem.2008.07.004. PubMed PMID: 19857406. [DOI] [PubMed] [Google Scholar]
- 45. Shepherd G, Velez LI, Keyes DC. . Intentional bupropion overdoses. J Emerg Med. 2004; 27 2: 147- 51. DOI: 10.1016/j.jemermed.2004.02.017. PubMed PMID: 15261357. [DOI] [PubMed] [Google Scholar]
- 46. Jefferson JW, Pradko JF, Muir KT. . Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations. Clin Ther. 2005; 27 11: 1685- 95. DOI: 10.1016/j.clinthera.2005.11.011. PubMed PMID: 16368442. [DOI] [PubMed] [Google Scholar]
- 47. Kara H, Ak A, Bayır A, Acar D, Istanbulluoğlu R, Değirmenci S. . Seizures after overdoses of bupropion intake. Balkan Med J. 2013; 30 2: 248- 9. DOI: 10.5152/balkanmedj.2012.094. PubMed PMID: 25207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jepsen F, Matthews J, Andrews FJ. . Sustained release bupropion overdose: an important cause of prolonged symptoms after an overdose. Emerg Med J. 2003; 20 6: 560- 1. DOI: 10.1136/emj.20.6.560. PubMed PMID: 14623854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dunner DL, Zisook S, Billow AA, Batey SR, Johnston JA, Ascher JA. . A prospective safety surveillance study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry. 1998; 59 7: 366- 73. PubMed PMID: 9714265. [DOI] [PubMed] [Google Scholar]
- 50. Knowles SR, Shapiro LE, Shear NH. . Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf. 1999; 21 6: 489- 501. PubMed PMID: 10612272. [DOI] [PubMed] [Google Scholar]
- 51. Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011; 124 7: 588- 97. DOI: 10.1016/j.amjmed.2011.01.017. PubMed PMID: 21592453. [DOI] [PubMed] [Google Scholar]
- 52. Meik S, Arias M, Fernandez Mago L, Lopez Sontoro MC, Abeldano A, Pellerano G. . Anticonvulsant hypersensitivity syndrome (DRESS Syndrome): Report of 4 cases. Dermatol Argent. 2010; 16 4: 272- 7. [Google Scholar]
- 53. Fong MK, Sheng B. . DRESS syndrome: A case of cross-reactivity with lacosamide? Epilepsia Open. 2017; 261916 2: 273- 5. DOI: 10.1002/epi4.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Piñana E, Lei SH, Merino R, Melgosa M, De La Vega R, Gonzales-Obeso E, et al. DRESS-syndrome on sulfasalazine and naproxen treatment for juvenile idiopathic arthritis and reactivation of human herpevirus 6 in an 11-year-old Caucasian boy. J Clin Pharm Ther. 2010; 35 3: 365- 70. DOI: 10.1111/j.1365-2710.2009.01081.x. PubMed PMID: 20831538. [DOI] [PubMed] [Google Scholar]
- 55. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007; 156 3: 609- 11. DOI: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- 56. Bocquet H, Bagot M, Roujeau JC. . Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. 1996; 15 4: 250- 7. DOI: 10.1016/S1085-5629(96)80038-1. PubMed PMID: 9069593. [DOI] [PubMed] [Google Scholar]
- 57. Walsh SA, Creamer D. . Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2010; 36 1: 6- 11. DOI: 10.1111/j.1365-2230.2010.03967.x. PubMed PMID: 21143513. [DOI] [PubMed] [Google Scholar]
- 58. Rzany B, Correia O, Kelly JP, Naldi L, Auquier A, Stern R. . Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy: a case-control study. Study Group of the International Case Control Study on Severe Cutaneous Adverse Reactions. Lancet. 1999; 353 9171: 2190- 4. PubMed PMID: 10392983. [DOI] [PubMed] [Google Scholar]
- 59. Criado PR, Criado RFJ, de Avancini JM, Santi CG. . Drug reaction with eosinophilia and systemic symptoms (DRESS) / drug-induced hypersensitivity syndrome (DIHS): a review of current concepts. An Bras Dermatol. 2012; 87 3: 435- 49. PubMed PMID: 22714760. [DOI] [PubMed] [Google Scholar]
- 60. Kano Y, Hiraharas K, Sakuma K, Shiohara T. . Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol. 2006; 155 2: 301- 6. DOI: 10.1111/j.1365-2133.2006.07238.x. PubMed PMID: 16882166. [DOI] [PubMed] [Google Scholar]
- 61. Seishima M, Yamanaka S, Fujisawa T, Tohyama M, Hashimoto K. . Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006; 155 2: 344- 9. DOI: 10.1111/j.1365-2133.2006.07332.x. PubMed PMID: 16882173. [DOI] [PubMed] [Google Scholar]
- 62. Chung W-H, Hung S-I, Hong H-S, Hsih M-S, Yang L-C, Ho H-C, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004; 428 6982: 486 DOI: 10.1038/428486a. PubMed PMID: 15057820. [DOI] [PubMed] [Google Scholar]
- 63. Hung S-I, Chung W-H, Liou L-B, Chu C-C, Lin M, Huang H-P, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005; 102 11: 4134- 9. DOI: 10.1073/pnas.0409500102. PubMed PMID: 15743917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moling O, Tappeiner L, Piccin A, Pagani E, Rossi P, Rimenti G, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir – a hypothesis. Med Sci Monit. 2012; 18 7: CS57- 62. DOI: 10.12659/MSM.883198. PubMed PMID: 22739739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leckband SG, Kelsoe JR, Dunnenberger HM, George AL, Tran E, Berger R, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin Pharmacol Ther. 2013; 94 3: 324- 8. DOI: 10.1038/clpt.2013.103. PubMed PMID: 23695185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Novartis Pharmaceuticals Corporation. Tegretol (carbamazepine) tablet, chewable, suspension, extended release tablet [rev 2015 Aug; cited 2017 July 17]. In: DailyMed [Internet]. 2005 [about 25 p.]. Bethesda (MD): National Library of Medicine (US) Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8d409411-aa9f-4f3a-a52c-fbcb0c3ec053 [Google Scholar]
- 67. Knowles SR, Dewhurst N, Shear NH. . Anticonvulsant hypersensitivity syndrome: an update. Expert Opin Drug Saf. 2012; 11 5: 767- 78. DOI: 10.1517/14740338.2012.705828. PubMed PMID: 22794330. [DOI] [PubMed] [Google Scholar]
- 68. Shear NH. . Defining the drug hypersensitivity syndrome: pharmacological basis. : Dyall-Smith D, Marks R, . Dermatology at the millennium. New York: Parthenon; 1999. p 394- 5. [Google Scholar]
- 69. Kim D, Steinhart B. . Seizures induced by recreational abuse of bupropion tablets via nasal insufflation [abstract]. CJEM. 2010; 12 2: 158- 161. DOI: 10.1017/S1481803500012203. [DOI] [PubMed] [Google Scholar]
- 70. Richard MA, Fiszenson F, Jreissati M, . Jean Pastor MJ, Grob JJ. [Cutaneous adverse effects during selective serotonin reuptake inhibitors therapy: 2 cases]. Ann Dermatol Venereol. 2001; 128 6-7: 759- 61. PubMed PMID: 11460042. [PubMed] [Google Scholar]
- 71. Herstowska M, Komorowska O, Cubała WJ, Jakuszkowiak-Wojten K, Gałuszko-Węgielnik M, Landowski J. . Severe skin complications in patients treated with antidepressants: a literature review. Postepy Dermatol Alergol. 2014; 31 2: 92- 7. DOI: 10.5114/pdia.2014.40930. PubMed PMID: 25097474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kälviäinen R, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013; 54 3: 551- 63. DOI: 10.1111/epi.12074. PubMed PMID: 23350722. [DOI] [PubMed] [Google Scholar]
- 73. Bromfield EB, Dworetzky BA, Wyszynski DF, Smith CR, Baldwin EJ, Holmes LB. . Valproate teratogenicity and epilepsy syndrome. Epilepsia. 2008; 49 12: 2122- 4. DOI: 10.1111/j.1528-1167.2008.01696.x. PubMed PMID: 18557775. [DOI] [PubMed] [Google Scholar]
- 74. Lamer V, Lipozencić J, Turcić P. . Adverse cutaneous reactions to psychopharmaceuticals. Acta Dermatovenerol Croat. 2010; 18 1: 56- 67. PubMed PMID: 20361889. [PubMed] [Google Scholar]
- 75. Seitz CS, Pfeuffer P, Raith P, Bröcker EB, Trautmann A. . Anticonvulsant hypersensitivity syndrome: cross-reactivity with tricyclic antidepressant agents. Ann Allergy Asthma Immunol. 2006; 97 5: 698- 702. DOI: 10.1016/S1081-1206(10)61103-9. PubMed PMID: 17165282. [DOI] [PubMed] [Google Scholar]
- 76. Cornell SL, DiBlasi D, Arora NS. . Drug reaction with eosinophilia and systemic symptoms: DRESS following initiation of oxcarbazepine with elevated human herpesvirus-6 titer. Case Rep Dermatol Med. 2014; 2014: 853281 DOI: 10.1155/2014/853281. PubMed PMID: 24826354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kaufman KR. . Antiepileptic drugs in the treatment of psychiatric disorders. Epilepsy Behav. 2011; 21 1: 1- 11. DOI: 10.1016/j.yebeh.2011.03.011. PubMed PMID: 21498130. [DOI] [PubMed] [Google Scholar]
- 78. Boylan LS, Devinsky O, Barry JJ, Ketter TA. . Psychiatric uses of antiepileptic treatments. Epilepsy Behav. 2002; 3 5S: S54- 9. PubMed PMID: 12609323. [DOI] [PubMed] [Google Scholar]
- 79. Cavanna AE, Seri S. . Psychiatric adverse effects of zonisamide in patients with epilepsy and mental disorder comorbidities. Epilepsy Behav. 2013; 29 2: 281- 4. DOI: 10.1016/j.yebeh.2013.08.024. PubMed PMID: 24070880. [DOI] [PubMed] [Google Scholar]
- 80. Piedad J, Rickards H, Besag FMC, Cavanna AE. . Beneficial and adverse psychotropic effects of antiepileptic drugs in patients with epilepsy: a summary of prevalence, underlying mechanisms and data limitations. CNS Drugs. 2012; 26 4: 319- 35. DOI: 10.2165/11599780-000000000-00000. PubMed PMID: 22393904. [DOI] [PubMed] [Google Scholar]
- 81. Ketter TA, Post RM, Theodore WH. . Positive and negative psychiatric effects of antiepileptic drugs in patients with seizure disorders. Neurology. 1999; 53 5 Suppl 2: S53- 67. PubMed PMID: 10496235. [PubMed] [Google Scholar]
- 82. Thigpen J, Miller SE, Pond BB. . Behavioral side effects of antiepileptic drugs. US Pharm. 2013; 38 11: HS15- 20. [Google Scholar]
- 83. Lexicomp Online®. Lexi-Drugs®. Hudson (OH): Lexi-Comp, Inc.; August 24, 2017. [Google Scholar]
- 84. Weintraub D, Buchsbaum R, Resor SR, Hirsch LJ. . Psychiatric and behavioral side effects of the newer antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2007; 10 1: 105- 10. DOI: 10.1016/j.yebeh.2006.08.008. PubMed PMID: 17079191. [DOI] [PubMed] [Google Scholar]
- 85. Landolt H. . [Psychic disorders in epilepsy. Clinical and electroencephalographic research]. Dtsch Med Wochenschr. 1962; 87: 446- 52. DOI: 10.1055/s-0028-1111776. PubMed PMID: 14461985. [DOI] [PubMed] [Google Scholar]
- 86. Kugaya A, Sanacora G. . Beyond monoamines: glutamatergic function in mood disorder [abstract]. CNS Spectr. 2005; 10 10: 808- 19. DOI: 10.1017/S1092852900010403. PubMed PMID: 16400244. [DOI] [PubMed] [Google Scholar]
- 87. Coccaro EF, Lee R, Vezina P. . Cerebrospinal fluid glutamate concentration correlates with impulsive aggression in human subjects. J Psychiatr Res. 2013; 47 9: 1247- 53. DOI: 10.1016/j.jpsychires.2013.05.001. PubMed PMID: 23791397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. US Food and Drug Administration. Suicidal behavior and ideation and antiepileptic drugs [updated 2009 May 9; cited 2017 Jul 6] Available from: https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm100190.htm
- 89. Britton JW, Shih JJ. . Antiepileptic drugs and suicidality. Drug Healthc Patient Saf. 2010; 2: 181- 9. DOI: 10.2147/DHPS.S13225. PubMed PMID: 21701630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. . Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Reports. 2015; 4: 79- 81. DOI: 10.1016/j.ebcr.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rickles NM, Gidal BE, Collins M, Hermanson MK, Sheth RD, Rutecki PA. . Possible association of behavioral disturbances with levetiracetam: report of a case series. Epilepsia. 2001; 42 suppl 7: 259. [Google Scholar]
- 92. Noguchi T, Fukatsu N, Kato H, Oshima T, Kanemoto K. . Impact of antiepileptic drugs on genesis of psychosis. Epilepsy Behav. 2012; 23 4: 462- 5. DOI: 10.1016/j.yebeh.2012.01.012. PubMed PMID: 22406094. [DOI] [PubMed] [Google Scholar]